Open Access Article
Open Access ArticleSelf-assembly of an MRI responsive agent under physiological conditions provides an extended time window for in vivo imaging†
Nishanth D.
Tirukoti
a,
Liat
Avram
 b,
Reut
Mashiach
a,
Hyla
Allouche-Arnon
a and
Amnon
Bar-Shir
b,
Reut
Mashiach
a,
Hyla
Allouche-Arnon
a and
Amnon
Bar-Shir
 *a
*a
aDepartment of Molecular Chemistry and Materials Science, Weizmann Institute of Science, Rehovot 7610001, Israel. E-mail: amnon.barshir@weizmann.ac.il
bDepartment of Chemical Research Support, Weizmann Institute of Science, Rehovot 7610001, Israel
First published on 15th September 2022
Abstract
An MRI-responsive agent that spontaneously self-assembles to a large supramolecular structure under physiological conditions was designed. The obtained assembly provides an extended time window for in vivo studies, as demonstrated for a fluorine-19 probe constructed to sense Zn2+ with 19F-iCEST MRI, in the future.
Small molecular MRI responsive agents, also known as smart contrast agents,1,2 are rationally designed to sense an analyte (i.e., metal ion, metabolite, etc.), condition (i.e., redox, pH, etc.), or enzymatic activity through a change in the obtained MRI signal. Among these agents, those designed to map metal ions were extensively developed in the last two decades,3 and although designed for a range of ions,4 those agents aimed to sense Zn2+
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and Ca2+,6,7 have shown promising results.8 For some applications, their fast response was not compromised by their fast clearance and allowed the spatial monitoring of Zn2+ secretion from functioning pancreatic tissues9 or glucose-stimulated Zn2+ excretion from non-tumoral prostate.10 Nevertheless, the fast washout rates of some MRI-responsive agents required continuous infusion throughout the study period to map ion-mediated brain activity6 or brain pathology.7 In addition, fluorine-19-based Zn2+-responsive agents developed for in vivo mapping of the metal ion in the brain11 and in an animal model of prostate cancer,12 using the 19F-iCEST approach,13 required relatively long acquisition times, and thus, continuous infusion of the synthetic probe. To overcome the limitation of fast washout of the delivered MRI-responsive agent, alternatives are needed.
5 and Ca2+,6,7 have shown promising results.8 For some applications, their fast response was not compromised by their fast clearance and allowed the spatial monitoring of Zn2+ secretion from functioning pancreatic tissues9 or glucose-stimulated Zn2+ excretion from non-tumoral prostate.10 Nevertheless, the fast washout rates of some MRI-responsive agents required continuous infusion throughout the study period to map ion-mediated brain activity6 or brain pathology.7 In addition, fluorine-19-based Zn2+-responsive agents developed for in vivo mapping of the metal ion in the brain11 and in an animal model of prostate cancer,12 using the 19F-iCEST approach,13 required relatively long acquisition times, and thus, continuous infusion of the synthetic probe. To overcome the limitation of fast washout of the delivered MRI-responsive agent, alternatives are needed.
One strategy to prevent the use of injectable probes is to develop and implement genetically encoded biosensors for MRI detection of metal ions.14 Another alternative to prevent the rapidly washed out small molecular probes is the use of large-sized MRI responsive agents. This includes ion responsive agents of a type of dendrimers15 or iron-oxide nanoparticles16,17 that could be detected for a long time at the region of interest after their injection, even when delivered at sub-microliter quantities. Inspired by the demonstrations that water-soluble benzene-1,3,5-tricarboxamides (BTAs) conjugates self-assemble under elevated temperatures to obtain supramolecular polymers,18 we show here a novel strategy for very slow washout of relatively small MRI probes. Specifically, we developed a 19F-MRI-responsive agent for Zn2+ that spontaneously self-assembled to large structures only upon its delivery to the tissue of interest, enabling the detection of a robust 19F-MRI signal from the imaging agent, even 20 hours after its delivery.
