Multi-functional metal–organic frameworks for detection and removal of water pollutions
Yang
Li
a,
Jiandong
Pang
 *ab and
Xian-He
Bu
*ab and
Xian-He
Bu
 *abc
*abc
aSchool of Materials Science and Engineering, National Institute for Advanced Materials, TKL of Metal and Molecule-Based Material Chemistry, Nankai University, Tianjin 300350, P. R. China. E-mail: buxh@nankai.edu.cn; jdpang@nankai.edu.cn
bHaihe Laboratory of Sustainable Chemical Transformations, Tianjin, 300192, P. R. China
cState Key Laboratory of Elemento–Organic Chemistry, College of Chemistry, Nankai University, Tianjin 300071, P. R. China
First published on 24th June 2022
Abstract
Water pollutions have caused serious threats to the aquatic environment and human health, it is of great significance to monitor and control their contents in water. Compared with the traditional single treatment method, multiple treatment can simultaneously realize detection and removal of water pollutions on one material, which brings convenience and promotes comprehensive treatment for water remediation. Metal–organic frameworks (MOFs) are versatile porous materials that hold huge potential in wastewater treatments. This feature article reviews the recent advances in MOFs for multifunctional water treatment. First, strategies to design the sensing, removal and degradation functions onto MOFs are introduced. Then, the sensing, removal and degradation effects on various inorganic and organic water pollutions based on multifunctional MOF materials are summarized, and the interaction mechanisms are discussed in detail. Finally, the current challenges and the future research trends on MOF materials for wastewater treatment are discussed as well.
1. Introduction
In the past decades, the humanity knowledge and transformation ability of nature further strengthen along with the swift and violent development of science and technology. However, rapid industrialization, accelerated urbanization, surging exploitation of resources, direct discard of wastes and so forth caused by human activities have placed an enormous burden on the environment, especially on water resources.1 As a result, a variety of contaminants have lowered the water quality extensively and brought about water pollution. Generally, Water pollution consist of miscellaneous inorganic and organic substances such as halide ion, heavy metals, radionuclides, oxysalt, synthetic dyes, herbicides, pesticides, persistent organic pollutants (POPs), endocrine disrupting chemicals (EDCs), pharmaceuticals and personal care products (PPCPs) and emerging organic contaminants (EOCs), etc.2–9 These hazardous contaminants are relatively stable in water and can possess carcinogenic, teratogenic and mutagenic effects, leading to adverse impact to both environment and human health.10,11 It is of great significance to detect and remove water pollutions effectively for the sake of preventing their repercussions.Up to now, A variety of wastewater treatments have been carried out for the remediation of aqueous environment, most of which can be summarized as the following strategies: sensing, removal and degradation. Sensing refers to methods for the determination of water pollutions with high sensitivity and selectivity, among which electrochemical, optical and electronic signals are common outputs.12 Numerous materials, for instance, small molecules, polymeric materials, nanomaterials and porous materials are exploited for various water contaminants detecting with low limit of detection and high selectivity.13–15 Removal is the basic function in water treatment to eliminate pollutions from aqueous environment via multifarious functional materials. It contains a number of techniques viz. coagulation, membrane filtration, chemical precipitation, ion exchange, electrodialysis, flocculation and adsorption.16–19 Adsorption is one of the most widely used approaches among them, due to the ease of operation, high efficiency, relatively low cost and reusability. Adsorbents play a crucial role in this method and various adsorbents, namely activated carbons, zeolites, graphene-based materials, clay minerals, resins and porous materials have been developed for eliminating all kinds of water pollutions with large adsorption capacity and high removal efficiency.20–25 Degradation is an option for water treatment by decomposing organic pollutions or oxidizing/reducing metal ions to a low toxicity state. Diversified methods, e.g., photocatalysis, electrocatalysis, Fenton reaction, ozonation and chlorination are widely explored for water pollution detoxication.26–30 Semiconductor materials like TiO2, WO3, CuS, CdS, ZnO and Fe2O3, etc., are typical catalytically active substances. In the last decades, organic materials, for instance, graphene, carbon nanotube and g-C3N4 and inorganic–organic hybrid materials such as metal–organic frameworks are burgeoning materials that reveal great potential for wastewater degradation.31–34
Impressing progress has been made for water pollution control. However, most research focus on realizing one function of sensing, removal or degradation. As the research and practice is continually comprehensive and in-depth, single-function treatment of wastewater has its limitation. For example, sensing probes commonly lack sufficient adsorption capacity, and additional adsorption material is needed to remove the pollutions after their detection; the concentrated adsorbed pollutions on adsorbent need to be carefully treated in order to avoid secondary pollution to the environment;35 Traditional catalyst, e.g., TiO2 often suffers from low surface area and shows low abundance catalytic sites toward analytes, thus displaying reduced catalytic efficiency.36 Integration of different functions to a single system, viz. construction of multifunctional materials can combine their respective advantages and implement multi-purpose applications, gradually developed in energy, biomedicine, materials science, etc.37–39 For water pollution treatment, exploiting materials with more than one function of sensing, removal or degradation to the specific target can help simultaneously detect and control pollutions in water, which is a promising way for comprehensive treatment of water pollutions. Nevertheless, this kind of strategy requires higher designability and versatility of materials, for which advanced materials are desired.
Metal–organic frameworks (MOFs) represent a class of porous inorganic–organic hybrid materials coming forth in the last decades that are synthesized through the assembly of secondary building units (SBUs), viz., organic linkers and metal cations/clusters via coordination bond.40 The crystalline structure, high porosity, high surface area, atomic-level precision design and multiplicate chemical properties enable MOFs to be utilized in copious applications involving energy storage, separation, bioimaging, catalysis, sensing, and so on.41–44 MOFs also demonstrate huge potential in water treatment field and a number of articles and reviews are reported.45–47 However, most studies mainly focus on one function, namely, sensing, removal or degradation for water pollution disposal. Synthesizing functional MOFs with multiple processing to a certain pollutant is of great significance to detect and eliminate water pollutions on one platform. Such integrated water treatment platform puts forward elegant design request for MOF-based materials.
In this feature article, we investigate the design strategy to accomplish the sensing, removal and degradation functions in MOFs and summarize the near-term advances in multifunctional MOFs for water pollution treatment. Furthermore, the current challenges for the multiple treatment of water pollution are discussed, and the outlook on strategies for further developments are recommended (Scheme 1).
2. MOFs design for water pollution treatment
MOFs have been explored for water pollution treatment in the past 15 years, while the first MOF for sensing was reported (Cu2+ ion) in aqueous solution in 2010,48 the first MOF for the removal of methyl orange (dye) from water in 201049 and the first MOF for degradation of phenol (industrial product) in 2007.50 Since then, MOFs are vastly developed as wastewater treatment materials duo to the following features: (I) high surface area, facilitating adsorption process;51 (II) accurate design at atomic level, where sensing/catalytic sites towards the analytes can be precisely embedded into the structure;52 (III) accessible and functionalized pores, where host–guest interactions with selectivity may occur;53 (IV) crystalline feature, which is convenient to explore the mechanism;54 (V) stability, with which a number of water stable MOFs can be achieved for water treatment;55 (VI) processibility, where MOFs can be manufactured to various devices.56 In recent years, MOFs with multifunction for water pollution treatment have been reported. MOFs are ideal materials for water pollution treatment and by virtue of their atom level accurate design and intrinsic porosity, specific interaction towards analytes can be realized. On the basis of adsorption of targets on MOFs, sensing and degradation functions can be conducted on the same material by rational and elegant design of the monomers. Design strategy of MOFs for sensing, removal and degradation of water pollutions are discussed below.2.1 MOFs for sensing
Sensing is a sensitive way to detect water pollutions with selectivity. MOFs are good candidates for water pollution sensing, since the constitutional units (ligand, metal ions or clusters) of MOFs have greatly variability and tunability that may generate electro/optical signals change after the interactions with analytes.57 Besides, the intrinsic porosity of MOFs plays an important role in sensing the targets. On one hand, the pores allow the analytes to enter into the framework and confine their motion to some extent, facilitating the interactions between MOFs and analytes.58 On the other hand, the pores can be elegantly tuned both in size and chemical environment via the regulation of MOFs construction through bottom-up or post-modification strategy.59,60 As a result, a variety of MOFs are synthesized by rationally designing metal ions/clusters and organic ligands for sensing water pollution.612.2 MOFs for removal
Removal of pollutions from aqueous solution is a particularly important mode in water treatment. MOFs exhibit unique characteristics in water pollution removal as such materials combine porosity, crystallinity and functionality. Porosity can help accommodate more analytes and increase removal efficiency. It is worth noting that pore size and surface area of MOFs can be tuned by changing the connectivity and structure of the organic ligand.62 MOFs with suitable pores can be synthesized to meet the removal requirements of pollutions with different size. Crystallinity enables MOFs to be determined at atomic precision, and the interactions with the targets can be revealed. A better understanding of the removal mechanism is hence given.63 Functionality is a fascinating feature in MOFs, where the diversity of organic linkers can be applied to the MOFs structure and brings about binding sites to the analytes. As a result, various interactions, for example, electrostatic interaction, hydrogen bonding, π–π interaction, acid–base interaction and hydrophobic interaction can be induced to the framework through organic ligands design to enhance the adsorption ability of the analytes in water. 54 Besides organic linkers, inorganic metals can also contact with the analytes via the interactions between their uncoordinated orbitals and the heteroatoms (O, N, S, etc.) of the adsorbates.642.3 MOFs for degradation
Degradation of water pollutions is a considerable approach as such process can transform pollutions to less toxic or nontoxic form. MOFs exhibit satisfactory performance in the degradation of water pollutions as their porosity can offer a suitable space for the chemical reactions to take place. Besides, MOFs consist of metal/metal clusters and organic ligands, both of which can be used as catalytic sites via meticulous design.65,66 Through the functional adjustment of organic ligands, e.g., introducing –NH2, –OH, –SH, halogen or heteroatom, the bandgap of MOFs can be tuned and a better catalytic performance can be acquired.67,68 In addition, doping is another efficient strategy to obtain effective catalyst for water pollution treatment based on MOFs. Noble metals like Au, Pd and Pt,69,70 metal semiconductors such as TiO271 and ZnO72 and carbon materials, for example, graphene oxide73 and g-C3N474 are integrated with MOFs to enhance the catalytic performance towards water pollution.Adsorption plays a critical role in water pollution treatment as such process enables analytes to separate from aqueous environment and attach to the adsorbent, after which the sensing or catalysis procedure can occur efficiently. The high surface area and porosity of MOFs make it good candidates to perform adsorption process towards analytes and promising platform to carry out removal, sensing and degradation functions. Removal process caused by adsorption is a fundamental function in water pollution treatment, however, analytes absorbed on MOFs may not necessarily lead to sensing or degradation response. By judicious choice of metal type, connectivity, ligand size, structure and function, multi-functional water pollution treatment materials based on MOFs can be accessed. In addition, removal, sensing and degradation of the same target help to monitor and control the specific pollution in water simultaneously, and is mainly discussed in this feature article.
3. Multifunctional MOFs for water pollution treatment
Scientists have synthesized a number of MOFs for wastewater treatment and achieved decent progress, however, pursuing MOFs with multiple-modality in water pollution treatment is far from over. Considering the diversity of water pollutions, the realization of sensing, removal and degradation of a specific contaminant on MOFs/MOF composites, and the exploitation of various functional MOFs for multiple treatment of different analytes, is expected (Fig. 1).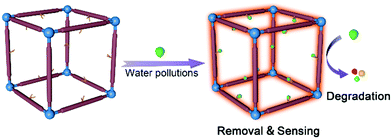 | ||
| Fig. 1 Illustration for the sensing, removal and degradation functions realized on multifunctional MOFs for water pollutions treatment. | ||
3.1. MOFs for multiple treatments of inorganic pollutions in water
Water pollutions have a diversity of sources and are numerous in variety.75–77 According to the constitution, water pollutions can be divided to organic and inorganic contaminants. Inorganic pollutions mainly include heavy metals, halide ion, oxyanions and radioactive elements.78,79 Adsorptive removal and sensing are frequently-used methods to detect and control inorganic water pollutions. As most inorganic pollutions are hard to degrade, some catalytic redox reactions can make the valence of metal transform to a low-toxic state, providing another way for inorganic pollution treatment.80,81 | ||
| Fig. 2 Schematic illustration of the structure of LMOF-261 for adsorption and sensing of Hg ion. Reproduced from ref. 84 with permission from American Chemical Society, copyright 2016. | ||
Ligand exchange is also an elegant approach to acquire functional MOFs.85 In a study by Wang and co-workers, the solvent-assisted ligand exchange approach was conducted to construct a mixed-ligand NH2-UiO66-SH (NSU66) via introducing the thiol containing ligands to NH2-UiO-66.86 The obtained NSU66 displayed a large adsorption capacity of 265.29 mg g−1 and a removal rate within 60 min together with qualified selectivity. NSU66 showed a fluorescence quenching effect towards Hg2+ with a low detection limit of 3.50 × 10−2 μM and a wide linear range of 1.00–99.7 μM. NSU66 also demonstrated high specificity and strong anti-interference capability during the sensing process. Compared with the NH2-UiO-66, NSU66 showed a significant enhancement in both sensing and adsorption ability, which benefits from the interactions between the incorporated thiol groups and Hg2+. The photo-induced electron transfer (PET) mechanism was proposed to explain the sensing process. Considering the good stability in water and recyclability, such MOFs can serve as multifunctional platform for sensing and removal of Hg2+ ion. In another work by the same group, a series of classic luminescence MOFs were synthesized based on the same organic ligand, NH2-H2BDC, and different central metals (Ti4+, Fe3+, Cr3+, Zr4+ and Al3+).87 Among these MOFs, NH2-MIL-53(Al) displayed good sensing ability towards Hg2+ with the response range of 1–17.3 μM, low detection limit of 0.15 μM, good selectivity and wide pH adaptation of 4.0–10.0. Meanwhile, the NH2-MIL-53(Al) showed an adsorption capacity of 153.85 mg g−1 and an uptake kinetic within 60 min. NH2-MIL-53(Al) demonstrated excellent stability due to the formation of strong coordination bonds between high oxidation state aluminium (Al3+) and organic carboxylate ligands. The satisfactory reusability endowed the NH2-MIL-53(Al) a promising application prospect for sensing and removal of Hg2+ simultaneously in aqueous solution.
