Open Access Article
Open Access ArticleCatalytic methoxylation of aryl halides using 13C- and 14C-labeled CO2†
Alexia
Ohleier
abc,
Antoine
Sallustrau
 b,
Bouchaib
Mouhsine
b,
Fabien
Caillé
b,
Bouchaib
Mouhsine
b,
Fabien
Caillé
 *c,
Davide
Audisio
*c,
Davide
Audisio
 *b and
Thibault
Cantat
*b and
Thibault
Cantat
 *a
*a
aUniversité Paris-Saclay, CEA, CNRS, NIMBE, Gif-sur-Yvette 91191, France. E-mail: thibault.cantat@cea.fr
bUniversité Paris-Saclay, Service de Chimie Bio-organique et Marquage (SCBM), CEA/DRF/JOLIOT, Gif sur Yvette 91191, France. E-mail: davide.audisio@cea.fr
cUniversité Paris-Saclay, Inserm, CNRS, CEA, Laboratoire d’Imagerie Biomédicale Multimodale Paris-Saclay, Orsay 91401, France. E-mail: fabien.caille@cea.fr
First published on 6th October 2022
Abstract
The functionalization of carbon dioxide (CO2) into high-value building blocks is a relevant topic in carbon isotope labeling, where CO2 is the primary carbon source. A catalytic methoxylation of aryl halides directly from [13C] and [14C]CO2 is reported. Relying on the intermediacy of the methoxyborane BBN-OCH3, as a new secondary nucleophilic labeled source, this strategy allowed labeling of a series of substrates, including challenging pharmaceuticals containing tertiary alkyl amine substituents.
The interest in CO2 transformations has brought tremendous progress in terms of conversion into high-value building blocks and development of mild reaction conditions, especially regarding low temperature and pressure.1 Such developments are highly relevant for the field of carbon isotope labeling, as CO2 is the most accessible primary source of carbon-14 (14C).2,3 These isotopes have widespread applications from medical imaging to ADME (absorption, distribution, metabolism, excretion) studies for drug development.4 However, the thermodynamic stability of CO2, has dramatically hampered the availability of 14C-labeled radiotracers. Indeed, [14C]CO2 is generally incorporated at the beginning of costly ([14C]CO2: 1600 € mmol−1) and time-consuming multistep syntheses at the expense of large amounts of long-lived radioactive waste (half-life: 5730 years). In addition, chemoselectivity is an important issue, as most functional groups do not withstand the harsh conditions often required to functionalize CO2.5 In this context, it becomes essential to develop late-stage labeling strategies relying on fast, selective and mild CO2 transformations to access a broader range of highly functionalized radiolabeled molecules.
The O-methyl group is one of the most popular structural features in medicinal chemistry and in the top-sold pharmaceuticals (see Scheme 1, top).6 It is therefore relevant to introduce a 14C radioisotope on the methoxy position. This approach is frequently utilized for preclinical in vitro experiments, and in early-stage developments of novel potential drugs.5 Access to labeled O-[14C]methylated substrates is traditionally granted by electrophilic methylation in the presence of [14C]methyl iodide.7 [14C]CH3I is prepared by LiAlH4 reduction of [14C]CO2 to [14C]CH3OH, followed by thermic treatment with HI,8 and subsequently employed for the synthesis of methyl ethers through the Williamson reaction. However, this strategy encounters selectivity issues in the presence of nucleophilic aliphatic amines, where the competitive N-methylation leads to the formation of quaternary ammonium salts, low yields and laborious purifications.9 For example, the selective O-[14C]methylation of dextromethorphan required the orthogonal vinyl chloroformate protection of the alcohol and the tertiary amine.10
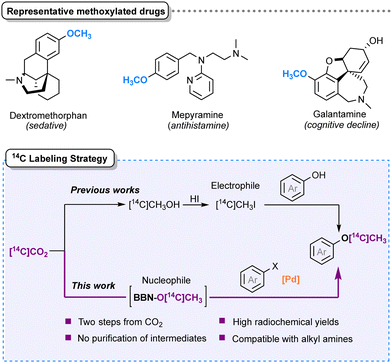 | ||
| Scheme 1 Representative drugs containing O-methyl groups (top) and new strategy to introduce a labeled –O[14C]CH3 synthon (bottom). | ||
These limitations encouraged us to develop a reversed polarity methoxylation strategy starting from labeled [14C]CO2.