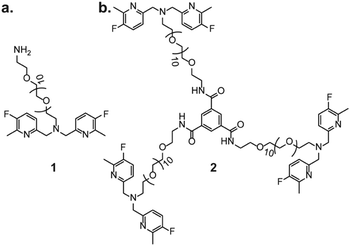
We have started by synthesizing a potential 19F-iCEST probe for Zn2+ MRI, as responsive agents of this type required prolonged infusion in order to allow robust 19F-MRI of the delivered agent.11,12 As a putative Zn2+ recognition moiety of the designed assembly, compound 1 was first synthesized with a PEG-10 as a linker for further conjugations to a BTA backbone, through a reductive amination (Fig. 1). 19F-NMR studies of 1 in the presence of Zn2+ showed characteristic 19F-NMR peaks of free and Zn2+-bound 1 with a Δω (related to the free 1, set to 0 ppm) of +5 ppm, and indeed, a pronounced 19F-iCEST effect of 25% was observed for a buffered solution containing 3 mM 1 and 60 μM Zn2+ at 37 °C (Fig. S1, ESI†). Then, 1 was reacted with benzene-1,3,5-tricarbonyl trichloride to form the tripod molecule 2 (Fig. 1). 2 has the BTA backbone conjugated to three hydrophilic moieties, and thus, the potential to form large supramolecular assemblies based on π–π interactions and hydrogen bonding18–20 between adjacent molecules (Fig. S2, ESI†).
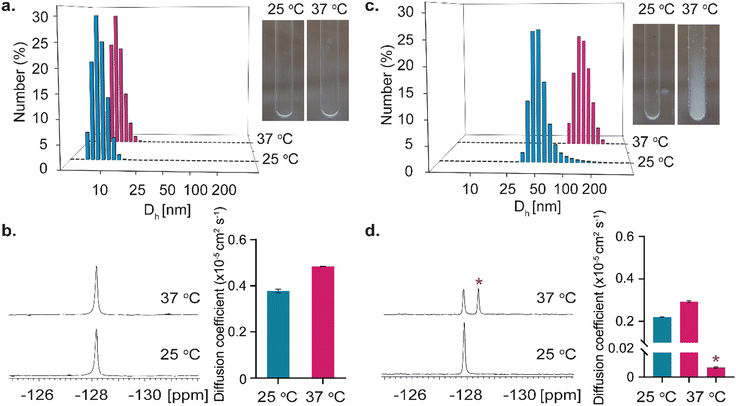
The ability of tripod 2 to form nano-sized supramolecular assemblies in aqueous solution was then studied and compared to the one-unit probe 1 at both 25 °C and 37 °C (Fig. 2). To this end, dynamic light scattering (DLS) measurements were first performed on aqueous solutions of 1 or 2 at either 25 °C or 37 °C. As expected, 1 showed a comparable size (Dh = 8.1 ± 1.6 nm and Dh = 9.2 ± 1.9 nm, at 25 °C and 37 °C, respectively) and a transparent solution at both studied temperatures (Fig. 2a), implying no formation of large assemblies even at an elevated temperature. Interestingly, a different observation was found for 2 when DLS measurements were performed at 25 °C (DH = 54 ± 20.2 nm) or 37 °C (DH = 163.3 ± 38.2 nm), indicating the formation of large assemblies when the solution of 2 was heated to a physiological temperature, 37 °C (Fig. 2c). Such large sizes obtained at 37 °C could also be detected by the naked eye, as shown from the pictures of tubes containing 2 at the two studied temperatures with white-colored assemblies appearing at 37 °C (Fig. 2c). Importantly, these assemblies are not just large-size precipitates, as they spontaneously disassembled when the solution was cooled to 25 °C to obtain their initial size and a clear solution (Fig. S3, ESI†).
