Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
AIE-active iridium(III) complex integrated with upconversion nanoparticles for NIR-irradiated photodynamic therapy†
Shengnan
Liu‡
 a,
Jiahong
Han‡
a,
Yulei
Chang
a,
Jiahong
Han‡
a,
Yulei
Chang
 *b,
Weijin
Wang
a,
Runlin
Wang
a,
Ziwei
Wang
a,
Guangzhe
Li
*c,
Dongxia
Zhu
*b,
Weijin
Wang
a,
Runlin
Wang
a,
Ziwei
Wang
a,
Guangzhe
Li
*c,
Dongxia
Zhu
 *a and
Martin R.
Bryce
*a and
Martin R.
Bryce
 *d
*d
aKey Laboratory of Nanobiosensing and Nanobioanalysis at Universities of Jilin Province, Department of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun, Jilin Province 130024, P. R. China. E-mail: zhudx047@nenu.edu.cn
bState Key Laboratory of Luminescence and Applications, Changchun Institute of Optics, Fine Mechanics and Physics, Chinese Academy of Sciences, Changchun, Jilin Province 130033, China. E-mail: yuleichang@ciomp.ac.cn
cJilin Provincial Science and Technology Innovation Center of Health Food of Chinese Medicine, Changchun University of Chinese Medicine, Changchun, Jilin Province 130117, P. R. China. E-mail: 1993008106@qq.com
dDepartment of Chemistry, Durham University, Durham, DH1 3LE, UK. E-mail: m.r.bryce@durham.ac.uk
First published on 22nd August 2022
Abstract
The integration of an aggregation induced emission (AIE)-active Ir(III) complex and upconversion nanoparticles (UCNPs) has achieved a NIR-irradiated photosensitizer (PS), UCNPs@Ir-2-N. This PS has satisfactory biocompatibility, excellent phototoxicity, good accumulation in cells and high 1O2 generation ability, thereby effectively killing 4T1 mouse cancer cells in vitro. This work has potential for future photodynamic therapy (PDT) applications.
Photodynamic therapy (PDT) is an efficient anticancer treatment of particular interest.1–3 In the PDT process light irradiates photosensitizers (PSs) to generate highly cytotoxic reactive oxygen species (ROS) causing the ablation of cancer cells.4–6 Traditional PSs suffer from several major drawbacks:7–10 (i) low intersystem crossing (ISC) ability; (ii) poor photostability; (iii) aggregation-caused quenching (ACQ) of emission; (iv) unsatisfactory ROS production efficiency. Moreover, the short-wavelength irradiation light results in shallow tissue penetration and photodamage to cells and tissue.11–13 Hence, the clinical application of PDT requires a solution to these problems.
Tang and co-authors have recently reported that aggregation-induced emission (AIE) luminogens show excellent aggregation-induced ROS generation activity.14 However, how to make the most of accelerating the ISC process to boost ROS generation still remains a key challenge. Inspired by the above facts, a few AIE-active Ir(III) complexes with efficient ISC processes, long excited-state lifetime, excellent photostability and tunable ligand modification have been developed as substitutes to traditional materials for efficient PDT.7,15–18
Nevertheless, the absorption bands of Ir(III) complexes are usually in the UV to visible range and not in the near-infrared (NIR) region,19,20 which limits their therapeutic effect in deep tissue. Upconversion nanoparticles (UCNPs) are desirable photoconversion materials for biosensing and biomedicine on account of their capability of transforming the NIR photons to UV/visible photons.11,13,21 Two reports have corroborated that PSs combining cationic Ir(III) complexes and UCNPs can overcome the problems caused by short wavelength irradiation in PDT.22,23 Possibly owing to the lack of a reliable molecular design strategy or facile synthetic routes, to the best of our knowledge, there have not been any reports of PSs based on AIE-active Ir(III) complexes and UCNPs.
