Biocatalytic dynamic reductive kinetic resolution of aryl α-chloro β-keto esters: divergent, stereocontrolled synthesis of diltiazem, clentiazem, and siratiazem†
Abstract
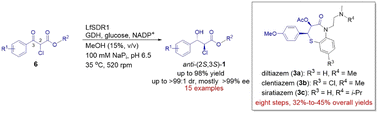
The first systematic study of ketoreductase (KRED)-catalyzed dynamic reductive kinetic resolution (DYRKR) on aryl α-chloro β-keto esters was performed, and 15 structurally diverse chiral anti-aryl α-chloro β-hydroxy esters were synthesized in 74–98% isolated yields, along with moderate-to-excellent diastereoselectivity (up to >99 : 1 dr) and good-to-excellent enantioselectivity (mostly >99% ee). LfSDR1-catalyzed complete reduction of 100 g L−1 of substrate 6b at a ten-gram scale was achieved with a continuous fed-batch strategy, affording anti-(2S,3S)-1b, the key intermediate of diltiazem, in a record-breaking space-time yield of 96 g L−1 d−1. An eight-step synthesis of diltiazem, clentiazem, and siratiazem was accomplished in 32–45% overall yields, featuring this versatile biocatalytic reduction reaction as well as an efficient, green chlorination reaction in flow.
- This article is part of the themed collection: Biocatalysis: A cross-journal collection


 Please wait while we load your content...
Please wait while we load your content...