Apart from the interaction between the sulphur and mercury, other heteroatoms such as nitrogen and oxygen can also have interactions with Hg2+.13 Morsali and co-workers introduced S linker which has a malonamide functional group into the Cu-based MOFs to form TMU-46S, -47S and -48S, respectively, via a solvent-assisted ligand exchange (SALE) method.88 The exchanged MOFs showed a large adsorption capacity of 714 mg g−1 towards Hg2+ and a limit of detection of 0.1 ppm with the quenching constants (Ksv) of 186![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 087 M−1, and the performance is superior to the original MOFs. The synergistic effects of both hydrophilic chelating malonamide and acylamide functional groups contributed a lot in recognizing mercury ion through theoretical calculations. The research represented an effective way to develop multi-functional MOFs in mercury ion treatment.
087 M−1, and the performance is superior to the original MOFs. The synergistic effects of both hydrophilic chelating malonamide and acylamide functional groups contributed a lot in recognizing mercury ion through theoretical calculations. The research represented an effective way to develop multi-functional MOFs in mercury ion treatment.
Visual monitoring is a direct and convenient method to detect mercury ions.89 In a research work by Roy's group, a Ni-based MOF was synthesized using an organic ligand that had the uncoordinated sulphur atom.90 The constructed MOFs had uncoordinated sulphur sites and can form strong bond with mercury due to the soft center of S. The Ni-MOF had a large Hg adsorption capacity of 713 mg g−1 in aqueous environment. The colour of the MOF changed from green to grey, thus giving a visual detection of Hg. A number of experiment data and theoretical studies revealed that Hg formed coordinate bonds with S atoms of the SCN− group. However, one drawback of this system is that the colour change of this MOFs is irreversible and limits its practical use in aqueous solution.
In another research by El-Sewify's group, a novel Al-MOF was synthesized with high surface area to support it as a microporous substrate for further embedding a thioketone derivative chromophore.91 The thioketone Al-MOF monitors (TAM) were employed through immobilization of chromophore on Al-MOFs’ surface. The TAM system had a colour change from yellow to green when contacted with Hg2+ ion in water, and the phenomenon can be seen clearly by the naked eye within a few seconds. The sensitivity of TAM to Hg2+ can reach 0.8 ppb, which is lower than the WHO permissible drinking water limits. In addition, a very large adsorption capacity of 1110 mg g−1 was obtained by TAM in aqueous environment. What is more important is that TAM could be reused several times without losing the sensing and removal ability. The fabricated TAM was used to sense and remove Hg2+ in well water samples and achieve good effects, exhibiting great potential for multi-treatment for Hg2+ ion in water.
Electrochemical detection is a sensitive sensing method apart from optical approach.92 Wang et al. reported a mercaptan functionalized MOFs (Zr-DMBD MOFs) by coordination between Zr(IV) and 2,5-dimercaptoterephthalic acid.93 The MOFs was then combined with kenaf stem-derived carbon (3D-KSC) to form a nanocomposite for electrochemical sensing and removal of Hg(II) simultaneously. The Zr-DMBD MOFs showed the adsorption capacity of 171.5 mg g−1, whereas the Zr-DMBD MOFs/3D-KSC displayed decreased adsorption capacity of 19.3 mg g−1, mainly due to the poor adsorption ability of 3D-KSC towards Hg ion. Zr-DMBD MOFs/3D-KSC demonstrated that the sensitivity of Hg2+ was 324.58 μA μM−1 cm−2, the linear range was 0.25–3.5 μM and the limit of detection was 0.05 μM. The porous Zr-DMBD MOFs can disperse uniformly on microporous 3D-KSC and offered a great deal of mercaptan groups to enrich Hg2+ on the electrode. The Zr-DMBD MOFs/3D-KSC materials showed excellent anti-interference performance, good stability and satisfactory reusability. The S atoms of mercaptan groups on MOFs facilitate the binding process towards Hg ion. The 3D-KSC displayed macroporous structure and high porosity, and Zr-DMBD MOFs were able to grew uniformly on its surface. The mass transfer was thus enhanced and the preconcentration process of Hg ion was accelerated. Furthermore, the Zr-DMBD MOFs/3D-KSC could work efficiently in real water sample, and the treated Hg ion in water was lower than national discharge standard of industrial wastewater.
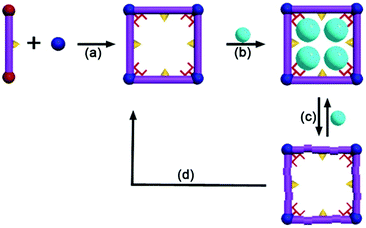 | ||
| Fig. 3 A regenerative MOF for reversible uptake of Cd(II): (a) self assembly 3D framework from organic ligand and hard metal ion; (b) effective absorption with high capacity; (c) absorption and desorption; (d) reconstruction of the used sample into a fresh sample. Reproduced from ref. 96 with permission from Royal Society of Chemistry, copyright 2016. | ||
Composite materials can integrate each function of their component and acquire multi functions, thus leading to synergistic effects.99 He's group developed a Fe3O4/MOF/cysteine composite by synthesizing Cu-based MOFs outside nano Fe3O4, following by modifications of L-cysteine.100 The Fe3O4 part in the material help realize fast separation from water with the help of a magnet. The maximum adsorption capacity was 248.2 mg g−1, and the Fe3O4/MOF/cysteine can also be regarded as a fluorescent quenching sensor for detection of Cd2+ with the detection of limits of 0.94 ng mL−1. In this composite, the high surface area and pore volume of MOFs offered a space to accommodate the target and L-cysteine, which had three functional groups (–SH, –NH2, –COOH) that may offer binding site toward Cd2+. Besides, L-cysteine help increase the fluorescent intensity of the MOF, which facilitate the Fe3O4/MOF/cysteine to be used as a turn off probe for Cd detection. The materials maintained above 90% efficiency after 5 times of reuse. Furthermore, a magnetic solid-phase extraction method was established using the MOF composites to detect trace Cd2+ ion in water. The limit of detection of 10.6 ng mL−1 was achieved. Fe3O4/MOF/cysteine was used to extract Cd2+ in tap water and lake water and showed potential for practical applications.
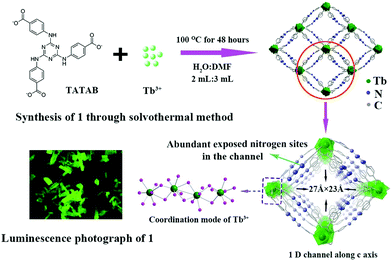 | ||
| Fig. 4 Schematic for the formation of Tb-MOF for the adsorption and detection of uranium from aqueous solution. Reproduced from ref. 106 with permission from American Chemical Society, copyright 2017. | ||
Ratiometric detection hold advantage in sensing. The same group of Wang and co-workers reported a ratiometric monitoring of Thorium contamination in natural water using a dual luminescent Eu-MOF.107 The obtained MOF ThP-1 showed highly sensitive and selective self-calibrated sensing of Th4+, with the emission intensity increased at 617 nm and decreased at 365 nm. ThP-1 showed a wide detection range (0–300 mg L−1) and low detection limit (24.2 μg L−1), much lower than the thorium contamination standard of 246 μg L−1 in drinking water defined by the World Health Organization. ThP-1 also displayed selectivity of Th4+ towards Na+, K+, Cs+, Mg2+, Ba2+, Sr2+ and Ca2+. ThP-1 demonstrated Langmuir adsorption of Th4+ with the adsorption capacity of 25.82 mg g−1 and the adsorption also showed good selectivity towards the metal ions above. Experiment results combined with DFT calculations revealed that the selective uptake of Th4+ by the host ThP-1 through inner-sphere coordination were responsible for the sensing and removal process. Furthermore, ThP-1 could be used in real water samples, offering an option for simultaneous detection and removal of Th4+ in aqueous solution.
Single-crystal to single-crystal (SC–SC) transformation of MOFs has caused great interest, as it allows creation of new materials and postsynthetic functionalization of MOFs in a stepwise and visualizable manner.108 Lin and co-workers synthesized a cationic thorium MOFs showing triple SC-SC transformation in organic solvents, water and NaIO3 solution.109 Thereinto, the SC–SC transformation in NaIO3 solution can cause turn-off sensing of IO3− ion. The Th-MOF showed the detection limit of 0.107 μg kg−1 of iodate and demonstrated good anti-interference towards a serious of cation and anion ions (BO33−, SO42−, CO32−, F−, Cl−, HCO3−, NO3−, Na+, K+, Ba2+, Sr2+, Ca2+, Zn2+, Mg2+ and Al3+). Besides sensing, Th-MOF also displayed efficient removal of IO3− with the adsorption capacity of 152.6 mg g−1 and the fast adsorption equilibrium within 30 min. The sensing mechanism can be explained as the SC–SC transition induced by IO3− caused the large modifications of the ligand conformation and turned the ligand-based emission off. Besides, the host–guest interaction between the luminescent framework and IO3− leads to heavy atom induced intersystem crossing, resulting in a selective and static quenching by a distinct mechanism. The SC–SC transition on such Th-MOF offered an alternative way to simultaneous detect and remove IO3− in aqueous solution.
MOF-based composites materials can also assist in radionuclide treatment. Wang's group designed a composite material made of a magnetic core and a zeolite imidazolate framework (ZIF-8) shell surrounded by carbon dots (CDs) to simultaneous sensing and removal of uranium ion in water.110 Fe3O4-CMC@ZIF-8@CDs had a large adsorption capacity of 606.06 mg g−1. Moreover, the fluorescence intensity decreased close to detection limit as the U(VI) concentration increased to 100 mg L−1. Experimental data showed that ZIF-8@CDs part offered large surface area and abundant nitrogen/oxygen sites on the surface, and the main interaction mechanisms are diffusion and coordination. Na2CO3 containing H2O2 solution can serve as desorption agents, and the Fe3O4-CMC@ZIF-8@CDs can be reused for at least 5 cycles with high adsorption efficiency, giving such material great potential in uranyl ion multiple treatment.
Ratiometric detection induced by dual emissions can eliminate interferences from the surrounding environment and is widely used in ions sensing.114 In another research of Zr-based MOFs synthesized by Choi and co-workers, two types of dyes, 7-amino-4-methylcoumarin and resorufin with two different emissions were introduced into a MOF consist of fumarate and Zr cluster, viz., MOF-801 (Fig. 5).115 The dyes offered a dual fluorescence emission for the ratiometric detection and the porous MOF-801 was conducive to effect removal of the target. The obtained Dyes⊂MOF-801 material showed a Cr2O72− adsorption capacity of 83 mg g−1 and the process can be finished within 3 min, even in the presence of excess metal ions and anions (Cl−, NO3−, CO32−, SO42−, PO43−, and citrate) with 10-fold excess concentrations. Besides, fluorescence intensity ratio of coumarin over resorufin in Dyes⊂MOF-801 was used as ratiometric detection for Cr2O72−, as the two dye molecules within MOF-801 can both detect Cr2O72− at different emissions. A detection limit of 0.03 mM was achieved and a high selectivity was attained even in the presence of a 260-fold excess of interfering ions on Dyes⊂MOF-801, exhibiting strong anti-interference capability.