Herein, AIE-active non-charged Ir(III) complexes integrated with UCNPs are employed as effective PSs for PDT for the first time. Taking advantage of the tunable ligand structure of Ir(III) complexes,24 a Schiff base was introduced as the ancillary N^O ligand due to its simple synthesis, high yield and proven coordination to achieve AIE-active Ir(III) complexes,16,25 giving Ir-1-N (structure in ESI†) and Ir-2-N (Scheme 1). Compared with Ir-1-N, the absorption of Ir-2-N was extensively enhanced through extending the π-conjugation of the C^N ligands. Furthermore, Ir-2-N exhibits bright luminescence in the aggregated state and high singlet oxygen (1O2) generation ability. The UCNPs@Ir-2-N nanoparticles (NPs) were formulated by encapsulating the Ir-2-N and UCNPs within D-α-tocopherol polyethylene glycol 1000 succinate (TPGS) to obtain better stability and biocompatibility. UCNPs@Ir-2-N is the main focus of the current study.
 | ||
| Scheme 1 Structure of Ir-2-N, preparation of UCNPs@Ir-2-N and schematic of UCNPs@Ir-2-N as a PS for PDT. | ||
The synthetic routes to Ir-1-N and Ir-2-N are shown in the ESI.† Their structures were validated by proton nuclear magnetic resonance (1H NMR) spectroscopy (Fig. S1–S6, ESI†), 13C NMR spectroscopy (Fig. S7 and S8, ESI†) and high-resolution mass spectrometry (HRMS) (Fig. S9 and S10, ESI†). The UCNPs (NaYF4@NaF:Yb,Tm@NaYF4) were prepared by the chloride solvothermal method as reported before26 (see ESI†).
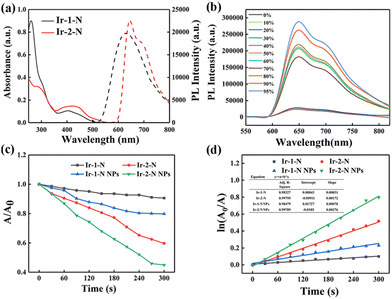
The photophysical properties of Ir-1-N and Ir-2-N were investigated (Fig. 1a and Fig. S11, Table S1, ESI†). In UV-vis absorption spectra, both of the Ir(III) complexes showed two absorption bands at 250–350 nm, corresponding to spin-allowed π–π* transitions at the ligand centers, and at 350–500 nm from metal-to-ligand charge transfer (1MLCT), ligand-to-ligand charge transfer (1LLCT), spin-forbidden metal-to-ligand charge-transfer (3MLCT) and spin-forbidden ligand-to-ligand charge-transfer (3LLCT).19,27,28 The photoluminescence (PL) spectra show that Ir-1-N emits at λmax 640 nm, and Ir-2-N at 660 nm. These results establish that the extended conjugation of the C^N ligands leads to a significant redshift in the PL of Ir-2-N and almost 1.5 times increase of the molar absorption coefficient of Ir-2-N relative to Ir-1-N. Moreover, these Ir(III) complexes are almost non-emissive in pure CH3CN (Fig. 1b and Fig. S12, ESI†). On the contrary, the emission intensity of Ir-1-N and Ir-2-N was greatly enhanced when the water fraction reached respectively to 80% and 50% demonstrating their typical AIE feature. Ir-2-N showed a brighter luminescence indicating the effective restriction of nonradiative transition processes, as found in AIE luminogens.29–31 Meanwhile, there are clear level-off tails at higher wavelengths in the UV-vis absorption spectra with the enhancement of water faction because of the Mie scattering effect from the aggregated suspensions32 (Fig. S13, ESI†). Transmission electron microscopy (TEM) provided further evidence for molecular aggregates of Ir-1-N and Ir-2-N in the 99% H2O/CH3CN mixture (Fig. S14, ESI†). Both complexes Ir-1-N and Ir-2-N exhibited a long excited-state lifetime of 0.56 μs and 0.62 μs, respectively, and high photoluminescence quantum yield (PLQY) of nearly 20% in the aggregated state (Table S1, ESI†).