We previously investigated the organocatalyzed CO2 reduction into BBN-OCH32, using the hydroborane 9-BBN (1) as a reductant.11 Among the organic bases catalyzing the reaction, Verkade's proazaphosphatrane (VBMe) appeared well-suited as it ensured fast, selective and stoichiometric CO2 conversion. In the presence of 9-BBN 1 and 5 mol% VBMe, [13C]CO2 was fully converted into BBN-O[13C]CH3[13C]2 within 30 min at 60 °C or 3 h at RT (Scheme 2A). We reasoned that the nucleophilic secondary carbon source BBN-OCH32, might represent an alternative opportunity to insert a methoxy moiety. In this work, we explored the palladium-catalyzed Suzuki coupling between aryl halides and BBN-OCH32, acting as a nucleophilic methoxylation source. We further developed a sequential one-pot protocol, which allows inserting methoxy moieties (–OCH3) starting directly from labeled CO2, unlocking the O-methylation of substrates bearing N-alkyl substituents. The versatility of this approach was first demonstrated using non-radioactive 13C on small molecules and pharmaceutical derivatives and subsequently applied to radiosyntheses with 14C.
A variety of metal-catalyzed C–O bond forming reactions have emerged over the last two decades.12 Although couplings involving primary and secondary alcohols are now well-established,13 direct methoxylations of aryl halides still remain scarce. Initiated by Buchwald in 2001, the palladium-catalyzed synthesis of anisoles from methanol, was limited to the highly electron deficient 3-nitroanisole.14 By varying the methanol source and by using bulky ligands, Clarke15 and Beller16 managed to improve the reaction conditions and significantly broadened the scope. However, most protocols require large excess of methanol,17 which is incompatible with labeling prospects. Interestingly, Novák reported an efficient cross coupling between aryl chlorides and equimolar amounts of tetramethoxyborate salts MB(OR)4 in the presence of Pd2dba3/tBuXPhos.18 Inspired by these achievements, we sought to develop the palladium catalyzed Suzuki coupling reaction between BBN-OCH32 and aromatic halides 3.
At first, the reaction conditions were optimized using 4-bromotoluene 3a and commercially available BBN-OCH32 in the presence of 10 mol% tBuXPhos and 5 mol% Pd2dba3 (Scheme 2B). We examined the influence of the base: the addition of 1.5 eq. of KOMe, generating in situ K[BBN-(OCH3)2], a surrogate of Novák's borate salt, led to 96% conversion within 1 h at 80 °C. Alongside 80% 4-methylanisole 4a, homocoupled product as well as toluene and formaldehyde, originating from β-hydride elimination, were formed. Though efficient, these conditions are not applicable to 13C and 14C labeling, as the use of unlabeled KOMe would isotopically dilute the desired product. While they differentiate both transmetalating moieties, the use of KOiPr and KOtBu generated 4-methoxyanisole 4a in only 54% and 62% yield respectively, with small amounts of isopropoxy- and tert-butoxyanisole. The use of 2 eq. of CsF turned out to be an efficient alternative, reaching 66% yield without additional side-products. A solvent screening confirmed that THF provided the best conversions, while being fully compatible with the CO2 reduction conditions into BBN-OCH32 (see ESI†). Interestingly, as previously observed by Novák,18 the Suzuki coupling performs better in the presence of aryl chlorides compared to aryl bromides (10% yield improvement) and works sluggishly with iodide derivatives (see ESI† for details), where homocoupling and dehalogenation products are preferentially formed. At present, we speculate that the relative rates between the oxidative addition19 and β-hydride elimination20 steps might explain this difference in reactivity.