To further elaborate on this observation, 19F-NMR experiments were then performed on aqueous solutions of 1 or 2 at 25 °C and 37 °C. As depicted in Fig. 2b, for the solution of 1, the same single peak was obtained in the 19F-NMR spectrum at both studied temperatures. In contrast, for tripod 2, a different observation could be detected at 25 °C and 37 °C. (Fig. 2d). While a single 19F-NMR peak was observed at 25 °C, an additional peak, resonating 0.7 ppm upfield, was obtained when the sample was heated to 37 °C. Such an observation can be explained by the formation of a new chemical environment of the 19F-entities of 2 upon temperature elevation and the assembly formation. To further explore this difference between the 19F-NMR of 1 and 2, and for a better understanding of the additional peak observed for 2 upon heating the solution to 37 °C, diffusion NMR experiments were performed as such studies are frequently used to determine the formation of large assemblies and differentiate them from the adjacent monomers in solution.21 For 1, a larger diffusion coefficient (D) of 0.48 ± 0.01 × 10−5 cm2 s−1 was obtained at 37 °C, compared to that obtained at 25 °C, i.e., 0.38 ± 0.01 × 10−5 cm2 s−1 (Fig. 2b). This observation is expected for freely diffusing molecules that experience a faster Brownian motion at an elevated temperature and do not assemble to obtain larger structures. Interestingly, for tripod 2, two different observations were obtained from the diffusion NMR experiments. While the 19F-NMR peak that resonated at −128 ppm represents a monomeric diffusing moiety, which shows an increased diffusion coefficient upon heating the sample from 25 °C (D = 0.22 ± 0.01 × 10−5 cm2 s−1) to 37 °C (D = 0.29 ± 0.01 × 10−5 cm2 s−1), the additional peak appeared at 37 °C (0.7 ppm upfield) reflected a diffusion characteristic of a much larger structure (Fig. 2d). Significantly, the diffusion 19F-NMR experiments of 2 revealed that this peak (marked with * in Fig. 2d) represented a molecular structure that had a very low diffusion coefficient (D = 0.008 ± 0.001 × 10−5 cm2 s−1). This slow diffusion rate, 38 times slower compared to that obtained at 37 °C for the molecular entity represented by the peak at −128 ppm (Fig. 2d), strengthened our conclusion that a large assembly of 2 is obtained at 37 °C. Importantly, the ability of 2 to self-assemble to large entities was not affected by pH values that are expected in biological systems (Fig. S4 and S5, ESI†).
Then, to examine the feasibility of 2 for use in future studies to sense Zn2+, its ability to bind the metal ion was studied with 19F-NMR. To this end, an aqueous solution containing 2 mM of 2 (i.e., 6 mM of chelating units) and 1.2 mM of Zn2+ (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chelate
1 chelate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
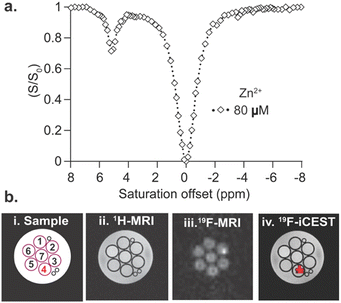
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn2+ ratio) was examined and an additional peak was obtained with a large and specific chemical shift offset relative to the resonance of free ligand 2 (Δω = +5 ppm, Fig. S6, ESI†). Note that at higher Zn2+ concentrations, beyond those expected in biological systems, the peak of 2-Zn2+ complex is clearly observed in the 19F-NMR spectrum but the peak that is assigned with the large assembly is reduced (Fig. S8, ESI†). Importantly, at such high Zn2+ levels even the presence of higher concentrations of Cu2+ (2 times more than Zn2+), the 19F-NMR peak of 2-Zn2+ complex was still preserved (Fig. S9, ESI†). Then, as one of the main advantages of 19F-iCEST is to amplify signals of low levels of ions, the Zn2+ concentration was further diluted to obtain a solution with 2 mM 2 and 80 μM of Zn2+ and 19F-iCEST experiments were performed (Fig. 3a). Here, where the Zn2+ ion was 75 times more diluted than the chelating units (3 units per one tripod 2), a pronounced 27% 19F-iCEST effect was obtained. Such an effect that was obtained with the sensitivity of 12 mM of 19F-nuclei (150 times more concentrated than Zn2+ set at 80 μM) corresponded to a 40 times signal amplification effect in the 19F-MRI framework. This 19F-iCEST effect obtained for 2 and Zn2+, although milder than that obtained with a more flexible 19F-chelate,11 was much larger than that obtained with a 19F-chelates based on the BAPTA backbone.12
Zn2+ ratio) was examined and an additional peak was obtained with a large and specific chemical shift offset relative to the resonance of free ligand 2 (Δω = +5 ppm, Fig. S6, ESI†). Note that at higher Zn2+ concentrations, beyond those expected in biological systems, the peak of 2-Zn2+ complex is clearly observed in the 19F-NMR spectrum but the peak that is assigned with the large assembly is reduced (Fig. S8, ESI†). Importantly, at such high Zn2+ levels even the presence of higher concentrations of Cu2+ (2 times more than Zn2+), the 19F-NMR peak of 2-Zn2+ complex was still preserved (Fig. S9, ESI†). Then, as one of the main advantages of 19F-iCEST is to amplify signals of low levels of ions, the Zn2+ concentration was further diluted to obtain a solution with 2 mM 2 and 80 μM of Zn2+ and 19F-iCEST experiments were performed (Fig. 3a). Here, where the Zn2+ ion was 75 times more diluted than the chelating units (3 units per one tripod 2), a pronounced 27% 19F-iCEST effect was obtained. Such an effect that was obtained with the sensitivity of 12 mM of 19F-nuclei (150 times more concentrated than Zn2+ set at 80 μM) corresponded to a 40 times signal amplification effect in the 19F-MRI framework. This 19F-iCEST effect obtained for 2 and Zn2+, although milder than that obtained with a more flexible 19F-chelate,11 was much larger than that obtained with a 19F-chelates based on the BAPTA backbone.12
Having confirmed the formation of nano-sized assemblies of 2 at a physiological temperature of 37 °C (Fig. 2) and its Zn2+ binding capability (Fig. 3), we examined whether our designed supramolecular probe could be used as a Zn2+-specific 19F-iCEST MRI agent when compared to other cations. For that purpose, a phantom composed of seven different test tubes containing 80 μM of biologically relevant cations (e.g., K+, Mg2+, Cu2+, Zn2+, Ca2+, Na+, or no ion) in the presence of 2 mM 2 was prepared, and MRI was performed on a 9.4 T MRI scanner at 37 °C. Note here that the addition of these cations at the expected biological levels did not affect the assembly process of 2 (Fig. S10 and S11, ESI†). As depicted in Fig. 3b, 1H-MRI showed no signal difference between the examined tubes, confirming that the assembly of 2 did not give rise to contrast changes. A similar observation was made for 19F-MRI where identical 19F-signal was detected for all 7 examined tubes. Nevertheless, a 19F-iCEST MRI experiment that was performed on the very same phantom resulted in a clear signal reduction at Δω = +5 ppm, only from the Zn2+ containing tube. Overlaying the 19F-iCEST contrast on the 1H-MR image represented the spatial distribution of the cation and presented as a Zn2+-map (Fig. 3b, z-spectrum shown in Fig. S12, ESI†).