In view of the promising optical properties of Ir-1-N and Ir-2-N, their PDT applications were investigated. Indocyanine green (ICG), for which the absorption band at 790 nm will decrease after encountering 1O2, was utilized as a 1O2 generation indicator.7 In Fig. S15 (ESI†), the absorption of ICG shows negligible change in the control groups. And there are also no changes in the absorption of the Ir(III) complexes upon irradiation (425 nm, 20 mW cm−2) implying their good photostability. In contrast, the absorption of ICG at 790 nm rapidly decreased in intensity under 425 nm light irradiation in the presence of 15 μg mL−1 of Ir-2-N. However, irradiating the Ir-1-N solution at the same concentration did not bring a significant change of the spectra (Fig. S15c and d, ESI†). Fig. 1d illustrates that the 1O2 generation of the complexes follows first-order kinetics and the slope of Ir-2-N is much higher than that of Ir-1-N, suggesting the higher 1O2 generation ability of Ir-2-N, which could originate from the higher absorption coefficient of Ir-2-N.33 The NPs of Ir-1-N and Ir-2-N (Ir-1-N NPs and Ir-2-N NPs) were self-assembled with poloxamer (F127) (ESI†), and their 1O2 generation ability was obviously enhanced compared to Ir-1-N and Ir-2-N (Fig. 1c, d and Fig. S16, ESI†). This enhancement is attributed to the AIE features of the Ir(III) complexes. Contemporaneously, Ir-2-N NPs exhibited higher 1O2 generation ability due to its stronger AIE features compared to Ir-1-N NPs, meaning Ir-2-N has the capacity to be utilized as a PS in the aggregated state. Therefore, Ir-2-N was used for subsequent experiments.
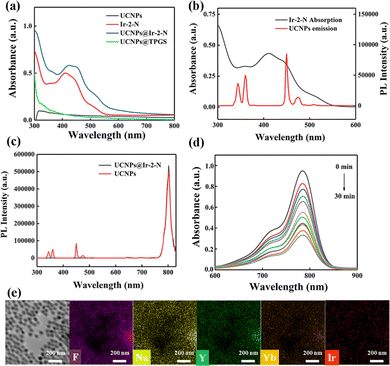
To overcome the drawbacks of the short wavelength absorption bands, Ir-2-N and UCNPs were integrated to improve the PS performance. Ir-2-N was encapsulated with UCNPs within TPGS to construct UCNPs@Ir-2-N (see ESI†) as PSs for the ensuing in vitro experiments. A scanning electron microscope (SEM) image showed a uniform morphology and size of the UCNPs (Fig. S17, ESI†). TPGS not only enables the integration of Ir-2-N and UCNPs, but also increases the biocompatibility of NPs.34 The UV-vis absorption spectra of UCNPs@Ir-2-N showed a significant absorption at about λmax 425 nm corresponding to that of Ir-2-N (Fig. 2a). Energy dispersive spectroscopy (EDS) confirmed the even distribution of the elements: F, Na, Y, Yb, Ir in UCNPs@Ir-2-N (Fig. 2e). The loading content of Ir-2-N in UCNPs@Ir-2-N was calculated to be 70% according to UV-vis spectral analysis (Fig. S18, ESI†). The hydrodynamic size and polydispersity index (PDI) of UCNPs@Ir-2-N were 113 nm and 0.256, respectively, detected by dynamic light scattering (DLS) (Fig. S19a, ESI†). The TEM images imply the average size of UCNPs@Ir-2-N is around 100 nm. Furthermore, one-week monitoring of the size and PDI of UCNPs@Ir-2-N revealed its excellent stability (Fig. S19b, ESI†) and hence potential applicability in PDT.
To ensure that the energy transfer between Ir-2-N and UCNPs can be realized and UCNPs@Ir-2-N could be irradiated by NIR light, the PL spectrum of UCNPs was measured under laser irradiation (980 nm). The absorption band of Ir-2-N is in the UV and blue regions overlapping with the emission bands of UCNPs (Fig. 2b). Next, the PL spectra of original UCNPs and UCNPs@Ir-2-N were compared. As shown in Fig. 2c and Fig. S18 (ESI†), the 345, 360, 450 and 470 nm bands nearly disappeared, due to strong absorption by Ir-2-Nvia ULRET (upconversion luminescence resonance energy transfer). This process is a prerequisite to use UCNPs@Ir-2-N as a PS for PDT upon NIR light irradiation.