Next, we investigated the feasibility of direct CO2 reduction into BBN-OCH32, followed by palladium-catalyzed Suzuki coupling for the methoxylation of aryl chlorides. Despite extensive optimization, the one-pot procedure could not be implemented, as 9-BBN 1 transmetalates faster than BBN-OCH32, thus leading to the formation of large amounts of toluene. Furthermore, both catalytic systems VBMe and tBuXPhos/Pd2dba3 appear to be incompatible. Decreasing the Verkade's base loading, employing easily removable low boiling point alternatives, as phosphazene BTPP or Suzuki friendly CsF, and preforming the active palladium species did not prevent catalyst poisoning.
Inspired by Skrydstrup's work on the commercial COware technology,21 we designed a two-chamber system to physically separate both reaction steps. The optimized set-up (Scheme S2, ESI†) is a double chamber, and can be connected to the RC Tritec manifold that allows handling and transferring controlled amounts of [13C] and [14C]CO2 into a reaction vessel. CO2 reduction was carried out in the first chamber, a distillation step was introduced to transfer BBN-OCH32 into the second vessel, pre-charged with the reagents and catalyst of the Suzuki coupling. BBN-OCH32 being a high boiling point liquid (230 °C < b.p. < 260 °C)22 efficient distillation was performed by heating under static vacuum (0.05 mbar).
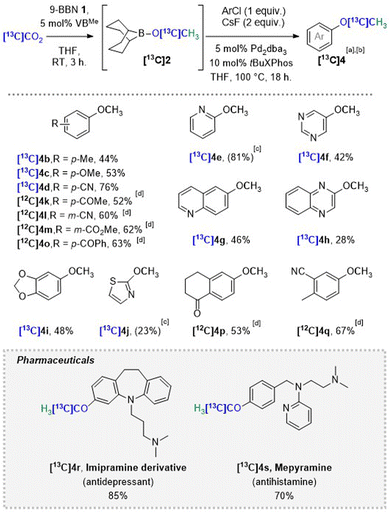
The complete reaction sequence was validated starting from 1 eq. of labeled [13C]CO2, generating in situ BBN-O[13C]CH3[13C]2, which was reacted with 1 eq. of model substrate 4-chlorotoluene 3b. After 18 h at 100 °C, 44% isolated yield of 4-methylanisole [13C]4b was obtained with respect to [13C]CO2 as limiting reagent, thus highlighting the stoichiometric conversion of the labeled starting material. Next, the substrate scope of the reaction sequence was investigated on a 1 mmol scale using the double chamber set-up and [13C]CO2 as a convenient and handy surrogate of [14C]CO2. A variety of aryl and heteroaryl chlorides could successfully be coupled in good to excellent yields with in situ generated BBN-O[13C]CH3[13C]2 (Scheme 3). The reaction resulted in a higher yield in the presence of an electron-withdrawing group (76% and 60% for [13C]4d and [12C]4l), whereas increasing the strength of electron-donating substituents gradually decreased it (44% for [13C]4b and 53% for [13C]4c).
The developed methodology was applied to nitrogen containing heterocycles.23 In this way, 2-chloropyridine 3e, 6-chloroquinoline 3g and 2-chloroquinoxaline 3h could all be converted into their methoxylated counterparts with up to 81% yield. The position 5 on the pyrimidine cycle 3f being the less electron deficient one and thus the less reactive towards nucleophiles, 5-methoxypyrimidine [13C]4f was obtained with 42% yield. In a similar fashion, electron-rich 5-chlorobenzo[1,3]dioxole 3i was transformed into [13C]5-methoxybenzo[1,3]dioxole [13C]4i, a sesamol derivative, in 48% isolated yield. Surprisingly, highly activated 2-chlorothiazole 3j was partially converted and generated only 23% NMR yield [13C]4j.