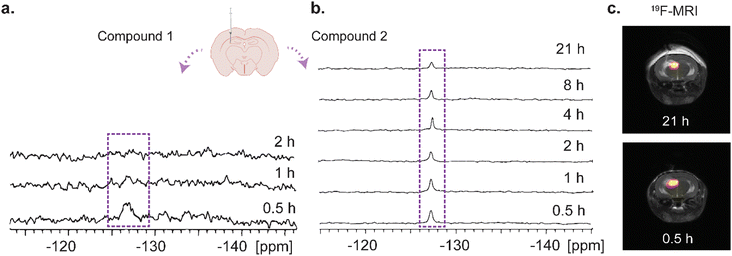
Finally, after confirming the stability of the large-assembly of 2 in biological media for 24 hours (Fig. S13, ESI†), we aimed to examine whether the transition of 2 to a large assembly under physiological condition would affect its washout profile in vivo. For that purpose, 1 μL of either 1 or tripod 2 (dissolved in DMSO, which did not affect the assembly and 19F-iCEST appearance of 2, Fig. S14, ESI†) at an equal fluorine-19 concentration was intracranially injected into the hippocampus of the mouse brain. The examined mice were placed in a 15.2 T MRI scanner and longitudinal 19F-MR studies were performed (Fig. 4). As expected from low-molecular-weight imaging agents, such as 1 (Fig. 2a and b), the 19F-NMR signal could be detected only 30 min after the intracranial injection of the solution of 1, with much of the probe washed out as fast as one hour after the injection (Fig. 4a). This fast washout does not allow for sufficient time to perform 19F-MRI experiments (Fig. S15, ESI†) and requires the use of continued infusions of the agent, as previously shown for small probes.11,12 In contrast, the 19F-NMR signal of the injected probe 2 was preserved for more than 20 hours after its delivery (Fig. 4b), although injected at the same concentration as 1. 19F-MRI experiments performed 30 min and 21 hours after its delivery showed the localization of 2 at the injection site even one day after its injection, confirming its very slow washout (Fig. 4c) without significant damage of the tissue (Fig. S17 and S18, ESI†). These results confirm that the spontaneous assembly of 2 to a large moiety upon its delivery, prevented its clearance from the injected site.
To summarize, we showed here a conceptually novel approach to slow down the washout rate of imaging agents from their region of delivery through their spontaneous self-assembly. By conjugating a 19F-iCEST probe (1) to an aromatic BTA motif to obtain a tripod type molecular architecture (2), the tendency of the obtained structure to form nano-assemblies at 37 °C was demonstrated. In vivo experiments of an injected 2 revealed a prolonged 19F-MRI signal without the need for continuous infusion strategies used currently in MRI studies of low-molecular-weight responsive probes.6,7,11,12 Adopting the principles used in this study, a similar molecular design can be applied to a wide range of imaging sensors that require prolonged imaging times or for those that cannot tolerate fast washout rates.
This work was supported by the Israel Science Foundation (ISF 1329/20) and the Minerva Foundation.
Conflicts of interest
There are no conflicts to declare.Notes and references
- G. Angelovski and E. Toth, Chem. Soc. Rev., 2017, 46, 324–336 RSC.
- J. Wahsner, E. M. Gale, A. Rodriguez-Rodriguez and P. Caravan, Chem. Rev., 2019, 119, 957–1057 CrossRef CAS PubMed.
- W.-h Li, S. E. Fraser and T. J. Meade, J. Am. Chem. Soc., 1999, 121, 1413–1414 CrossRef CAS.
- E. L. Que and C. J. Chang, Chem. Soc. Rev., 2010, 39, 51–60 RSC.
- A. J. Lubag, L. M. De Leon-Rodriguez, S. C. Burgess and A. D. Sherry, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 18400–18405 CrossRef CAS PubMed.
- A. Barandov, B. B. Bartelle, C. G. Williamson, E. S. Loucks, S. J. Lippard and A. Jasanoff, Nat. Commun., 2019, 10, 897 CrossRef.
- T. Savic, G. Gambino, V. S. Bokharaie, H. R. Noori, N. K. Logothetis and G. Angelovski, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 20666–20671 CrossRef CAS.