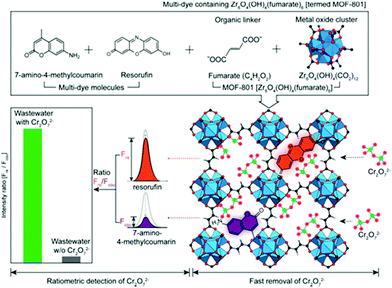 | ||
| Fig. 5 Schematic of the ratiometric detection and simultaneous removal of Cr2O72− using multi-dye (resorufin and 7-amino-4-methylcoumarin) containing MOF-801. Reproduced from ref. 115 with permission from Elsevier Inc, copyright 2019. | ||
Membranes play an important role in wastewater separation. MOFs based membranes can combine the high surface area, porosity and functionality of MOF and the easy maintenance, strong adaptability, small footprint and high separation efficiency of membrane, offering attractive prospect in water pollution treatment.116 Cui's group developed MOF-based mixed-matrix membranes (MMMs) for simultaneously removal and sensing of Cr2O72− in aqueous system.117 To be specific, a water stable cationic Eu-based MOF (Eu-mtb) was mixed with poly (vinylidene fluoride) to form MOF-based MMM. The obtained Eu-mtb MMM showed efficient removal ability towards Cr2O72− with the adsorption capacity of 33.34 mg g−1. In addition, fluorescence sensing behavior of Eu-mtb MMM to Cr2O72− was also achieved, with the detection limit of 5.73 nM. The MMMs helped Eu-mtb to distribute uniformly without aggregation and make it possible for the sufficient interactions between Cr2O72− and Eu-mtb MOF. As a result, the adsorption capacity and sensing sensitivity were both enhanced compared with bare Eu-mtb. The competitive adsorption and resonance energy transfer may response for the sensing behavior. Eu-mtb MMM also manifested good selectivity when anions like Cl−, NO3− and I− caused small interference in both adsorption and sensing towards Cr2O72−.
As non-degradable properties of metal ions, catalytic redox reactions that transform the metal valence to a low-toxic state offers an effective way for inorganic pollution treatment. For example, dichromate ions are highly toxic, but their reduction products, Cr3+ ions are much less toxic.118 Functional MOFs can serve as a catalytic media for the reduction of Cr6+ oxyanions.119 Gu and co-workers constructed a serious of UiO-66 type MOFs with different hydroxyl groups (UiO-66-OH, UiO66-(OH)2).120 The hydroxyl groups on organic ligands played a vital role in Cr(VI) treatment, as UiO66-(OH)2 showed an adsorption capacity of 53.8 mg g−1, larger than that in UiO-66-OH of 16.1 mg g−1. Meanwhile, a reduction process can occur in situ on the Cr(VI) adsorbed MOFs and Cr(III) was detected as a less toxic product. The abundant hydroxyl groups can serve as not only reductive sites for the conversion of Cr(VI) to Cr(III), but also intrinsic anchors for the enhanced adsorption of Cr(VI). When simulated industrial wastewater containing 5 mg L−1 Cr(VI) was treated with UiO66-(OH)2, less than 50 μg L−1 Cr(VI) could be detected, making this material promising candidate for removal and reduction of Cr(VI) in water. Afterwards, the same group developed a universal strategy of introducing thiol groups into MOFs for Cr(VI) removal and reduction in water.121 For details, thiol-containing Zr-MOFs was fabricated using mercaptosuccinic acid (MSA) and meso-dimercaptosuccinic acid (DMSA) as organic ligands. The thiol groups played an important role in simultaneous removal and reduction of Cr(VI), as Cr (VI)–thiolate complex formed to facilitate the adsorption process and the oxidation of thiol groups helped to reduce Cr(VI) to Cr(III), respectively. As a result, adsorption capacity of 202.0 and 138.7 mg g−1 was acquired for Zr-MSA and Zr-DMSA, respectively. By virtue of UV-Vis spectra and XPS measurement, Cr(III) was detected after the Cr(VI) was adsorbed on the MOF, indicating a reduction of Cr(VI) on MOFs. The thiol-containing MOFs showed good anti-interference when other ions coexisted. Moreover, such thiol-introducing strategy is universal for other MOFs such as UiO-66 and MOF-808, which provided a guideline for design Cr(VI) multiple treatment MOFs in aqueous environment. However, the catalytic reduction of Cr(VI) was not discussed in detail on the above two works.
Materials that can realize sensing, removal and degradation functions simultaneously are highly desirable, as the overall treatment from “birth” to “death” of water pollutions can be achieved on one material. However, such process made high requests for material design and preparation. To this end, Liu and co-workers made an effort to employ a multifunctional Zr-based MOFs for efficient detection and removal of Cr(VI) in water solution. In this work, a highly water stable luminescent MOF consists of 2,3,5,6-tetrakis(4-carboxyphenyl)pyrazine (H4TCPP) and Zr clusters, namely, JLU-MOF60 was successfully constructed (Fig. 6).122 The sqc topology Zr-MOF showed extensive pH stability from 0 to 11 and is ideal for applications in water. As Zr-bonded hydroxides have high affinity to Cr2O72−, JLU-MOF60 displayed a high adsorption capacity of 149 mg g−1 as well as fast adsorption rate of 38 mg g−1 min−1 towards Cr2O72−, and showed excellent anti-interfering without losing much of its adsorption capacity when competing anions such as Cl−, Br−, I−, NO3−, SO42− and PO43− coexisted in aqueous solution. Furthermore, with the help of the aggregation induced emission (AIE) effect of the organic H4TCPP ligands, JLU-MOF60 can not only sense Cr2O72− through luminescence quenching, but also photodegrade Cr(VI) to Cr(III) in water. For sensing, the detection limit can reach as low as 9.38 μM with good anti-interference ability of other anions, e.g., F−, Cl−, Br−, I−, PO43−, NO2−, NO3−, MoO42− and VO3−. For photocatalytic reduction, 98% Cr(VI) converted to Cr(III) after 70 min of light exposure, and the rate constant κ was determined to be 0.058 min−1, showing an effective photocatalytic property. This work offered an efficient method for design and synthesize multi-functional MOFs for simultaneously sensing, removal and degradation of Cr(VI) in water.
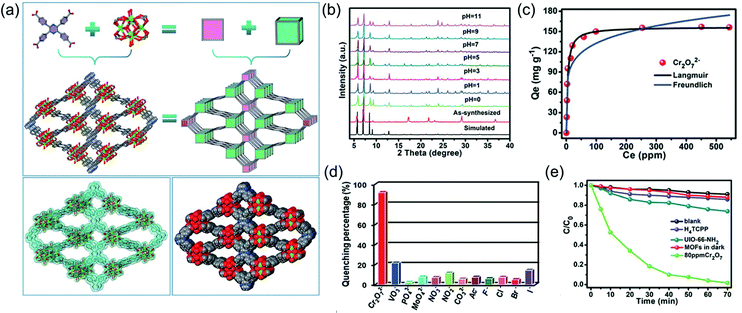 | ||
| Fig. 6 (a) The structure illustration of JLU-MOF60; (b) the stability of JLU-MOF60 in various pHs; (c) the removal ability; (d) the sensing ability and (e) the degradation ability of JLU-MOF60 for Cr2O72− treatment. Reproduced from ref. 122 with permission from Royal Society of Chemistry, copyright 2019. | ||
In a recent work, Li and co-workers explored a novel In-based MOF, In(tcpp) using a chromophore ligand, 2,3,5,6-tetrakis(4-carboxyphenyl)pyrazine (H4tcpp) (Fig. 7).126 The In(tcpp) showed dual functions for sensing and removal of fluorinated chemicals. Specifically, In(tcpp) displayed a turn-on detection of F− within 5 min and showed a limit of detection of 1.3 μg L−1. In(tcpp) also exhibited excellent anti- interference against various cations and anions such as Ag+, Cd2+, Co2+, Cu2+, Ni2+, Pb2+ Fe3+, Al3+, Cl−, Br−, NO2−, NO3−, SO42−, PO43−, CN− and acetate. Besides, In(tcpp) can serve as an adsorbent for the removal of F− with a adsorption capacity of 36.7 mg g−1. Experiments and DFT calculations revealed that the interaction between F− and In(tcpp) mainly occurred at the bridging –OH− offered by organic ligands. The reusability makes In(tcpp) promising materials for fluorinated chemicals treatment in aqueous solution.
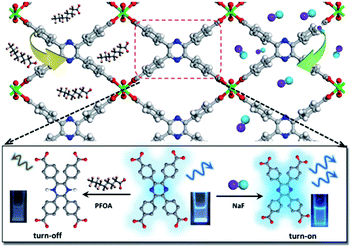 | ||
| Fig. 7 The structure of In(tcpp) and schematic presentation of its luminescence switchable turn-off response toward PFOA and turn-on response toward F ions. Reproduced from ref. 126 with permission from Royal Society of Chemistry, copyright 2021. | ||
MOFs and MOF-based composites have shown potentials for multiple treatment for inorganic pollutions, as more than one function of sensitive sensing, efficient removal and detoxication can be realized. However, some improvements can still be made, for example, exploiting sensitive turn on sensing modes and improving the reusability performance towards metal ions.
3.2. MOFs for multiple treatments of organic pollutions in water
Compared with inorganic pollutions, the organic pollutions are more abundant and diverse in water environment, including dyes, pharmaceuticals and personal care products (PPCP), pesticides, persistent organic pollutants (POPs), endocrine-disrupting compounds (EDCs) and emerging organic contaminants (EOCs).127 These organic pollutions can cause adverse effect to water environment and organisms and bring mutagenesis, carcinogenesis and teratogenesis to human.128 Therefore, detection and removal of organic water pollution is significant. MOFs have the advantages of porosity, designability, easy functionalization and tunability, which are ideal materials suited for introducing multiple functions on organic water pollution treatment.129In another work by Li's group, Fe-loaded MOF-545(Fe) was explored as peroxidase-like activity for removal and degradation of dyes in water.135 The experimental conditions were investigated in detail, and the degradation can be completed within 2 hours under optimized conditions in the presence of H2O2. Fe-loaded MOF-545(Fe) also displayed good removal ability, with the adsorption capacities of methylene blue and methyl orange reaching 382.35 and 803.66 mg g−1, respectively. The Fe-loaded MOF-545(Fe) demonstrated good reusability, which is crucial for practical application.
Yu's group constructed iron–nickel bimetallic MOFs for removal and photo-Fenton degradation of dyes in aqueous solution (Fig. 8).136 With the Ni doping in MIL-101(Fe), the obtained FeNi1/15-BDC showed 5.2 and 2.6 times larger in adsorption capacity for MB and MO, respectively, due to the enlarged pore volume and specific surface area. Slow induction stage and rapid oxidation stage on the degradation process of FeNi1/15-BDC was found to fit with pseudo-zero-order kinetics. And the increased degradation rate constants were observed by FeNi1/15-BDC (2.472 vs. 1.188 min−1 for MB; 0.616 vs. 0.421 min−1 for MO). Notably, the FeNi1/15-BDC displayed wide working pH range and reusability, and can be used for the reinforced removal and degradation of organic dyes.
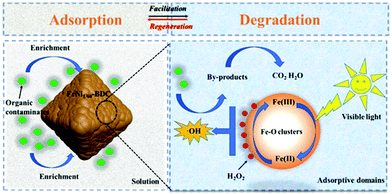 | ||
| Fig. 8 Schematic illustration of contaminant removal in FeNi1/60-BDC/H2O2/visible light system. Reproduced from ref. 136 with permission from Elsevier Inc, copyright 2021. | ||
MOF composite materials also contribute to the multiple treatment of dyes. Basu's group fabricated a silica supported bimetallic MOF, viz., MIL-53(Al-Fe)@SiO2 to realize adsorption and photocatalytic degradation of methylene blue (MB).137 SiO2 assisted in increasing the photocatalytic activity and the composites outperformed the pure MIL 53 (Al). As Fe, Al and SiO2 coexisted in MIL-53, the electron and hole recombination was inhibited and the band gap was reduced. As a result, 99.85% of MB was photodegraded with satisfactory recyclability. Besides, a large maximum adsorption capacity of 681.78 mg g−1 was acquired, also with good reusability.
Another example of MOF-based hybrids for dye treatment was reported by Meng and co-workers. Gold nanorods/MOFs (AuNRs/Fe-MOF) composite was successfully developed for sensing and degradation of MB (Fig. 9).138 The catalytic activity of Au NRs/Fe-MOF was notably improved compared with pure MOFs. AuNRs in the composites could facilitate electrons transferred from Au to MOF, resulting reduction of Fe(III) to Fe(II) on MOFs and Fenton reaction by Fe3+/Fe2+ was activated. Thereby, an excellent rate constant (k = 0.182 min−1) and good degradation rate (5 μM MB, completely degraded within 20 min) was observed with the presence of H2O2. Interestingly, Au NRs/Fe-MOF can display chemical and electromagnetic enhancement of Raman signals towards MB. Fe-MOF can accept electrons from Au NRs under localized surface plasmon resonance (LSPR) excitation and the electron–hole recombination is prohibited. Therefore, Raman signal is enhanced through a charge-transfer mechanism with the help of Au NRs. The detection limit of MB is 9.3 × 10−12 M, showing ultra-low sensitivity, thus causing enhancement on both catalytic and Raman signals. Considering the excellent stability and reusability, Au NRs/Fe-MOF can serve as a promising material for dye detection and degradation in water.