Further study of the scope revealed that the presence of substituted ketones was well tolerated. [12C]4k and 4o were isolated in 52 and 63%, and 1-tetralone derivative 4p was obtained in 53% yield. While the presence of free alcohols and carboxylic acids has failed to provide the desired products (see ESI†), simple protection of the acid in the form of a methylester restores the reactivity (4m, 62%). Finally, when both Csp2–Cl and Csp2–Br substituents are included in the substrate, the reactions provide a mixture of OMe products, difficult to separate. Next, we extend the methodology to drug-like scaffolds. Clomipramine derivative, [13C]3-methoxyimipramine [13C]4r and FDA approved antihistamine [13C]mepyramine [13C]4s could respectively be obtained in 85% and 70% isolated yield in only two steps starting from [13C]CO2. It is worth noting, that [13C]4r and [13C]4s bear tertiary alkyl amine substituents, which makes them both challenging to label by selective O-methylation with [13C]CH3I.9,10
This newly developed sequence was applied to 14C-labeling,24 as proven by the radiosynthesis of substrates 4d and 4s, using the same double chamber set-up and the RC Tritec (Scheme 4). Radiosyntheses were conducted on a 0.25 mmol scale to limit the amounts of handled radioactivity. First, as a proof of concept, model substrate [14C]4-methoxybenzonitrile [14C]4d was generated with 37% yield and a high molar activity (Am) of 1.99 GBq mmol−1. [14C]mepyramine [14C]4s was obtained in 35% yield and with an Am of 2.03 GBq mmol−1 (Scheme 4), which are suitable for use in biological studies. The lower yields can be attributed to the downscaling, which modifies the liquid gas equilibrium during the distillation step.
In conclusion, we devised a catalytic methoxylation of aryl halides from 13C- and 14C-labeled CO2. This strategy relies on an inverse polarity strategy using a new secondary nucleophilic source BBN-OCH3, derived from fast and stoichiometric CO2 conversion. The development of a palladium catalyzed Suzuki coupling between BBN-OCH3 and aryl halides and the use of a double chamber set up enabled the late-stage methoxylation of aromatic chlorides, in only two steps under mild conditions.25 A series of substrates, including drug-like scaffolds containing tertiary alkyl amine substituents, could be methoxylated in good to excellent yields starting from equimolar amounts of [13C]CO2. This methodology further proved its relevance and robustness in the radiolabeling of two 14C-labeled functionalized anisoles with good radiochemical yields and high molar activities, suitable to conduct in vivo biological studies.
Financial support was provided by CEA (DRF Impulsion 2017), CNRS, University Paris-Saclay, European Research Council (ERC Consolidator Grant Agreement no. 818260 (to TC) and no. 864576 (to DA)), ANR (ANR-19-CE07-0046). The authors thank D.-A. Buisson, A. Gaudet and S. Lebrequier for the excellent analytical support.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) L. Wu, Q. Liu, R. Jackstell and M. Beller, Top. Organomet. Chem., 2016, 53, 279–304 CrossRef CAS; (b) S. Bontemps, Coord. Chem. Rev., 2016, 308, 117–130 CrossRef CAS.
- (a) R. A. Bragg, M. Sardana, M. Artelsmair and C. S. Elmore, J. Labelled Compd. Radiopharm., 2018, 61, 934–948 CrossRef CAS; (b) C. S. Elmore and R. A. Bragg, Bioorg. Med. Chem. Lett., 2015, 25, 167–171 CrossRef CAS.
- While for stable isotope carbon-13 (13C), the primary building block available is carbon monoxide [13C]CO, [13C]CO2 is a very common starting material, as well.
- (a) Z. Tu and R. H. Mach, Curr. Top. Med. Chem., 2010, 10, 1060–1095 CrossRef CAS PubMed; (b) G. Boscutti, M. Huiban and J. Passchier, Drug Discovery Today: Technol., 2017, 25, 3–10 CrossRef.
- R. Voges, J. R. Heys and T. Moenius, Preparation of Compounds Labeled with Tritium and Carbon-14, John Wiley & Sons, New York, 2009, ch. 5, pp. 211–285 Search PubMed.
- https://njardarson.lab.arizona.edu/content/top-pharmaceuticalsposter: D. T. Smith, M. D. Delost, H. Qureshi and J. T. Njararson, J. Chem. Educ., 2010, 87, 1348 CrossRef ; Poster information gathered by the Njararson group with data from DrugTopics & Pharmacompass.