Subsequently, the 1O2 generation ability of UCNPs@Ir-2-N was assessed through ICG as indicator. Compared with the control groups, the absorption bands of ICG decreased in UCNPs@Ir-2-N solution with increasing the irradiation time under 980 nm light (Fig. 2d and Fig. S21a, b, ESI†). The data reveal an effective process to generate 1O2: (i) UCNPs absorb the NIR light at 980 nm and emit the UV/blue light which is absorbed by Ir-2-N; (ii) Ir-2-N is then excited and undergoes energy transfer through O2 to form 1O2. The absorption of UCNPs@Ir-2-N remains unchanged under irradiation (980 nm) over the same period (30 min) confirming its desirable photostability (Fig. S21c, ESI†). The 1O2 generation ability of UCNPs was tested as a control, which further confirmed that the good 1O2 generation ability of UCNPs@Ir-2-N was mainly from the Ir-2-N excited by UCNPs (Fig. S21d, ESI†). Hence, it has been demonstrated that the NIR-irradiated PSs obtained by the combination of UCNPs and Ir(III) complexes could ameliorate the problems coming from the short wavelength absorption of the Ir(III) complexes.
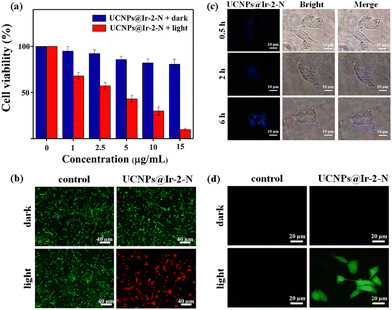
Encouraged by the 1O2 generation ability of NIR-irradiated UCNPs@Ir-2-N, their ability to kill cancer cells through PDT was investigated. In vitro photo-cytotoxicity experiments using UCNPs@Ir-2-N with concentrations from 0 to 15 μg mL−1 (in terms of the calculated concentration of Ir-2-N in NPs, see ESI† for details) were evaluated against 4T1 mouse breast cancer cells via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays (Fig. 3a). Without NIR irradiation, the >85% viability of 4T1 cells indicated UCNPs@Ir-2-N possess a satisfactory biocompatibility. After exposure to 980 nm laser irradiation, the viability of UCNPs@Ir-2-N-treated cells decreased to 9%, pointing to the considerable cytotoxicity of UCNPs@Ir-2-N (IC50 value = 3.68 μg mL−1). The same concentration of UCNPs was used to conduct MTT assays as a control experiment (Fig. S22, ESI†). The data suggested that only UCNPs have negligible phototoxicity to 4T1 cells. To further visually evaluate the therapeutic effect of UCNPs@Ir-2-N, a Calcein AM and propidium iodide (PI) co-staining assay was performed to stain viable and dead cells, respectively. There were negligible dead cells in control groups and the groups in dark conditions (Fig. 3b). In contrast, a majority of cells were dead in the group with UCNPs@Ir-2-N under 980 nm irradiation. This was in good accordance with the MTT assays. The data demonstrated that UCNPs@Ir-2-N could be employed as an NIR-irradiated PS and applied in efficient PDT.
The cellular uptake experiments further confirmed that UCNPs@Ir-2-N could be effectively ingested by 4T1 cells according to confocal laser scanning microscope (CLSM) imaging (Fig. 3c). The blue fluorescence signal (from the UCNPs in UCNPs@Ir-2-N) gradually enhanced with incubation time, implying a time-dependent uptake pathway and the efficient accumulation of UCNPs@Ir-2-N in cells. Furthermore, the in situ1O2 generation capability was evaluated using 2′,7′-dichlorofluorescein diacetate (DCFH-DA). As expected, UCNPs@Ir-2-N-treated cells exhibited strong green fluorescence under 980 nm irradiation, but almost no fluorescence was detected under dark conditions (Fig. 3d). The results are consistent with the fact that Ir-2-N could absorb the UV/blue light from NIR-irradiated UCNPs through ULRET and then generate 1O2. All the results illustrate the feasible strategy that UCNPs and AIE-active Ir-2-N are encapsulated together to achieve effective energy transfer, and hence exert their respective advantages to construct NIR-irradiated PSs, UCNPs@Ir-2-N which generate toxic ROS and thereby kill the cancer cells. This approach has effectively overcome the problems of short-wavelength absorption of Ir(III) complexes.