- V. Clavijo Jordan, C. D. G. Hines, L. T. Gantert, S. Wang, S. Conarello, C. Preihs, S. Chirayil, M. Klimas, J. L. Evelhoch and A. D. Sherry, Front. Endocrinol., 2021, 12, 641722 CrossRef PubMed.
- A. F. Martins, V. Clavijo Jordan, F. Bochner, S. Chirayil, N. Paranawithana, S. Zhang, S. T. Lo, X. Wen, P. Zhao, M. Neeman and A. D. Sherry, J. Am. Chem. Soc., 2018, 140, 17456–17464 CrossRef CAS PubMed.
- M. V. Clavijo Jordan, S. T. Lo, S. Chen, C. Preihs, S. Chirayil, S. Zhang, P. Kapur, W. H. Li, L. M. De Leon-Rodriguez, A. J. Lubag, N. M. Rofsky and A. D. Sherry, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E5464–E5471 CrossRef CAS PubMed.
- N. D. Tirukoti, L. Avram, T. Haris, B. Lerner, Y. Diskin-Posner, H. Allouche-Arnon and A. Bar-Shir, J. Am. Chem. Soc., 2021, 143, 11751–11758 CrossRef CAS.
- Y. Yuan, Z. Wei, C. Chu, J. Zhang, X. Song, P. Walczak and J. W. M. Bulte, Angew. Chem., Int. Ed., 2019, 58, 15512–15517 CrossRef CAS PubMed.
- A. Bar-Shir, A. A. Gilad, K. W. Chan, G. Liu, P. C. van Zijl, J. W. Bulte and M. T. McMahon, J. Am. Chem. Soc., 2013, 135, 12164–12167 CrossRef CAS PubMed.
- H. F. Ozbakir, A. D. C. Miller, K. B. Fishman, A. F. Martins, T. E. Kippin and A. Mukherjee, ACS Sens., 2021, 6, 3163–3169 CrossRef CAS PubMed.
- S. Gunduz, N. Nitta, S. Vibhute, S. Shibata, M. E. Mayer, N. K. Logothetis, I. Aoki and G. Angelovski, Chem. Commun., 2015, 51, 2782–2785 RSC.
- A. Bar-Shir, L. Avram, S. Yariv-Shoushan, D. Anaby, S. Cohen, N. Segev-Amzaleg, D. Frenkel, O. Sadan, D. Offen and Y. Cohen, NMR Biomed., 2014, 27, 774–783 CrossRef CAS.
- S. Okada, B. B. Bartelle, N. Li, V. Breton-Provencher, J. J. Lee, E. Rodriguez, J. Melican, M. Sur and A. Jasanoff, Nat. Nanotechnol., 2018, 13, 473–477 CrossRef CAS PubMed.
- N. M. Matsumoto, R. P. M. Lafleur, X. Lou, K. C. Shih, S. P. W. Wijnands, C. Guibert, J. van Rosendaal, I. K. Voets, A. R. A. Palmans, Y. Lin and E. W. Meijer, J. Am. Chem. Soc., 2018, 140, 13308–13316 CrossRef CAS PubMed.
- X. Lou, R. P. M. Lafleur, C. M. A. Leenders, S. M. C. Schoenmakers, N. M. Matsumoto, M. B. Baker, J. L. J. van Dongen, A. R. A. Palmans and E. W. Meijer, Nat. Commun., 2017, 8, 15420 CrossRef PubMed.
- R. P. M. Lafleur, S. M. C. Schoenmakers, P. Madhikar, D. Bochicchio, B. Baumeier, A. R. A. Palmans, G. M. Pavan and E. W. Meijer, Macromolecules, 2019, 52, 3049–3055 CrossRef CAS.
- L. Avram and Y. Cohen, Chem. Soc. Rev., 2015, 44, 586–602 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and supplementary figures. See DOI: https://doi.org/10.1039/d2cc03126d |
| This journal is © The Royal Society of Chemistry 2022 |