- For selected examples see: (a) D. H. R. Barton, G. W. Kirby, J. B. Taylor and G. M. Thomas, J. Chem. Soc., 1963, 4545–4558 RSC; (b) J. Liu, G. G. Miller, L. Huang, Z. Diwu, J. W. Lown, K. Brown, R. B. Moore, J. Tulip and M. S. McPhee, J. Labelled Compd. Radiopharm., 1995, 36, 815–823 CrossRef CAS; (c) Y. Cai, W. Baer-Dubowska, M. J. Ashwood-Smith, O. Ceska, S. Tachibana and J. DiGiovanni, Chem. Res. Toxicol., 1996, 9, 729–736 Search PubMed; (d) R. T. Brown, V. L. Murrell, A. McMordie, M. Sriram, K. G. Pinney, S. Sharma and D. J. Chaplin, J. Labelled Compd. Radiopharm., 2009, 52, 567–570 CAS.
- (a) A. Murray and A. R. Ronzio, J. Am. Chem. Soc., 1952, 74, 2408–2412 CrossRef CAS; (b) T. Nagasaki, Y. Katsuyama and M. Yoshioka, J. Labelled Compd. Radiopharm., 1987, 24, 65–71 CrossRef CAS.
- C. G. M. Janssen, J. B. A. Thijssen and W. J. M. Verluyten, J. Labelled Compd. Radiopharm., 2002, 45, 841–855 CrossRef CAS.
- S. G. Senderoff, S. W. Landvatter and J. R. Heys, J. Labelled Compd. Radiopharm., 2000, 43, 1283–1288 CrossRef CAS.
- (a) E. Blondiaux, J. Pouessel and T. Cantat, Angew. Chem., Int. Ed., 2014, 53, 12186–12190 CrossRef CAS; (b) T. Cantat, C. Gomes, E. Blondiaux and O. Jacquet, CEA – IRAMIS, WO 2014162266, 2014 Search PubMed.
- (a) S. Enthaler and A. Company, Chem. Soc. Rev., 2011, 40, 4912–4924 RSC; (b) S. Bhadra, W. I. Dzik and L. J. Goossen, J. Am. Chem. Soc., 2012, 134, 9938–9941 CrossRef CAS PubMed; (c) J. P. Stambuli, RSC Catal. Ser., 2015, 21, 254–275 CAS; (d) K. Keerthi Krishnan, S. M. Ujwaldev, K. S. Sindhu and G. Anilkumar, Tetrahedron, 2016, 72, 7393–7407 CrossRef CAS.
- (a) G. Mann and J. F. Hartwig, J. Am. Chem. Soc., 1996, 118, 13109–13110 CrossRef CAS; (b) M. Palucki, J. P. Wolfe and S. L. Buchwald, J. Am. Chem. Soc., 1997, 119, 3395–3396 CrossRef CAS; (c) K. E. Torraca, S.-I. Kuwabe and S. L. Buchwald, J. Am. Chem. Soc., 2000, 122, 12907–12908 CrossRef CAS; (d) A. V. Vorogushin, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 8146–8149 CrossRef CAS PubMed; (e) S. Gowrisankar, A. G. Sergeev, P. Anbarasan, A. Spannenberg, H. Neumann and M. Beller, J. Am. Chem. Soc., 2010, 132, 11592–11598 CrossRef CAS PubMed; (f) X. Wu, B. P. Fors and S. L. Buchwald, Angew. Chem., Int. Ed., 2011, 50, 9943–9947 CrossRef CAS; (g) P. E. Maligres, J. Li, S. W. Krska, J. D. Schreier and I. T. Raheem, Angew. Chem., Int. Ed., 2012, 51, 9071–9074 CrossRef CAS PubMed; (h) L. Rotteveel, A. J. Poot, U. Funke, A. Pekosak, U. Filp, A. A. Lammertsma and A. D. Windhorst, J. Labelled Compd. Radiopharm., 2017, 60, 566–576 CrossRef CAS; (i) H. Zhang, P. Ruiz-Castillo and S. L. Buchwald, Org. Lett., 2018, 20, 1580–1583 CrossRef CAS.
- K. E. Torraca, X. Huang, C. A. Parrish and S. L. Buchwald, J. Am. Chem. Soc., 2001, 123, 10770–10771 CrossRef CAS PubMed.
- E. J. Milton, J. A. Fuentes and M. L. Clarke, Org. Biomol. Chem., 2009, 7, 2645–2648 RSC.
- S. Gowrisankar, H. Neumann and M. Beller, Chem. – Eur. J., 2012, 18, 2498–2502 CrossRef CAS.
- (a) P. Dash, M. Janni and S. Peruncheralathan, Eur. J. Org. Chem., 2012, 4914–4917 CrossRef CAS; (b) C. W. Cheung and S. L. Buchwald, Org. Lett., 2013, 15, 3998–4001 CrossRef CAS; (c) B. T. Inogoglia and S. L. Buchwald, Org. Lett., 2017, 19, 2853–2856 CrossRef.
- G. L. Tolnai, B. Pethő, P. Králl and Z. Novák, Adv. Synth. Catal., 2014, 356, 125–129 CrossRef CAS.
- D. Blakemore, Synthetic Methods in Drug Discovery: Volume 1, The Royal Society of Chemistry, 2016, vol. 1, pp. 1–69 Search PubMed.
- (a) L. M. Martínez-Prieto, E. Ávila, P. Palma, E. Álvarez and J. Cámpora, Chem. – Eur. J., 2015, 21, 9833–9849 CrossRef; (b) J. A. Mueller, C. P. Goller and M. S. Sigman, J. Am. Chem. Soc., 2004, 126, 9724–9734 CrossRef CAS PubMed.
- P. Hermange, A. T. Lindhardt, R. H. Taaning, K. Bjerglund, D. Lupp and T. Skrydstrup, J. Am. Chem. Soc., 2011, 133, 6061–6071 CrossRef CAS.
- (a) H. C. Brown and S. U. Kulkarni, J. Organomet. Chem., 1979, 168, 281–293 CrossRef CAS; (b) H. C. Brown, J. S. Cha, B. Nazer and C. A. Brown, J. Org. Chem., 1985, 50, 549–553 CrossRef CAS.
- J. Magano and J. R. Dunetz, Chem. Rev., 2011, 111, 2177–2250 CrossRef CAS PubMed.
- (a) N. Penner, I. J. Klunk and C. Prakash, Biopharm. Drug Dispos., 2009, 30, 185–203 CrossRef CAS PubMed; (b) E. M. Isin, C. S. Elmore, G. N. Nilsson, R. A. Thompson and L. Weidolf, Chem. Res. Toxicol., 2012, 25, 532–542 Search PubMed.
- While this work was in preparation, a copper-catalyzed reaction between MeO-9BBN and aryl bromides was reported, see: J.-R. Wang, Z.-Q. Song, C. Li and D.-H. Wang, Org. Lett., 2021, 23, 8450–8454 CrossRef CAS PubMed . Interestingly, alryl chlorides are poorly effective substrates under these reported conditions.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc03746g |
| This journal is © The Royal Society of Chemistry 2022 |

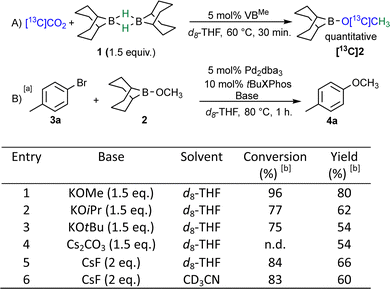
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Reaction conditions: 0.1 mmol 4-bromotoluene 3a, 0.1 mmol BBN-OCH32, 0.2 mL solvent.
Reaction conditions: 0.1 mmol 4-bromotoluene 3a, 0.1 mmol BBN-OCH32, 0.2 mL solvent.