In summary, two AIE-active Ir(III) complexes (Ir-1-N and Ir-2-N) were synthesized through rational molecular design. Extending the conjugation within the C^N ligands of Ir-2-N resulted in a redshift of its PL spectra and enhanced absorption. Moreover, Ir-2-N showed high 1O2 generation ability and its AIE properties lead to increased 1O2 generation in the aggregated state. UCNPs were applied as photoconversion materials to obtain UCNPs@Ir-2-N, in which ULRET between AIE-active Ir-2-N and UCNPs play a very important role under NIR irradiation. In vitro, NIR-irradiated UCNPs@Ir-2-N has satisfactory biocompatibility, excellent phototoxicity, good accumulation in cells and superior 1O2 generation ability. To the best of our knowledge, this is the first report of PSs that combine AIE-active Ir(III) complexes with UCNPs. The work represents a significant development for PSs based on readily-available, high-performance transition metal complexes.
This work was funded by NSFC (Grants 52073045, 51773195, 62075217), the Key Scientific and Technological Project of Jilin Province (20190701010GH), Jilin Provincial Department of Science and Technology (Grant 20210101148JC), the Development and Reform Commission of Jilin Province (2020C035-5), and Changchun Science and Technology Bureau (CC202110378310002101). M. R. B. thanks EPSRC (UK) grant EP/L02621X/1 for funding. D. Zhu is grateful for the support from the Key Laboratory of Nanobiosensing and Nanobioanalysis at the Universities of Jilin Province. The authors acknowledge the support from the Jilin Provincial Department of Education.
Conflicts of interest
There are no conflicts to declare.Notes and references
- D. W. Felsher, Nat. Rev. Cancer, 2003, 3, 375–380 CrossRef CAS PubMed.
- T. Yan, L. Ren, F. Li, F. Tian, C. Jiang, Q. Wang, X. Song and S. Zhang, Chem. Commun., 2022, 58, 1617–1620 RSC.
- X. Zhao, J. Liu, J. Fan, H. Chao and X. Peng, Chem. Soc. Rev., 2021, 50, 4185–4219 RSC.
- L. Huang, S. Zhao, J. Wu, L. Yu, N. Singh, K. Yang, M. Lan, P. Wang and J. S. Kim, Coord. Chem. Rev., 2021, 438, 213888 CrossRef CAS.
- Z. Zhang, M. Kang, H. Tan, N. Song, M. Li, P. Xiao, D. Yan, L. Zhang, D. Wang and B. Z. Tang, Chem. Soc. Rev., 2022, 51, 1983–2030 RSC.
- G. Jin, G. Feng, W. Qin, B. Z. Tang, B. Liu and K. Li, Chem. Commun., 2016, 52, 2752–2755 RSC.
- L. Zhang, Y. Li, W. Che, D. Zhu, G. Li, Z. Xie, N. Song, S. Liu, B. Z. Tang, X. Liu, Z. Su and M. R. Bryce, Adv. Sci., 2019, 6, 1802050 CrossRef PubMed.
- M. Liu, Y. Chen, Y. Guo, H. Yuan, T. Cui, S. Yao, S. Jin, H. Fan, C. Wang, R. Xie, W. He and Z. Guo, Nat. Commun., 2022, 13, 2179 CrossRef CAS PubMed.
- Y. Hong, J. W. Lam and B. Z. Tang, Chem. Commun., 2009, 4332–4353 RSC.
- J. Zhang, F. Fang, B. Liu, J. H. Tan, W. C. Chen, Z. Zhu, Y. Yuan, Y. Wan, X. Cui, S. Li, Q. X. Tong, J. Zhao, X. M. Meng and C. S. Lee, ACS Appl. Mater. Interfaces, 2019, 11, 41051–41061 CrossRef CAS PubMed.
- Z. Wang, B. Liu, Q. Sun, L. Feng, F. He, P. Yang, S. Gai, Z. Quan and J. Lin, ACS Nano, 2021, 15, 12342–12357 CrossRef CAS PubMed.
- P. S. Chelushkin, J. R. Shakirova, I. S. Kritchenkov, V. A. Baigildin and S. P. Tunik, Dalton Trans., 2022, 51, 1257–1280 RSC.