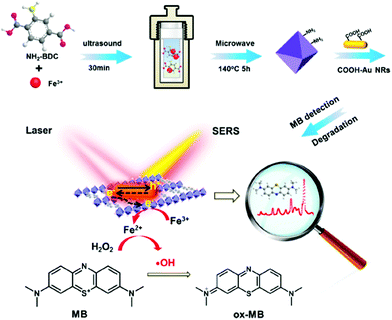 | ||
| Fig. 9 Schematic diagram of the preparation of Au NRs/Fe-MOFs for methylene blue in situ catalytic degradation and SERS detection. Reproduced from ref. 138 with permission from American Chemical Society, copyright 2022. | ||
Membrane materials can facilitate dye treatment as well. Balakrishna's group developed photocatalytic MOF-integrated polysulfone (PSF) membranes for separation and degradation of organic dyes in water (Fig. 10).139 NH2-MIL-101(Fe) was synthesized to enhance the optical properties and control the surface charge. This Fe-MOF was then embedded into PSF membrane, and the obtained MOF-integrated membrane was used for separation, removal and degradation of RhB and MO. The membrane could make NH2-MIL-101(Fe) devices and improved water flux and good rejection was achieved. Antifouling capacity was largely increased and above-par, reaching flux recovery ratio values above 95%. Compared with the bare membrane with 20–30% rejection and 0% degradation efficiency of dyes, the MOF-integrated membrane displayed around 80–100% rejection as well as 10 and 18% degradation efficiency to MO and RhB, respectively. The good water flux and antifouling capacity made such MOF membrane promising candidates for wastewater treatment.
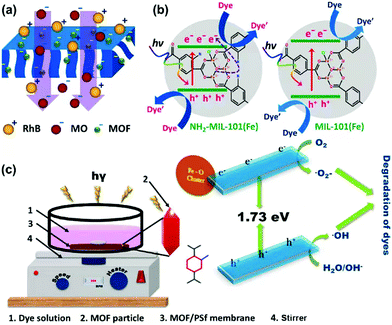 | ||
| Fig. 10 Schematic illustration of (a) rejection and (b) photocatalytic degradation of dye molecules over the NH2-MIL-101(Fe) and MIL-101(Fe) MOF-integrated membranes and (c) pictorial illustration of degradation setup and its mechanism. Reproduced from ref. 139 with permission from Elsevier Inc, copyright 2022. | ||
Apart from Fe-based MOFs, other MOFs can also serve as multifunction platform for anionic dye treatment. Zheng and co-workers developed SCNU-Z2 based on a heterotopic tripodal nitrogen-containing ligand and Co(II).140 The imidazole group was replaced by the tetrazole group and the framework charge is turned to anionic. The SCNU-Z2 showed high stability in wide pH range from 3 to 11, and the anionic character enables the efficient removal for cationic dyes, viz., Methylene Blue (MLB), Crystal Violet (CV) and Rhodamine B (RhB) with the large adsorption capacity of 455.6, 847.4 and 751.8 mg g−1, respectively. In contrast, SCNU-Z2 displayed extremely low adsorption to anionic dyes and can be used for cationic and anionic dyes separation. Interestingly, SCNU-Z2 demonstrated degradation behavior towards MB, and the degradation rate can reach 97% in the darkness within 15 min. However, A deep understanding of the degradation mechanism needs to be further explored.
Ce-based MOFs that suited for the treatment of both cationic and anionic dyes are explored by Cai's group. In this study, Ce-UiO-66 was explored as adsorbent to remove and degrade MB, MO, RhB and AO7.141 Ce-UiO-66 had an adsorption capacity of 243, 288, 583 and 413 mg g−1 to MB, MO, RhB and AO7, respectively, which is increased by 3–4 times compared with Zr-UiO-66. Experiment results and DFT calculations revealed that π–π interactions existed between the four dyes and Ce-UiO-66. Electrostatic interaction played vital role for the adsorption of cationic MB and MO in Ce-UiO-66. For anionic RhB and AO7, hydrogen bonding and Ce–O were the main mechanisms. Subsequently, the removal rate of MB, MO, RhB, and AO7 by Ce-UiO-66 was significantly improved, and the degradation rates were 81%, 89%, 99% and 98%, respectively, within 60 min under UV irradiation. Investigation of the degradation mechanism showed that the negative ligand-to-metal charge transfer (LMCT) accelerated the separation of electron–hole pairs and caused effective degradation of organic dyes. The reusability and water stability combined with removal and degradation ability endowed such MOFs potential practical applications in wastewater treatment.
The exploration of Indium-based MOF for removal and degradation of dyes was conducted by Zhai and co-workers. SNNU-110 was synthesized using an amino-functional linker and In clusters.142 Under irradiation, SNNU-110 exhibited good photocatalytic behavior toward RhB and MB, with the degradation efficiency of 94.6% for RhB and 93.1% for MB. The inorganic [In3O] cluster endowed SNNU-110 with good photocatalytic activity. The framework formation played a critical role in the degradation process, as in a control experiment, pure In2O3 displayed little photocatalytic activity to RhB and MB. Interestingly, SNNU-110 also showed fluorescence quenching performance for six nitroaromatics with good repeatability. SNNU-110 can be used as a multifunctional material for dyes elimination and nitroaromatics sensing.
Rare earth-based MOFs can also assist in dye elimination. Zhang and co-workers fabricated two rare earth MOFs from the reaction of Eu3+ and Tb3+ with an aromatic tetracarboxylic acid (TPTC).143 The two MOFs displayed pH stability in the range of 3–9 and the negative zeta potential enabled the Eu-TPTC and Tb-TPTC to selective adsorption and degradation of cationic and neutral dyes. Eu-TPTC showed removal efficiency of 96%, 92%, 93% and 85% for cationic MB, CV, RhB and neutral dye, neutral red (NR), respectively. The two MOFs also showed good photocatalytic degradation towards the dyes mentioned above, with all the degradation efficiency greater than 94%. The mechanism indicated that ˙OH and ˙O2− increased the sensitization of dyes and the separation of photogenerated carriers, and promoted the efficiency of photocatalytic degradation.
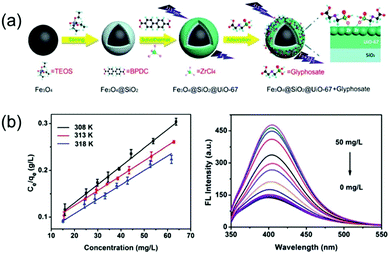 | ||
| Fig. 11 (a) A schematic illustration of the fabrication of the Fe3O4@-SiO2@UiO-67 MOF-based adsorbent. (b) The adsorption and the detection performance for glyphosate treatment. Reproduced from ref. 147 with permission from Royal Society of Chemistry, copyright 2018. | ||
Enzymes hold great potential in wastewater treatment, as they exhibit excellent selectivity, mild operating condition and high degradation efficiency.148 However, as enzymes are sensitive to external environment, inactivation easily occurs to enzymes, and careful measures should be taken to protect the enzymes.149 Due to their porosity, high surface area and functionality, MOFs can offer a good platform to encapsulate enzymes and improve their resistance to environment and the MOF–enzymes can hold promising applications in various fields.150,151 For this purpose, Deep and co-workers constructed Terbium-BTC MOF to encapsulate hexahistidine-tagged organophosphate hydrolase (OPH6His) enzyme.152 The MOF–enzyme was used to simultaneously detect and degrade an organophosphate pesticide, methyl parathion in water. The porous MOF played a part in protecting the enzyme and improving the adsorption ability towards the analyte. The OPH6His@Tb-BTC showed a 30% enhanced enzymatic activity compared with pure OPH6His and the good reusability for at least five cycles. An increased signal in UV-vis adsorption spectra was observed when methyl parathion was incubated with OPH6His@Tb-BTC, which is ascribe to the signal of the catalytic conversion products. A wide detection range of 0.1–1000 μM as well as a low detection limit of 2.6 nM was obtained. Moreover, a less toxic metabolite was formed via the catalytic conversion of methyl parathion, indicating a promising role of OPH6His@Tb-BTC in the detection and elimination of pesticide in aqueous environment.
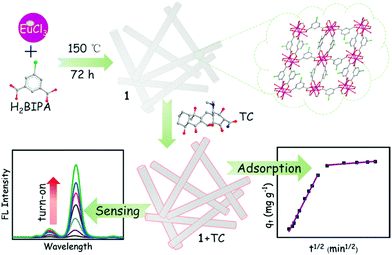 | ||
| Fig. 12 Schematic procedure for the preparation of Eu-MOF for simultaneously sensing and removal of tetracycline. Reproduced from ref. 158 with permission from Elsevier Inc, copyright 2021. | ||
Another work to eliminate TC was reported by Yang and co-workers.159 Depending on the catalytic behavior of Fe, three Fe-based MOFs, namely, Fe-MIL-101, Fe-MIL-100 and Fe-MIL-53 were synthesized to explored the degradation abilities of TC on Fe-based MOFs. The experiment results showed that Fe-MIL-101 had the best removal efficiency of 96.6% compared to that of 57.4% in Fe-MIL-100 and 40.6% in Fe-MIL-53, respectively. Moreover, through the investigation of degradation conditions, Fe-MIL-101 showed excellent photocatalytic degradation performance when TC concentration was 50 mg L−1. ˙O2−, ˙OH and h+ were the main active species in photocatalytic degradation process of TC. The materials can be reused three times without losing its removal and degradation abilities.
MOF composites can also assist in TC treatment. Dai's group fabricated a p-type semiconductor-MOFs composite (CBO@ZIF-8) to sense and degrade TC in aqueous solution (Fig. 13).160 Core-shell structure was obtained with a CuBi2O4 core and ZIF-8 shell. CBO@ZIF-8 showed an excellent fluorescence turn on effect towards TC at 517 nm emission, and a good linear range from 0 μM to 45 mM was obtained. The detection limit was as low as 26 nM and a fast response within 120 s, which can be applied for real-time monitoring of TC. The interfering experiment showed that antibiotics such as penicillin, cefradine, and levofloxacin caused little fluorescence change on CBO@ZIF-8, indicating good selectivity of the material. The good sensing performance can be ascribed to the formation of a Zn-TC coordination compound between TC and ZIF-8. The photodegradation behavior of CBO@ZIF-8 displayed that 75.2% TC was decomposed after 120 min. The mechanism revealed that photogenerated electrons generated on CBO@ZIF-8 can be separated effectively to produce reactive oxygen species (ROS), which can further oxidize TC. In general, the ZIF-8 shell could generate sensing performance towards TC and enrich TC using its porosity, thus enhancing the degradation efficiency of CBO. CBO@ZIF-8 showed good repeatability in both sensing and degradation process and demonstrated a great potential for wastewater multiple treatment.
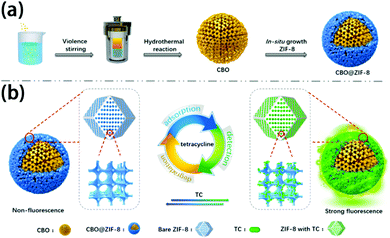 | ||
| Fig. 13 Schematic illustration of (a) synthesis procedure of the CBO@ZIF-8 and (b) corresponding strategies of TC adsorption, detection, and degradation over CBO@ZIF-8. Reproduced from ref. 160 with permission from American Chemical Society, copyright 2020. | ||
In addition to TC, other antibiotics can also be treated by multifunctional MOFs. Wang and co-workers synthesized water-stable luminescent UiO-type MOFs (Zr-fcu-sti) for simultaneous sensing and removal of two nitrofurans (NFs) antibiotics (nitrofurazone, NZF and nitrofurantoin, NFT).161 As shown in Fig. 14, the Zr-fcu-sti showed luminescence quenching toward the analyte and the detection limit was turned to be as low as 0.152 μM. The strong adsorption of nitrofurans at the excitation wavelength of Zr-fcu-sti, and the photo-induced electron transfer process from the organic ligand to nitrofurans are responsible for the sensing process. Interestingly, nitrofurans can lead to the bathochromic shift of the MOF's maximum emission, distinguishing them from other antibiotics, and this phenomenon can be used to detect nitrofurans qualitatively. As a result, Zr-fcu-sti showed a good selectivity while other antibiotics caused weaker luminescence quenching than nitrofurans. Moreover, the large surface area, proper pore size and the numerous hydrogen bonding sites in Zr-fcu-sti enabled such MOF to remove nitrofurans efficiently in water with the maximum adsorption capacity of 98 mg g−1 and 96 mg g−1 for nitrofurazone and nitrofurantoin, respectively. Zr-fcu-sti can be used as an effective material for monitoring and removal of nitrofurans in water.
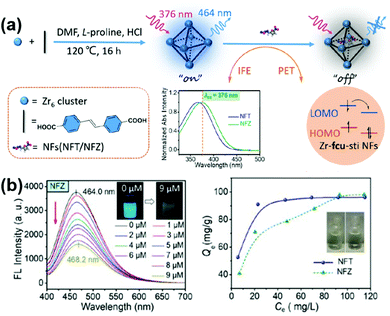 | ||
| Fig. 14 (a) Schematic illustration of detection of NFs Based on Zr-fcu-sti and (b) sensing and removal of NFs on Zr-fcu-sti. Reproduced from ref. 161 with permission from Elsevier Inc, copyright 2022. | ||
Ciprofloxacin is another typical antibiotic that is widely used in daily life. Basu's group constructed Fe–Cu binary MOF, MIL-53(Fe–Cu) through a single step solvothermal route to removal and degrade ciprofloxacin in water.162 MIL-53(Fe–Cu) displayed beneficial properties such as porosity, high surface area, thermal stability, functional sites in framework and photocatalytic activity. The MOF showed a maximum adsorption capacity of 190.4 mg g−1 for ciprofloxacin. Molecular calculation was conducted to demonstrate the molecular level adsorption and a contact angle of 34.5° and a minimun bond-length of 4.3 Å were observed. For degradation, the maximum degradation of 74.48 and 57.88%, and a kinetic rate of 0.010 and 0.012 min−1 were obtained in the presence of UV and visible light, respectively. The photoluminescence (PL) spectra showed that MIL-53(Fe–Cu) offers a reduced rate of electron-hole recombination, which is benefit to the degradation procedure. In addition, the reusability made MIL-53(Fe–Cu) potential material in practical application.
Yang and co-workers designed two flexible 3D Zn-based MOFs, namely, ROD-Zn1 and ROD-Zn2 to sense and remove several antibiotics such as TC, nitrofurans (NZF and NFT) and chloramphenicols (chloramphenicol, CAP and thiamphenicol, THI).163 The adsorption abilities on ROD-Zn1 and ROD-Zn2 follow a trend of TC > NZF > NFT > CAP > THI. The maximum adsorption capacities of TC on ROD-Zn1 and ROD-Zn2 were 142.9 and 125.0 mg g−1and the adsorption can be finished with 40 min. As all the studied antibiotics can be adsorbed in the cavities of ROD-Zn MOFs, π–π interactions can occur between the analytes and MOFs. The different adsorption capacities may be ascribed to different electronic behaviors of antibiotics and TC with –NH2 groups could generate hydrogen bonds with O–H on ligands of ROD-Zn MOFs, which may facilitate the adsorption process. ROD-Zn MOFs also displayed luminescence quenching towards the antibiotics, with the efficiencies followed the same order as in adsorption capacities. TC had quenching efficiencies of 96.4 and 95.1% on ROD-Zn1 and ROD-Zn2, respectively. ROD-Zn MOF had a good linear range of 0–45 μM towards TC with the detection limit of 0.11–0.12 μM. The experiment as well as DFT calculations revealed that Förster resonance energy transfer (FRET) and photoinduced electron transfer (PET) made these antibiotics show a diverse fluorescence quenching effect. Furthermore, the MOF-melamine foam (MF) hybrid was prepared via one-pot synthesis. The resulting microporous–macroporous MOF@MF demonstrated high mechanical stabilities, good recyclability and enhanced adsorption abilities, endowing such material with great potential in practical applications.
Besides antibiotics, other pharmaceuticals may also cause adverse effect to environment and human health, and are need to be treated. Yu and co-workers synthesized MIL-53(Fe) for remove and degrade two pharmaceuticals, viz., clofibric acid (CA) and carbamazepine (CBZ) from aqueous solution.164 MIL-53(Fe) displayed good adsorption performance with the adsorption capacities of CA and CBZ of 0.80 and 0.57 mmol g−1, respectively. The π–π interaction mainly contributed for the adsorption of CBZ, whereas electrostatic interactions are responsible for the CA adsorption. Furthermore, the photocatalytic activity was studied. MIL-53(Fe) showed significantly improved photocatalytic efficiency up to 90% for both CA and CBZ with the addition of small amount of H2O2, and outperformed those of traditional catalysts such as Fe(II)/H2O2 and TiO2 under visible light. Mechanism study revealed that upon irradiation, the charge carriers directly generated in MIL-53(Fe) and the synergistic effect of H2O2 accelerate the Fenton-like reaction. For practical application, MIL-53(Fe) was used for remove CA and CNZ in real municipal wastewater and river water, and exhibited good performance for wastewater treatment.
Expect that the metal/metal clusters can offer catalytic sites on MOFs, the organic ligand can also serve as a catalytic center for catalytic degradation. On this basis, Yu's group further explored a mixed-linker Zr-MOFs, PCN-134 to remove and degrade diclofenac (DF) in water.165 As Zr-MOFs allows the partial absence of linkers without destroying the framework integrity. The defect extent can be controlled by different TCPP ligand ratios in PCN-134 and best ratio balancing the porosity and detects was achieved. As a result, defect PCN-134 showed a maximum adsorption capacity of 2.04 mmol g−1 towards DF. PCN-134 displayed good photocatalytic activity to DF under visible light with degradation efficiency of above 99% in 5 hours. As porphyrin unit can generate 1O2, PCN-134 was dominated by type II photosensitization reaction, and the corresponding productivity was higher than that of single-linker PCN-224. Besides, the electron transformed between TCPP linker and Zr clusters also contributed to the photodegradation of DF. The enhanced behavior in PCN-224 mainly rely on the extra defect sites, open porous structure, synergistic effect between organic linkers and efficient energy transfer. Moreover, the good reusability and water stability made PCN-134 promising material for wastewater treatment.
Another example of porphyrin MOFs for phenolic compounds multiple treatment was presented by Gu's group (Fig. 15). In this work, a Zr-MOF, NU-1000 with mesopores was synthesized for removal and degradation of 4-chlorophenol (4-CP).170 carboxylic β-cyclodextrin (CMCD) was incorporated into NUS-1000 to assist adsorbing 4-CP. 4-CP were inclined to form a stable inclusion complex with CMCD, which facilitate the adsorption process. As a result, a large adsorption capacity of 296 mg g−1 was obtained. The degradation experiments showed that CMCD@NU-1000 can detoxified 4-CP within 60 min, together with a half-life time of only 5.98 min under sunlight irradiation. The MOF containing porphyrin units could generate 1O2 to oxidize the analyte under irradiation and played a vital role in the photodegradation of 4-CP through adsorption–degradation integrator. In addition, CMCD@NU-1000 can still keep 96% of its adsorption ability after five regeneration–adsorption cycles, indicating a good potential for practical use.
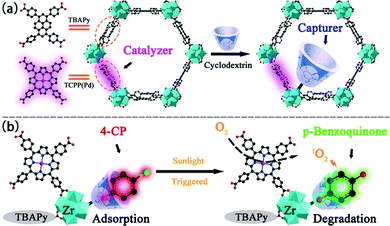 | ||
| Fig. 15 (a) The construction of CMCD@NU-1000-TCPP(Pd) and (b) schematic illustration of the adsorption and degradation processes toward 4-CP. Reproduced from ref. 170 with permission from American Chemical Society, copyright 2021. | ||
Enzymes have great potential in degrading pollutions as aforementioned. MOF–enzymes composites can also work on EDCs treatment. To this end, Wei and co-workers fabricated a laccase (LAC)-embedded MOF and its composite to sense and degrade phenolic pollutants.171 To be specific, ZIF-90 was firstly encapsulated with LAC, and then combined with bacterial cellulose (BC) and carboxylated multi-walled carbon nanotubes to prepare a cellulose membrane. The MOF composite showed an electrochemical detection ability towards catechol. A linear range of 20-400 μM and a detection limit of 1.86 μM was acquired, together with satisfactory reproducibility and selectivity. Besides, the MOF–enzyme composite displayed higher degradation efficiency to catechol than pure LAC, as the porous ZIF-90 could increase the adsorption ability to catechol and protect the LAC from reducing the enzyme activity. The degradation efficiency of 93.4% was obtained and the MOF–enzyme membrane composite was successfully used in enzyme membrane reactor, holding a practical application prospect in the phenolic wastewater treatment.
MOF and MOF composites have been reported to hold promising potential in organic water pollution treatment. The porosity, designability, tunability and functionality make MOFs ideal platform to realize more than one function of removal, sensing and degradation towards organic pollutions. Nevertheless, there is still space for improving sensing sensitivity, reusability and mechanism explanation. Table 1 lists the representative studies of MOFs for water pollution multiple treatment.
| Targets | MOF | Treatment model | Adsorption capacity | Limit of detection | DEa (%) | Ref. |
|---|---|---|---|---|---|---|
| a Degradation efficiency. b Removal. c Sensing. d Degradation. e Not mentioned. f No function. | ||||||
| Hg2+ | LMOF-263 | Rb & Sc | 380 mg g−1 | 3.3 μg L−1 | —f | 84 |
| Hg2+ | NH2-UiO66-SH | R & S | 265.29 mg g−1 | 0.035 μM | — | 85 |
| Hg2+ | NH2-MIL-53(Al) | R & S | 153.85 mg g−1 | 0.15 μM | — | 87 |
| Hg2+ | TMU-46∼48S | R & S | 714 mg g−1 | 0.1 mg L-1 | — | 88 |
| Hg2+ | Ni-MOF | R & S | 713 mg g−1 | Nme | — | 90 |
| Hg2+ | Al-MOF monitors | R & S | 1110 mg g−1 | 0.8 μg L−1 | — | 91 |
| Hg2+ | Zr-DMBD MOFs | R & S | 171.5 mg g−1 | 0.05 μM | — | 93 |
| Cd2+ | FJI-H9 | R & S | 225 mg g−1 | 10 mg L−1 | — | 96 |
| Cd2+ | Fe3O4/MOF/cysteine | R & S | 248.2 mg g−1 | 0.94 μg L−1 | — | 100 |
| Pb2+ & Cu2+ | NH2-MIL-101(Fe) | R & S | 0.9 and 1.1 mM g−1 | 1.6–5.2 μM | — | 101 |
| UO22+ | Tb-MOF | R & S | 179.08 mg g−1 | 0.9 μg L−1 | — | 106 |
| Th4+ | ThP-1 | R & S | 25.82 mg g−1 | 24.2 μg L−1 | — | 107 |
| IO3− | Th-MOF | R & S | 152.6 mg g−1 | 0.107 μg kg−1 | — | 109 |
| UO22+ | Fe3O4@ZIF-8@CDs | R & S | 606.06 mg g−1 | Nm | — | 110 |
| Cr2O72− | BUT-39 | R & S | 215 mg g−1 | 1.5 μM | — | 113 |
| Cr2O72− | Dyes⊂MOF-801 | R & S | 83 mg g−1 | 30 μM | — | 115 |
| Cr2O72− | MOF-MMMs | R & S | 33.34 mg g−1 | 5.73 nM | — | 117 |
| Cr2O72− | UiO66-(OH)2 | R & Dd | 53.8 mg g−1 | — | Nm | 120 |
| Cr2O72− | Zr-MSA | R & D | 202.0 mg g−1 | — | Nm | 121 |
| Cr2O72− | JLU-MOF60 | R & S& D | 149 mg g−1 | 9.38 μM | 98 | 122 |
| F− | NH2-MIL-53(Al) | R & S | 203 mg g−1 | 0.31 μM | — | 125 |
| F− | In-TCPP | R & S | 36.7 mg g−1 | 1.3 μg L−1 | — | 126 |
| AO7 | Fe-based MOFs | R & D | 31.4–153.4 mg g−1 | — | 3.8–33.2 | 134 |
| MB & MO | Fe loaded MOF-545(Fe) | R & D | 382.4 and 803.7 mg g−1 | — | >99 | 135 |
| MB & MO | Ni-doping MIL-101(Fe) | R & D | About 40 and 80 mg g−1 | — | >90 | 136 |
| MB | MIL-53(Al-Fe)@SiO2 | R & D | 681.78 mg g−1 | — | 99.85 | 137 |
| MB | Gold nanorods/MOFs | S & D | — | 9.3 pM | 60 | 138 |
| RhB & MO | NH2-MIL-101(Fe) membrane | R & D | Membrane 80–100% rejection | — | 10–18 | 139 |
| CV, MB, RhB | SCNU-Z2 | R & D | 455–847 mg g−1 | — | 97 | 140 |
| MB, MO, RhB and AO7 | Ce-UiO-66 | R & D | 249–427 mg g−1 | — | 81–99 | 141 |
| RhB & MB | SNNU-110 | R & D | 51.6 mg g−1 | — | 93.1–94.6 | 142 |
| CR & RhB | Eu/Tb-MOF | R & D | Nm | — | >99 | 143 |
| glyphosate | Fe3O4@SiO2@UiO-67 | R & S | 256.54 mg g−1 | 93 μg L−1 | — | 147 |
| Methyl parathion | OPH6His@Tb-BTC | S & D | — | 2.6 nM | 82 | 152 |
| TC | Eu-MOF | R & S | 387 mg g−1 | 3 nM | — | 158 |
| TC | Fe-MIL-MOF | R & D | Nm | — | 96.6 | 159 |
| TC | CBO@ZIF-8 | S & D | — | 26 nM | 75.2 | 160 |
| NZF & NFT | UiO-type MOF | R & S | 96 and 98 mg g−1 | 0.152 μM | — | 161 |
| CP | MIL-53(Fe–Cu) | R & D | 190.4 mg g−1 | — | 74.5 | 162 |
| TC, CP, NFs and THI | ROD-Zn | R & S | 142.9 mg g−1 | 0.11–1.39 μM | — | 163 |
| CA & CBZ | MIL-53(Fe) | R & D | 0.57 and 0.8 mmol g−1 | — | >90 | 164 |
| DF | PCN-134 | R & D | 2.04 mmol g−1 | — | 99 | 165 |
| BPA | PCN-222 | R & D | 487 mg g−1 | — | 99 | 169 |
| 4-CP | β-CD-NU-1000 | R & D | 296 mg g−1 | — | >90 | 170 |
| Catechol | LAC-embedded MOF | S & D | — | 1.86 μM | 93.4 | 171 |
4. Conclusions and outlook
MOFs, as a shining star in porous coordination materials, have been widely exploited in various fields and drawn fascinating attention in the past decades. For water pollution treatment, realizing multiple function treatment towards one analyte simultaneously is of great convenience and might be the future trend. This feature article firstly discussed the importance of sensing, removal and degradation treatments towards water pollutions. Then, the attention was focused on MOFs, which displayed porosity, designability, stability and functionality, as an ideal platform to achieve multiple treatment to water pollutions. Afterwards, examples of MOFs/MOF composites for the multifunction treatment to inorganic/organic water pollution were summarized. Despite that MOFs possessed tremendous potential in wastewater treatment, some deficiencies may still restrain their further application. Hence, the challenges as well as opportunities should be thought about in the future as follows:(1) The water stability of MOFs is still a problem. Although some typical MOFs showed stability in water, more MOFs tend to decompose in aqueous system and acid/base solution, and the instability in water largely limits their practical applications. In fact, the MOFs for wastewater treatment as summarized above mainly centered on the UiO, MIL and ZIF serious typical MOFs that are stable in water. Considering the diversification of water pollutions, the current water stable MOFs are insufficient for the sensitive and selective treatment of various water pollutions, especially organic pollutions. Accordingly, enhancement in MOFs water stability is of vital importance in the future research. Exploiting water stable MOFs or fabricating MOF composites to improve their water resistance may be the possible ways.172–176 Generally, improving the strength of the metal-ligand bond may help enhance the water resistance, for which HSAB offers a helpful guidance. To be specific, azolate-based ligands and low-valency transition metal ions, or carboxylate-based ligands and high-valency metal ions tend to form relative strong metal–ligand bond.177 Besides, tuning the pore environments of MOFs, e.g., constructing hydrophobic MOFs via delicate design to exclude the infusion of water is another conducive way to improve the water stability of MOFs.
(2) The deep understanding of structure–property relationship needs to improve. At present, how to choose MOFs for the treatment of a specific target lacks general guidance. Different water pollutions have varied size, hydrophilicity, polarity, electrical property and functional group, and there are a hundred thousand of MOFs with all kinds of topologies, pore size, center metal/metal clusters, connectivity and functional groups been explored. The current research of the analytes response on MOFs mostly relies on experiment screening, which may lead to huge amount of work and low efficiency. High-throughput computational screening may provide an optional plan.178 As several databases have been established containing numerous MOFs structures, the required pores, binding sites or catalytic sites can be pre-programmed according to the property of the target molecules, and MOFs with potential interactions towards analytes can be screened out.
(3) The reusability needs more attention. Though many MOFs displayed regeneration and reusability, their removal and degradation performances usually decreased when the reuse cycles are above 3. During the recycle process, MOFs have the risk of leaking out into the water, and some MOFs containing toxic metals may cause second pollution in the environment. Thereby, to monitor the MOFs during the recycle process and to pay more attention to MOF reusability during design is necessary. Besides, the current research works usually investigate the reusability of MOFs with less than 10 cycles, and long-term efficiency of MOFs for water treatment need to be further explored, ensuring MOFs as a long-tested material for practical application.
(4) The mechanisms of MOFs for water pollution multiple treatment should be investigated more deeply. Many works discussed the removal, sensing and degradation mechanisms by experiment measurement or computational calculations, and explained the behavior to some extent. However, MOFs have the unique advantages compared with covalent organic frameworks or other porous materials as MOFs can obtain single crystal form, which is effective for revealing the mechanisms. Therefore, if the crystal form of MOF-analyte combinations is acquired, there will be convincing evidence to illustrate the mechanisms.
Although there are still challenges to exploit the MOFs as multifunctional materials in wastewater treatment, it is undeniable that the exploration of MOFs brings more promising thoughts to the researchers. They will keep devoting themselves to designing and constructing suitable MOFs and multifunctional MOFs for wastewater treatment will have a bright future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support of the National, Natural Science Foundation of China (Grant 22035003), Nature Science Fund of Tianjin, China (Grant 19JCZDJC37200), Fundamental Research Funds for the Central Universities (63223020) and the Haihe Laboratory of Sustainable Chemical Transformations (YYJC202101).Notes and references
- F. G. Acién, C. Gómez-Serrano, M. M. Morales-Amaral, J. M. Fernández-Sevilla and E. Molina-Grima, Appl. Microbiol. Biotechnol., 2016, 100, 9013–9022 CrossRef PubMed.
- S. Bolisetty, M. Peydayesh and R. Mezzenga, Chem. Soc. Rev., 2019, 48, 463–487 RSC.
- J. Li, X. X. Wang, G. X. Zhao, C. L. Chen, Z. F. Chai, A. Alsaedi, T. Hayat and X. K. Wang, Chem. Soc. Rev., 2018, 47, 2322–2356 RSC.
- D. Banerjee, D. Kim, M. J. Schweiger, A. A. Kruger and P. K. Thallapally, Chem. Soc. Rev., 2016, 45, 2724–2739 RSC.
- M. Nasiri, H. Ahmadzadeh and A. Amiri, TrAC, Trends Anal. Chem., 2020, 123, 115772 CrossRef CAS.
- K. H. Chu, Y. A. J. Al-Hamadani, C. M. Park, G. Lee, M. Jang, A. Jang, N. Her, A. Son and Y. Yoon, Chem. Eng. J., 2017, 327, 629–647 CrossRef CAS.
- M. Lorenzo, J. Campo and Y. Picó, TrAC, Trends Anal. Chem., 2018, 103, 137–155 CrossRef CAS.
- S. Rojas and P. Horcajada, Chem. Rev., 2020, 120, 8378–8415 CrossRef CAS PubMed.
- Y. H. Pi, X. Y. Li, Q. B. Xia, J. L. Wu, Y. W. Li, J. Xiao and Z. Li, Chem. Eng. J., 2018, 337, 351–371 CrossRef CAS.
- R. Das, C. D. Vecits, A. Schulze, B. Cao, A. F. Ismail, X. Lu, J. Chen and S. Ramakrishna, Chem. Soc. Rev., 2017, 46, 6946–7020 RSC.
- M. Patel, R. Kumar, K. Kishor, T. Mlsna, C. U. Pittman and D. Mohan, Chem. Rev., 2019, 119, 3510–3673 CrossRef CAS.
- S. Mao, J. Chang, G. Zhou and J. Chen, Small, 2015, 11, 5336–5359 CrossRef CAS.
- D. Dai, J. Yang, Y. Wang and Y. W. Yang, Adv. Funct. Mater., 2021, 31, 2006168 CrossRef CAS.
- B. Bansod, T. Kumar, R. Thakur, S. Rana and I. Singh, Biosens. Bioelectron., 2017, 94, 443–455 CrossRef CAS.
- S. Y. Chen, Z. Li, K. Li and X. Q. Yu, Coord. Chem. Rev., 2021, 429, 213691 CrossRef CAS.
- B. O. Okesola and D. K. Smith, Chem. Soc. Rev., 2016, 45, 4226–4251 RSC.
- M. Gao, G. Liu, Y. Gao, G. Chen, X. Huang, X. Xu, J. Wang, X. Yang and D. Xu, TrAC, Trends Anal. Chem., 2021, 137, 116226 CrossRef CAS.
- C. Jung, J. Oh and Y. Yoon, Environ. Sci. Pollut. Res., 2015, 22, 10058–10069 CrossRef CAS.
- F. Dixit, R. Dutta, B. Barbeau, P. Berube and M. Mohseni, Chemosphere, 2021, 272, 129777 CrossRef CAS PubMed.
- J. Völker, M. Stapf, U. Miehe and M. Wagner, Environ. Sci. Technol., 2019, 53, 7215–7233 CrossRef.
- N. Jiang, R. Shang, S. G. J. Heijman and L. C. Rietveld, Water Res., 2018, 144, 145–161 CrossRef CAS PubMed.
- M. K. Uddin, Chem. Eng. J., 2017, 308, 438–462 CrossRef CAS.
- P. L. Yap, M. J. Nine, K. Hassan, T. T. Tung, D. N. H. Tran and D. Losic, Adv. Funct. Mater., 2021, 31, 2007356 CrossRef CAS.
- T. H. Boyer, Y. Fang, A. Ellis, R. Dietz, Y. J. Choi, C. E. Schaefer, C. P. Higgins and T. J. Strathmann, Water Res., 2021, 200, 117244 CrossRef CAS PubMed.
- F. Yu, X. Bai, M. Liang and J. Ma, Chem. Eng. J., 2021, 405, 126960 CrossRef CAS.
- Y. Zhu, W. Fan, W. Feng, Y. Wang, S. Liu, Z. Dong and X. Li, J. Hazard. Mater., 2021, 414, 125517 CrossRef CAS PubMed.
- J. Wang and Z. Bai, Chem. Eng. J., 2017, 312, 79–98 CrossRef CAS.
- S. W. Nam, Y. Yoon, D. J. Choi and K. D. Zoh, J. Hazard. Mater., 2015, 285, 453–463 CrossRef CAS.
- V. Choudhary, K. Vellingiri, M. I. Thayyil and L. Philip, Environ. Sci.: Nano, 2021, 8, 1133–1176 RSC.
- S. Wu, Y. Lin and Y. H. Hu, J. Mater. Chem. A, 2021, 9, 2592–2611 RSC.
- M. Pedrosa, J. L. Figueiredo and A. M. T. Silva, J. Environ. Chem. Eng., 2021, 9, 104930 CrossRef CAS.
- H. Sun, C. Kwan, A. Suvorova, H. M. Ang, M. O. Tadé and S. Wang, Appl. Catal., B, 2014, 154–155, 134–141 CrossRef CAS.
- X. Liu, R. Ma, L. Zhuang, B. Hu, J. Chen, X. Liu and X. K. Wang, Crit. Rev. Environ. Sci. Technol., 2021, 51, 751–790 CrossRef CAS.
- V. K. Sharma and M. Feng, J. Hazard. Mater., 2019, 372, 3–16 CrossRef CAS PubMed.
- Y. Zhou, L. Tang, G. Zeng, J. Chen, Y. Cai, Y. Zhang, G. Yang, Y. Liu, C. Zhang and W. Tang, Biosens. Bioelectron., 2014, 61, 519–525 CrossRef CAS PubMed.
- H. Dong, G. Zeng, L. Tang, C. Fan, C. Zhang, X. He and Y. He, Water Res., 2015, 79, 128–146 CrossRef CAS PubMed.
- Q. Hua, J. Sun, H. Liu, R. Bao, R. Yu, J. Zhai, C. Pan and Z. L. Wang, Nat. Commun., 2018, 9, 244 CrossRef.
- J. N. Tiwari, S. Sultan, C. W. Myung, T. Yoon, N. Li, M. Ha, A. M. Harzandi, H. J. Park, D. Y. Kim, S. S. Chandrasekaran, W. G. Lee, V. Vij, H. Kang, T. J. Shin, H. S. Shin, G. Lee, Z. Lee and K. S. Kim, Nat. Energy, 2018, 3, 773–782 CrossRef CAS.
- P. Cheng and K. Pu, Nat. Rev. Mater., 2021, 6, 1095–1113 CrossRef CAS.
- H. Li, M. Eddaoudi, M. O’Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS.
- X. Zhao, Y. Wang, D. S. Li, X. Bu and P. Feng, Adv. Mater., 2018, 30, 1705189 CrossRef PubMed.
- H. Y. Li, S. N. Zhao, S. Q. Zhang and J. Li, Chem. Soc. Rev., 2020, 49, 6364–6401 RSC.
- X. Liu, T. Yue, K. Qi, Y. Qiu, B. Y. Xia and X. Guo, Chin. Chem. Lett., 2020, 31, 2189–2201 CrossRef CAS.
- Y. Du, X. Gao, S. Li, L. Wang and B. Wang, Chin. Chem. Lett., 2020, 31, 609–616 CrossRef CAS.
- G. R. Xu, Z. H. An, K. Xu, Q. Liu, R. Das and H. L. Zhao, Coord. Chem. Rev., 2021, 427, 213554 CrossRef CAS.
- V. Kumar, V. Singh, K. H. Kim, E. E. Kwon and S. A. Younis, Coord. Chem. Rev., 2021, 447, 214148 CrossRef CAS.
- R. J. Drout, L. Robison, Z. Chen, T. Islamoglu and O. K. Farha, Trends Chem., 2019, 1, 304–317 CrossRef CAS.
- Y. Xiao, Y. Cui, Q. Zheng, S. Xiang, G. Qian and B. Chen, Chem. Commun., 2010, 46, 5503–5505 RSC.
- M. Alvaro, E. Carbonell, B. Ferrer, F. X. Llabrés i Xamena and H. Garcia, Chem. – Eur. J., 2007, 13, 5106–5112 CrossRef CAS.
- E. Haque, J. E. Lee, I. T. Jang, Y. K. Hwang, J. S. Chang, J. Jegal and S. H. Jhung, J. Hazard. Mater., 2010, 181, 535–542 CrossRef CAS.
- Z. Chen, K. O. Kirlikovali, P. Li and O. K. Farha, Acc. Chem. Res., 2022, 55, 579–591 CrossRef CAS PubMed.
- C. C. Wang, J. R. Li, X. L. Lv, Y. Q. Zhang and G. Guo, Energy Environ. Sci., 2014, 7, 2831–2867 RSC.
- A. Karmakar, P. Samanta, A. V. Desai and S. K. Ghosh, Acc. Chem. Res., 2017, 50, 2457–2469 CrossRef CAS PubMed.
- Z. Hasan and S. H. Jhung, J. Hazard. Mater., 2015, 283, 329–339 CrossRef CAS PubMed.
- C. Wang, X. Liu, N. K. Demir, J. P. Chen and K. Li, Chem. Soc. Rev., 2016, 45, 5107–5134 RSC.
- J. Liu, C. Chen, K. Zhang and L. Zhang, Chin. Chem. Lett., 2021, 32, 649–659 CrossRef CAS.
- P. Samanta, A. V. Desai, S. Sharma, P. Chandra and S. K. Ghosh, Inorg. Chem., 2018, 57, 2360–2364 CrossRef CAS PubMed.
- W. P. Lustig, S. Mukherjee, N. D. Rudd, A. V. Desai, J. Li and S. K. Ghosh, Chem. Soc. Rev., 2017, 46, 3242–3285 RSC.
- J. Jin, J. Xue, Y. Liu, G. Yang and Y. Y. Wang, Dalton Trans., 2021, 50, 1950–1972 RSC.
- S. Li, Y. Li and B. Yan, CrystEngComm, 2021, 23, 5345–5352 RSC.
- X. Fang, B. Zong and S. Mao, Nano-Micro Lett., 2018, 10, 64 CrossRef.
- W. Lu, Z. Wei, Z. Y. Gu, T. F. Liu, J. Park, J. Park, J. Tian, M. Zhang, Q. Zhang, T. Gentle, M. Bosch and H. C. Zhou, Chem. Soc. Rev., 2014, 43, 5561–5593 RSC.
- C. Negro, H. Martínez, P. Cejuela, E. F. Simó-Alfonso, J. M. Herrero-Martínez, R. Bruno, D. Armentano, J. Ferrando-Soria and E. Pardo, ACS Appl. Mater. Interfaces, 2021, 13, 28424–28432 CrossRef CAS.
- Y. Chen, B. Wang, X. Wang, L. H. Xie, J. Li, Y. Xie and J. R. Li, ACS Appl. Mater. Interfaces, 2017, 9, 27027–27035 CrossRef CAS.
- Y. Li, G. Hou, J. Yang, J. Xie, X. Yuan, H. Yang and M. Wang, RSC Adv., 2016, 6, 16395–16403 RSC.
- Q. Li, D. X. Xue, Y. F. Zhang, Z. H. Zhang, Z. Gao and J. Bai, J. Mater. Chem. A, 2017, 5, 14182–14189 RSC.
- C. Zhou, C. Lai, D. Huang, G. Zeng, C. Zhang, M. Cheng, L. Hu, J. Wan, W. Xiong, M. Wen, X. Wen and L. Qin, Appl. Catal., B, 2018, 220, 202–210 CrossRef CAS.
- E. Flage–Larsen, A. Røyset, J. H. Cavka and K. Thorshaug, J. Phys. Chem. C, 2013, 117, 20610–20616 CrossRef.
- R. Liang, F. Jing, L. Shen, N. Qin and L. Wu, Nano Res., 2015, 8, 3237–3249 CrossRef CAS.
- R. Liang, S. Luo, F. Jing, L. Shen, N. Qin and L. Wu, Appl. Catal., B, 2015, 176-177, 240–248 CrossRef CAS.
- T. Zeng, D. Shi, Q. Cheng, G. Liao, H. Zhou and Z. Pan, Environ. Sci.: Nano, 2020, 7, 861–879 RSC.
- X. Wang, J. Liu, S. Leong, X. Lin, J. Wei, B. Kong, Y. Xu, Z. X. Low, J. Yao and H. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 9080–9087 CrossRef CAS PubMed.
- M. A. Ahsan, V. Jabbari, M. A. Imam, E. Castro, H. Kim, M. L. Curry, D. J. Valles-Rosales and J. C. Noveron, Sci. Total Environ., 2020, 698, 134214 CrossRef CAS PubMed.
- W. Huang, N. Liu, X. Zhang, M. Wu and L. Tang, Appl. Surf. Sci., 2017, 425, 107–116 CrossRef CAS.
- X. S. Miao, F. Bishay, M. Chen and C. D. Metcalfe, Environ. Sci. Technol., 2004, 38, 3533–3541 CrossRef CAS PubMed.
- Y. Luo, W. Guo, H. H. Ngo, L. D. Nghiem, F. I. Hai, J. Zhang, S. Liang and X. C. Wang, Sci. Total Environ., 2014, 473-474, 619–641 CrossRef CAS PubMed.
- F. Murphy, C. Ewins, F. Carbonnier and B. Quinn, Environ. Sci. Technol., 2016, 50, 5800–5808 CrossRef CAS PubMed.
- J. A. Camargo and Á. Alonso, Environ. Int., 2006, 32, 831–849 CrossRef CAS.
- S. Dutta, S. Let, S. Sharma, D. Mahato and S. K. Ghosh, Chem. Rec., 2021, 21, 1666–1680 CrossRef CAS PubMed.
- N. Sharma, A. K. Dey, R. Y. Sathe, A. Kumar, V. Krishnan, T. J. D. Kumar and C. M. Nagaraja, Catal. Sci. Technol., 2020, 10, 7724–7733 RSC.
- M. E. Mahmoud, S. M. Elsayed, S. E. M. E. Mahmoud, R. O. Aljedaani and M. A. Salam, J. Mol. Liq., 2022, 347, 118274 CrossRef CAS.
- F. Zahir, S. J. Rizwi, S. K. Haq and R. H. Khan, Environ. Toxicol. Pharmacol., 2005, 20, 351–360 CrossRef CAS PubMed.
- L. D. Hylander and M. E. Goodsite, Sci. Total Environ., 2006, 368, 352–370 CrossRef CAS PubMed.
- N. D. Rudd, H. Wang, E. M. A. Fuentes-Fernandez, S. J. Teat, F. Chen, G. Hall, Y. J. Chabal and J. Li, ACS Appl. Mater. Interfaces, 2016, 8, 30294–30303 CrossRef CAS.
- T. Li, Mark T. Kozlowski, E. A. Doud, M. N. Blakely and N. L. Rosi, J. Am. Chem. Soc., 2013, 135, 11688–11691 CrossRef CAS PubMed.
- L. Zhang, J. Wang, H. Wang, W. Zhang, W. Zhu, T. Du, Y. Ni, X. Xie, J. Sun and J. Wang, Nano Res., 2021, 14, 1523–1532 CrossRef CAS.
- L. Zhang, J. Wang, T. Du, W. Zhang, W. Zhu, C. Yang, T. Yue, J. Sun, T. Li and J. Wang, Inorg. Chem., 2019, 58, 12573–12581 CrossRef CAS.
- L. Esrafili, M. Gharib and A. Morsali, New J. Chem., 2019, 43, 18079–18091 RSC.
- G. Chen, Z. Guo, G. Zeng and L. Tang, Analyst, 2015, 140, 5400–5443 RSC.
- S. Halder, J. Mondal, J. Ortega-Castro, A. Frontera and P. Roy, Dalton Trans., 2017, 46, 1943–1950 RSC.
- A. Radwan, I. M. El-Sewify, A. Shahat, H. M. E. Azzazy, M. M. H. Khalil and M. F. El-Shahat, ACS Sustainable Chem. Eng., 2020, 8, 15097–15107 CrossRef CAS.
- Y. Zhang, G. M. Zeng, L. Tang, J. Chen, Y. Zhu, X. X. He and Y. He, Anal. Chem., 2015, 87, 989–996 CrossRef CAS PubMed.
- H. Yang, C. Peng, J. Han, Y. Song and L. Wang, Sens. Actuators, B, 2020, 320, 128447 CrossRef CAS.
- P. A. Kobielska, A. J. Howarth, O. K. Farha and S. Nayak, Coord. Chem. Rev., 2018, 358, 92–107 CrossRef CAS.
- N. Lubick and D. Malakoff, Science, 2013, 341, 1443 CrossRef CAS PubMed.
- H. Xue, Q. Chen, F. Jiang, D. Yuan, G. Lv, L. Liang, L. Liu and M. Hong, Chem. Sci., 2016, 7, 5983–5988 RSC.
- Y. Wang, G. Ye, H. Chen, X. Hu, Z. Niu and S. Ma, J. Mater. Chem. A, 2015, 3, 15292–15298 RSC.
- Q. R. Fang, D. Q. Yuan, J. Sculley, J. R. Li, Z. B. Han and H. C. Zhou, Inorg. Chem., 2010, 49, 11637 CrossRef CAS PubMed.
- B. E. Meteku, J. Huang, J. Zeng, F. Subhan, F. Feng, Y. Zhang, Z. Qiu, S. Aslam, G. Li and Z. Yan, Coord. Chem. Rev., 2020, 413, 213261 CrossRef CAS.
- L. Fan, M. Deng, C. Lin, C. Xu, Y. Liu, Z. Shi, Y. Wang, Z. Xu, L. Li and M. He, RSC Adv., 2018, 8, 10561–10572 RSC.
- S. W. Lv, J. M. Liu, C. Y. Li, N. Zhao, Z. H. Wang and S. Wang, Chem. Eng. J., 2019, 375, 122111 CrossRef CAS.
- X. Zhang and Y. Liu, Environ. Sci.: Nano, 2020, 7, 1008–1040 RSC.
- K. Jin, B. Lee and J. Park, Coord. Chem. Rev., 2020, 427, 213473 CrossRef.
- S. Rapti, S. A. Diamantis, A. Dafnomili, A. Pournara, E. Skliri, G. S. Armatas, A. C. Tsipis, I. Spanopoulos, C. D. Malliakas, M. G. Kanatzidis, J. C. Plakatouras, F. Noli, T. Lazarides and M. J. Manos, J. Mater. Chem. A, 2018, 6, 20813–20821 RSC.
- S. Khan and S. K. Mandal, ACS Appl. Mater. Interfaces, 2021, 13, 45465–45474 CrossRef CAS PubMed.
- W. Liu, X. Dai, Z. Bai, Y. Wang, Z. Yang, L. Zhang, L. Xu, L. Chen, Y. Li, D. Gui, J. Diwu, J. Wang, R. Zhou, Z. Chai and S. Wang, Environ. Sci. Technol., 2017, 51, 3911–3921 CrossRef CAS PubMed.
- W. Liu, X. Dai, Y. Wang, L. Song, L. Zhang, D. Zhang, J. Xie, L. Chen, J. Diwu, J. Wang, Z. Chai and S. Wang, Environ. Sci. Technol., 2019, 53, 332–341 CrossRef CAS PubMed.
- M. I. Gonzalez, A. B. Turkiewicz, L. E. Darago, J. Oktawiec, K. Bustillo, F. Grandjean, G. J. Long and J. R. Long, Nature, 2019, 577, 64 CrossRef PubMed.
- Z. J. Li, M. Lei, H. Bao, Y. Ju, H. Lu, Y. Li, Z. H. Zhang, X. Guo, Y. Qian, M. Y. He, J. Q. Wang, W. Liu and J. Lin, Chem. Sci., 2021, 12, 15833–15842 RSC.
- X. Guo, Q. Liu, J. Liu, H. Zhang, J. Yu, R. Chen, D. Song, R. Li and J. Wang, Appl. Surf. Sci., 2019, 491, 640–649 CrossRef CAS.
- S. Prasad, K. K. Yadav, S. Kumar, N. Gupta, M. M. S. Cabral-pinto, S. Rezania, N. Radwan and J. Alam, J. Environ. Manage., 2021, 285, 112174 CrossRef CAS PubMed.
- C. E. Barrera-Díaz, V. Lugo-Lugo and B. Bilyeu, J. Hazard. Mater., 2012, 223–224, 1–12 CrossRef PubMed.
- T. He, Y. Z. Zhang, X. J. Kong, J. Yu, X. L. Lv, Y. Wu, Z. J. Guo and J. R. Li, ACS Appl. Mater. Interfaces, 2018, 10, 16650–16659 CrossRef CAS PubMed.
- S. H. Park, N. Kwon, J. H. Lee, J. Yoon and I. Shin, Chem. Soc. Rev., 2020, 49, 143–179 RSC.
- J. Yoo, U. Ryu, W. Kwon and K. M. Choi, Sens. Actuators, B, 2019, 283, 426–433 CrossRef CAS.
- S. Yu, H. Pang, S. Huang, H. Tang, S. Wang, M. Qiu, Z. Chen, H. Yang, G. Song, D. Fu, B. Hu and X. Wang, Sci. Total Environ., 2021, 800, 149662 CrossRef CAS PubMed.
- S. Zhang, H. Zheng, Y. Yang, G. Qian and Y. Cui, Front. Chem., 2022, 10, 852402 CrossRef CAS PubMed.
- H. P. Singh, P. M. S. Kaur, D. R. Batish and R. K. Kohli, Environ. Chem. Lett., 2013, 11, 229–254 CrossRef CAS.
- C. C. Wang, X. D. Du, J. Li, X. X. Guo, P. Wang and J. Zhang, Appl. Catal., B, 2016, 193, 198–216 CrossRef CAS.
- Z. Wang, J. Yang, Y. Li, Q. Zhuang and J. Gu, Chem. – Eur. J., 2017, 23, 15415–15423 CrossRef CAS PubMed.
- P. Yang, Y. Shu, Q. Zhuang, Y. Li and J. Gu, Langmuir, 2019, 35, 16226–16233 CrossRef CAS PubMed.
- J. Liu, Y. Ye, X. Sun, B. Liu, G. Li, Z. Liang and Y. Liu, J. Mater. Chem. A, 2019, 7, 16833–16841 RSC.
- X. Yang, Q. Zheng, M. He, B. Chen and B. Hu, Water Res., 2021, 202, 117401 CrossRef CAS PubMed.
- Y. Zhou, J. F. Zhang and J. Yoon, Chem. Rev., 2014, 114, 5511–5571 CrossRef CAS PubMed.
- D. H. Xie, X. Ge, W. X. Qin and Y. X. Zhang, Chin. J. Chem. Phys., 2021, 34, 227 CrossRef CAS.
- H. Q. Yin, K. Tan, S. Jensen, S. J. Teat, S. Ullah, X. Hei, E. Velasco, K. Oyekan, N. Meyer, X. Y. Wang, T. Thonhauser, X. B. Yin and J. Li, Chem. Sci., 2021, 12, 14189–14197 RSC.
- L. Joseph, B. M. Jun, M. Jang, C. M. Park, J. C. Muñoz-Senmache, A. J. Hernández-Maldonado, A. Heyden, M. Yu and Y. Yoon, Chem. Eng. J., 2019, 369, 928–946 CrossRef CAS.
- D. J. Lapworth, N. Baran, M. E. Stuart and R. S. Ward, Environ. Pollut., 2012, 163, 287–303 CrossRef CAS PubMed.
- S. Dhaka, R. Kumar, A. Deep, M. B. Kurade, S. W. Ji and B. H. Jeon, Coord. Chem. Rev., 2019, 380, 330–352 CrossRef CAS.
- J. B. DeCoste and G. W. Peterson, Chem. Rev., 2014, 114, 5695–5727 CrossRef CAS PubMed.
- B. O. Okesola and D. K. Smith, Chem. Soc. Rev., 2016, 45, 4226–4251 RSC.
- Y. Liu, Y. Zhao and J. Wang, J. Hazard. Mater., 2021, 404, 124191 CrossRef CAS PubMed.
- M. Cheng, C. Lai, Y. Liu, G. Zeng, D. Huang, C. Zhang, L. Qin, L. Hu, C. Zhou and W. Xiong, Coord. Chem. Rev., 2018, 368, 80–92 CrossRef CAS.
- X. Li, W. Guo, Z. Liu, R. Wang and H. Liu, Appl. Surf. Sci., 2016, 369, 130–136 CrossRef CAS.
- C. Zhang, H. Li, C. Li and Z. Li, Molecules, 2020, 25, 168 CrossRef CAS PubMed.
- Q. Wu, M. S. Siddique and W. Yu, J. Hazard. Mater., 2021, 401, 123261 CrossRef CAS PubMed.
- A. Chatterjee, A. K. Jana and J. K. Basu, Mater. Res. Bull., 2021, 138, 111227 CrossRef CAS.
- X. Zhao, T. Yang, D. Wang, N. Zhang, H. Yang, X. Jing, R. Niu, Z. Yang, Y. Xie and L. Meng, Anal. Chem., 2022, 94, 4484–4494 CrossRef CAS PubMed.
- K. Vinothkumar, M. S. Jyothi, C. Lavanya, M. Sakar, S. Valiyaveettil and R. G. Balakrishna, Chem. Eng. J., 2022, 428, 132561 CrossRef CAS.
- S. Q. Deng, Y. L. Miao, Y. L. Tan, H. N. Fang, Y. T. Li, X. J. Mo, S. L. Cai, J. Fan, W. G. Zhang and S. R. Zheng, Inorg. Chem., 2019, 58, 13979–13987 CrossRef CAS PubMed.
- D. Zhao and C. Cai, Dyes Pigm., 2021, 185, 108957 CrossRef CAS.
- H. P. Li, Z. Dou, S. Q. Chen, M. Hu, S. Li, H. M. Sun, Y. Jiang and Q. G. Zhai, Inorg. Chem., 2019, 58, 11220–11230 CrossRef CAS PubMed.
- M. T. Hang, Y. Chen, Y. T. Wang, H. Li, M. Q. Zheng, M. Y. He, Q. Chen and Z. H. Zhang, CrystEngComm, 2022, 24, 552–559 RSC.
- F. H. M. Tang, M. Lenzen, A. McBratney and F. Maggi, Nat. Geosci., 2021, 14, 206–210 CrossRef CAS.
- M. Syafrudin, R. A. Kristanti, A. Yuniarto, T. Hadibarata, J. Rhee, W. A. Al-onazi, T. S. Algarni, A. H. Alwarri and A. M. Al-Mohaimeed, Int. J. Environ. Res. Public Health, 2021, 18, 468 CrossRef CAS PubMed.
- G. Aragay, F. Pino and A. Merkçi, Chem. Rev., 2012, 112, 5317–5338 CrossRef CAS PubMed.
- Q. Yang, J. Wang, X. Chen, W. Yang, H. Pei, N. Hu, Z. Li, Y. Suo, T. Li and J. Wang, J. Mater. Chem. A, 2018, 6, 2184–2192 RSC.
- L. F. Stadlmair, T. Letzel, J. E. Drewes and J. Grassmann, Chemosphere, 2018, 205, 649–661 CrossRef CAS PubMed.
- M. Wang, S. K. Mohanty and S. Mahendra, Acc. Chem. Res., 2019, 52, 876–885 CrossRef CAS PubMed.
- W. Liang, P. Wied, F. Carraro, C. J. Sumby, B. Nidetzky, C. K. Tsung, P. Falcaro and C. J. Doonan, Chem. Rev., 2021, 121, 1077–1129 CrossRef CAS PubMed.
- X. Lian, Y. Fang, E. Joseph, Q. Wang, J. Li, S. Banerjee, C. Lollar, X. Wang and H. C. Zhou, Chem. Soc. Rev., 2017, 46, 3386–3401 RSC.
- J. Mehta, S. Dhaka, N. Bhardwaj, A. K. Paul, S. Dayananda, S. E. Lee, K. H. Kim and A. Deep, Sens. Actuators, B, 2019, 290, 267–274 CrossRef CAS.
- T. A. Ternes, A. Joss and H. Siegrist, Environ. Sci. Technol., 2004, 38, 392A–399A CrossRef CAS PubMed.
- Y. Yang, Y. S. Ok, K. H. Kim, E. E. Kwon and Y. F. Tsang, Sci. Total Environ., 2017, 596–597, 303–320 CrossRef CAS PubMed.
- Y. Xu, T. Liu, Y. Zhang, F. Ge, R. M. Steel and L. Sun, J. Mater. Chem. A, 2017, 5, 12001–12014 RSC.
- E. Jin, S. Lee, E. Kang, Y. Kim and W. Choe, Coord. Chem. Rev., 2020, 425, 213526 CrossRef CAS.
- F. Wang, F. Ren, P. Mu, Z. Zhu, H. Sun, C. Ma, C. Xiao, W. Liang, L. Chen and A. Li, J. Mater. Chem. A, 2017, 5, 11348–11356 RSC.
- Y. Zhao, Q. Wang, H. Wang, H. Zhangsun, X. Sun, T. Bu, Y. Liu, W. Wang, Z. Xu and L. Wang, Sens. Actuators, B, 2021, 334, 129610 CrossRef CAS.
- D. Wang, F. Jia, H. Wang, F. Chen, Y. Fang, W. Dong, G. Zeng, X. Li, Q. Yang and X. Yuan, J. Colloid Interface Sci., 2018, 519, 273–284 CrossRef CAS PubMed.
- Y. Gao, J. Wu, J. Wang, Y. Fan, S. Zhang and W. Dai, ACS Appl. Mater. Interfaces, 2020, 12, 11036–11044 CrossRef CAS PubMed.
- P. Su, A. Zhang, L. Yu, H. Ge, N. Wang, S. Huang, Y. Ai, X. Wang and S. Wang, Sens. Actuators, B, 2022, 350, 130865 CrossRef CAS.
- A. Chatterjee, A. K. Jana and J. K. Basu, New J. Chem., 2021, 45, 17196–17210 RSC.
- S. Gai, J. Zhang, R. Fan, K. Xing, W. Chen, K. Zhu, X. Zheng, P. Wang, X. Fang and Y. Yang, ACS Appl. Mater. Interfaces, 2020, 12, 8650–8662 CrossRef CAS PubMed.
- Y. Gao, G. Yu, K. Liu, S. Deng, B. Wang, J. Huang and Y. Wang, Chem. Eng. J., 2017, 330, 157–165 CrossRef CAS.
- Y. Gao, J. Xia, D. Liu, R. Kang, G. Yu and S. Deng, Chem. Eng. J., 2019, 378, 122118 CrossRef CAS.
- L. G. Kahn, C. Philippat, S. F. Nakayama, R. Slama and L. Trasande, Lancet Diabetes Endocrinol., 2020, 8, 703–718 CrossRef CAS PubMed.
- M. A. La Merrill, L. N. Vandenberg, M. T. Smith, W. Goodson, P. Browne, H. B. Patisaul, K. Z. Guyton, A. Kortenkamp, V. J. Cogliano, T. J. Woodruff, L. Rieswijk, H. Sone, K. S. Korach, A. C. Gore, L. Zeise and R. T. Zoeller, Nat. Rev. Endocrinol., 2020, 16, 45–57 CrossRef CAS PubMed.
- A. C. Gore, V. A. Chappell, S. E. Fenton, J. A. Flaws, A. Nadal, G. S. Prins, J. Toppari and R. T. Zoeller, Endocr. Rev., 2015, 36, 1–150 CrossRef PubMed.
- A. N. Meng, L. X. Chaihu, H. H. Chen and Z. Y. Gu, Sci. Rep., 2017, 7, 6297 CrossRef PubMed.
- W. Zhang, M. Gong, J. Yang and J. Gu, Langmuir, 2021, 37, 8157–8166 CrossRef CAS PubMed.
- D. Li, Y. Cheng, H. Zuo, W. Zhang, G. Pan, Y. Fu and Q. Wei, J. Colloid Interface Sci., 2021, 603, 771–782 CrossRef CAS PubMed.
- Y. Zhao, R. L. Wang, X. X. Wu and C. D. Yang, Chin. J. Struct. Chem., 2019, 38, 991–998 CAS.
- X. Qian, S. Deng, X. Chen, Q. Gao, Y. L. Hou, A. Wang and L. Chen, Chin. Chem. Lett., 2020, 31, 2211–2214 CrossRef CAS.
- L. Zhang, X. Y. Wu, C. Z. Lu and W. Z. Chen, Chin. J. Struct. Chem., 2016, 35, 1929–1935 Search PubMed.
- R. Wang, X. Y. Dong, H. Xu, R. B. Pei, M. L. Ma, S. Q. Zang, H. W. Hou and T. C. W. Mak, Chem. Commun., 2014, 50, 9153–9156 RSC.
- Z. W. Zhai, S. H. Yang, M. Cao, L. K. Li, C. X. Du and S. Q. Zang, Cryst. Growth Des., 2018, 18, 7173–7182 CrossRef CAS.
- S. Yuan, L. Feng, K. Wang, J. Pang, M. Bosch, C. Lollar, Y. Sun, J. Qin, X. Yang, P. Zhang, Q. Wang, L. Zou, Y. Zhang, L. Zhang, Y. Fang, J. Li and H. C. Zhou, Adv. Mater., 2018, 30, 1704303 CrossRef PubMed.
- Y. J. Colón and R. Q. Snurr, Chem. Soc. Rev., 2014, 43, 5735–5749 RSC.
| This journal is © The Royal Society of Chemistry 2022 |