- Y. Wang, Y. Li, Z. Zhang, L. Wang, D. Wang and B. Z. Tang, Adv. Mater., 2021, 33, 2103748 CrossRef CAS PubMed.
- Y. Li, R. Zhang, Q. Wan, R. Hu, Y. Ma, Z. Wang, J. Hou, W. Zhang and B. Z. Tang, Adv. Sci., 2021, 8, 2102561 CrossRef CAS PubMed.
- J. Liu, C. Jin, B. Yuan, X. Liu, Y. Chen, L. Ji and H. Chao, Chem. Commun., 2017, 53, 2052–2055 RSC.
- L. Li, L. Zhang, X. Tong, Y. Li, Z. Yang, D. Zhu, Z. Su and Z. Xie, Dalton Trans., 2020, 49, 15332–15338 RSC.
- D. Ding and B. Z. Tang, Adv. Healthcare Mater., 2021, 10, 2102499 CrossRef CAS.
- X. Cai, K. N. Wang, W. Ma, Y. Yang, G. Chen, H. Fu, C. Cui, Z. Yu and X. Wang, J. Nanobiotechnol., 2021, 19, 254 CrossRef CAS PubMed.
- Y. You and W. Nam, Chem. Soc. Rev., 2012, 41, 7061–7084 RSC.
- G. Li, D. Zhu, X. Wang, Z. Su and M. R. Bryce, Chem. Soc. Rev., 2020, 49, 765–838 RSC.
- Y. Feng, H. Chen, Y. Wu, I. Que, F. Tamburini, F. Baldazzi, Y. Chang and H. Zhang, Biomaterials, 2020, 230, 119637 CrossRef CAS PubMed.
- J. Zhao, S. Sun, X. Li, W. Zhang and S. Gou, ACS Appl. Bio Mater., 2020, 3, 252–262 CrossRef CAS PubMed.
- J. Zhao, X. Zhang, L. Fang, C. Gao, C. Xu and S. Gou, Small, 2020, 16, 2000363 CrossRef CAS PubMed.
- T. S. Teets, ChemPhotoChem, 2018, 2, 380–381 CrossRef CAS.
- P. Ghosh, S. K. Dey, M. H. Ara, K. Karim and A. B. M. N. Islam, Egypt. J. Chem., 2019, 62, 523–547 Search PubMed.
- H.-S. Qian and Y. Zhang, Langmuir, 2008, 24, 12123–12125 CrossRef CAS PubMed.
- C. You, D. Liu, J. Yu, H. Tan, M. Zhu, B. Zhang, Y. Liu, Y. Wang and W. Zhu, Adv. Opt. Mater., 2020, 8, 2000154 CrossRef CAS.
- H. F. Li, X. Q. Liu, C. Lyu, J. Gorbaciova, L. L. Wen, G. G. Shan, P. B. Wyatt, H. Q. Ye and W. P. Gillin, Light: Sci. Appl., 2020, 9, 32 CrossRef CAS PubMed.
- J. Luo, Z. Xie, J. W. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- J. Mei, Y. Hong, J. W. Lam, A. Qin, Y. Tang and B. Z. Tang, Adv. Mater., 2014, 26, 5429–5479 CrossRef CAS PubMed.
- F. Würthner, Angew. Chem., Int. Ed., 2020, 59, 14192–14196 CrossRef PubMed.
- B. Xu, W. Li, J. He, S. Wu, Q. Zhu, Z. Yang, Y. C. Wu, Y. Zhang, C. Jin, P. Y. Lu, Z. Chi, S. Liu, J. Xu and M. R. Bryce, Chem. Sci., 2016, 7, 5307–5312 RSC.
- X. Zheng, L. Wang, S. Liu, W. Zhang, F. Liu and Z. Xie, Adv. Funct. Mater., 2018, 28, 1706507 CrossRef.
- T. Y. Lu, W. F. Lu, Y. H. Wang, M. Y. Liao, Y. Wei, Y. J. Fan, E. Y. Chuang and J. Yu, ACS Appl. Mater. Interfaces, 2021, 13, 38074–38089 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, supporting figures and tables. See DOI: https://doi.org/10.1039/d2cc03622c |
| ‡ The authors contributed equally to the preparation of this work. |
| This journal is © The Royal Society of Chemistry 2022 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: