Open Access Article
Open Access ArticleProbing the cell delivery of synthetic diubiquitin chains†
Shaswati
Mandal
 and
Ashraf
Brik
and
Ashraf
Brik
 *
*
Schulich Faculty of Chemistry, Technion-Israel Institute of Technology, Haifa, 3200008, Israel. E-mail: abrik@technion.ac.il
First published on 15th July 2022
Abstract
In this study, the live-cell delivery of structurally different synthetic diubiquitin chains was examined. We found that the combination of structural variations of the Ub chains (intrinsic factors); nature of CPP and CPP-protein linkage (extrinsic factors) influence their delivery.
Ubiquitin (Ub) is a 76 amino acid long globular protein that is conserved in eukaryotes.1 Single Ub or chains of Ub (polyUb) can be attached covalently to cellular proteins in a process that is known as ubiquitination which controls a plethora of biological processes.1 Ub chains introduce an extra layer of complexity into the cellular signaling cascade by self-ubiquitination via an isopeptide bond or an amide bond between its C-terminus and the side chain of one of the seven Lys (K6, K11, K27, K29, K33, K48, or K63) or the N-terminus of the consecutive Ub, respectively.2,3
To understand the molecular basis of Ub signaling, several groups have recently reported the delivery of Ub, Ub activity-based probes (ABP), Ub variants (Ubv) for various studies such as probing the activity of enzymes in the Ub system, and also the delivery of small Ub like modifiers (e.g., SUMO) have been reported.4 Nevertheless, these studies have been mainly limited to monoUb analogues.
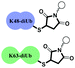
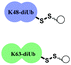
One interesting feature of polyUb chains is that they have unique structural topologies along with their distinctive signaling events.3 For example, several studies have shown that at neutral pH, while Lys48-linked Ub chains adopt a closed conformation, the Lys63-linked chains have an extended one.5 Yet, the effect of structural changes on their cellular uptake is still unclear. Therefore, we chose the Lys48 and Lys63-linked diUb chains as a model system for studying their delivery since they are structurally different yet have the same amino acid composition. Such a system offers a unique opportunity to study various aspects of diUb delivery with varying intrinsic properties (e.g., conformation, surface charge distribution, hydrophobicity, etc.) and extrinsic properties (e.g., choice of CPP, linkages).
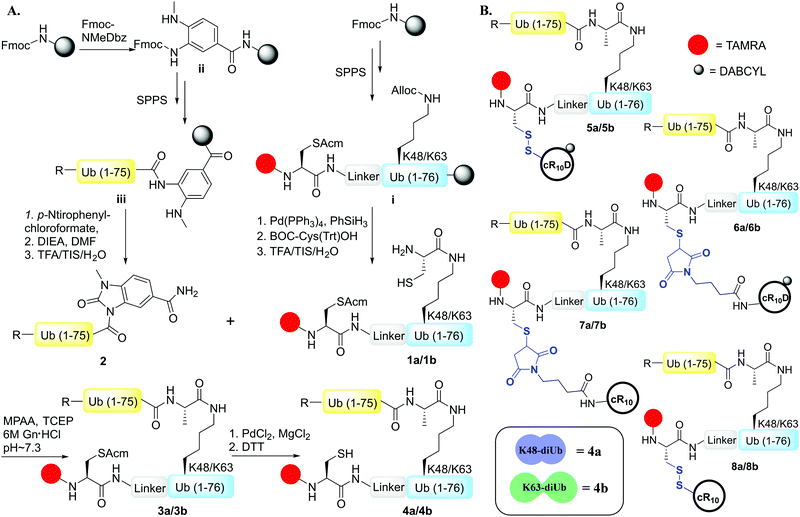
Herein, we report on the design and synthesis of eight Lys48 and Lys63-linked diUb-CPP conjugates for studying their live-cell delivery. We found that the choice of CPP along with the linkage connecting it with the cargo influences the delivery outcome apart from the structural properties of the specific Ub chain. We have successfully demonstrated the need for a combination of all the necessary elements to modulate the live-cell delivery of our synthetic Ub dimers.
To examine the effect of the structural differences along with the nature of CPPs on cell delivery, we aimed to study the combination of two types of CPPs i.e., cyclic deca-arginine (cR10) and DABCYL modified cR10 (cR10D)4d and linkages (disulfide and maleimide) and the two diUbs (Lys48 and Lys63). Therefore, eight different diUb-CPP conjugates had to be chemically prepared. Each diUb was designed to have a fluorophore attached to its N-terminus of the proximal Ub along with a Cys for the attachment of the CPP unit. The attachment of cR10D via a glutathione cleavable disulfide linkage was found to be superior to cR10 in the delivery of SUMO, monoUb, and their analogues.4d,e On the other hand, when attaching cR10 to Ub analogues via a stable thiazolidine linkage this allowed for acceptable cell delivery, which hints at the role of the linkage type between the CPP and POI on cell delivery.4c For better stability against enzymatic degradation of the dimers, the junction for linking both Ub's to afford the diUbs was mutated to Gly76Ala.6 Importantly this also facilitates the synthesis of the diUb by employing routine native chemical ligation (NCL).7,8 For this purpose, the distal Ub was designed with Gly76 deletion and the proximal Ub was designed with a Cys coupled at either Lys48 or Lys63 position, (ESI,† Section S9 and S10). Along with this, the proximal Ub was designed to carry a Cys(Acm) residue and 5-carboxytetramethylrhodamine (TAMRA) as a fluorophore. For keeping similarity in both Ub units, extra Ala was added to the N-terminus of the distal Ub. The addition of a Peg linker [2-[2-(Fmoc-amino)ethoxy]ethoxy]acetic acid after the Ub sequence stands as a spacer between the natural and unnatural parts in the diUb scaffold.
The distal Ub, Ub(1–75) was synthesized via SPPS employing the Fmoc-3-amino-4-(methylamino)benzoic acid (Fmoc-MeDbz) linker coupled to a Rink amide resin.4d,9 Upon cyclization, cleavage, and purification, the distal Ub unit was used as the component for 4-mercaptophenylacetic acid (MPAA) mediated NCL with the proximal Ub unit bearing the designed elements (Scheme 1A). One-pot desulfurization10 of the Cys at the ligation junction was achieved in the presence of Cys(Acm) (Fig. S4 and S5, ESI†). After purification, Pd mediated rapid Acm removal11 from the ligated diUb was commenced to generate the fluorescently labeled Lys48/Lys63-linked diUb model systems bearing a free Cys for CPP attachment (Scheme 1A, Fig. S6 and S7, ESI†).
The synthesis of Cys-cR10D and Cys-cR10 was carried out according to the previously reported procedure.4d We optimized the reaction conditions for the synthesis of Lys48/Lys63-linked diUb-cR10D conjugates with the asymmetric disulfide linkage by varying the pH of the reaction buffer along with the temperature and the same optimized conditions were employed to conjugate Cys-cR10 with the diUbs. We synthesized the maleimide-cR10 and maleimide-cR10D CPPs (ESI,† Section S13) and conjugated them with the diUbs via the Michael-addition reaction. The final constructs of the eight diUb-CPP conjugates are shown in Scheme 1B (ESI,† Section S16–S19).
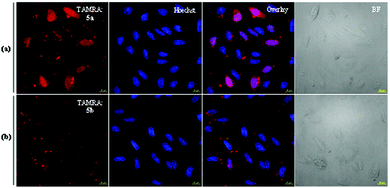
To examine the difference between the two diUbs and the effect of the cleavable linker with cR10D (i.e., 5a & 5b) over the cellular uptake efficiency, we probed their delivery to live U2OS cells using 4 μM of each construct at 37 °C. After washing with PBS and Heparin, the nuclear staining was commenced with Hoechst solution. From the live-cell confocal laser scanning microscope (CLSM) images, we could observe that the cell permeability of the Lys48-linked diUb (5a) was higher than the Lys63-linked diUb (5b) analogue (Fig. 1). Once the protein of interest (POI) penetrates the cell membrane, the passive diffusion through the nuclear pore (for small proteins, <60 kDa) will allow it to reach the nucleus, which reflects its cytosolic delivery.12 As a matter of fact, the nuclei are deprived of any compartments like endosomes/lysosomes, making it a better organelle to measure the live-cell delivery efficacy of the internalized POI.12
Therefore, to get a more quantitative output from these results, we ran a cell marking algorithm based on Hoechst nuclear staining and nuclear TAMRA intensity was calculated on all acquired confocal images (∼500 cells) using the FiJi software. The quantification result depicted nearly two-fold higher efficiency of Lys48-linked diUb delivery than Lys63-linked diUb when applying the cleavable disulfide-linked cR10D (Fig. 1 and Fig. S20, ESI†).
After exploring the disulfide linkage effect on diUb delivery, we aimed to examine the influence of the stable linker with the same CPP unit i.e., cR10D. We anticipated that the stable linkage in diUb-CPP analogues would provide subcellular localization of the conjugates inside the nucleoli.13 Therefore, we performed live-cell protein delivery experiments with conjugates 6a and 6b in U2OS cells using 4 μM of each analogue at 37 °C. Subsequent washing and Hoechst staining were done followed by confocal microscopy. Surprisingly, we did not see any substantial differences in the delivery as depicted by the TAMRA intensities (Fig. S21, ESI†). The quantification based on calculating nuclear TAMRA intensity showed that these analogues have a similar cellular uptake irrespective of their conformational differences. As the DABCYL can quench the TAMRA fluorescence,14 the cytosolic fluorescence signal was compromised. However, in our comparison study both the diUbs were having the same DABCYL and TAMRA units at the same positions, regardless of diUb conformation, therefore we believe the recorded data is sufficient for such a comparison.
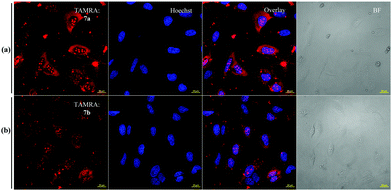
The observation that the delivery of Lys63-linked diUb in presence of the disulfide-linked cR10D was nearly half of that of Lys48-linked diUb and is equal to the maleimide linkage, prompted us to exclude DABCYL from the system to dissect its specific effect on each diUb chain. For this, we tested separately the delivery of analogues 7a and 7b bearing the stable maleimide-linked to cR10 and analogues 8a and 8b with the disulfide-linked cR10. First, we performed the cellular uptake studies using 7 μM of 7a and 7b. The confocal images showed substantial delivery of the non-cleavable conjugates based on the TAMRA intensities and were mainly localized in the nucleoli (Fig. 2). However, the nuclear TAMRA intensity measurement showed that the Lys63-linked diUb conjugate (7b) was delivered to a lesser extent compared to Lys48-linked diUb (7a). Indeed, the ratio of the intensities for 7a and 7b was 1·4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (Fig. S22, ESI†) which did not change with varying concentrations (Fig. S23, ESI†). Moreover, cellular uptake of 7a can also be achieved at 4 °C suggesting direct transduction of the conjugate (Fig. S24, ESI†).15
1, (Fig. S22, ESI†) which did not change with varying concentrations (Fig. S23, ESI†). Moreover, cellular uptake of 7a can also be achieved at 4 °C suggesting direct transduction of the conjugate (Fig. S24, ESI†).15
Next, we tested the difference between 8a and 8b to probe the effect of the disulfide bond on the delivery. Cells were incubated with each analogue at 4 μM at 37 °C and prepared for CLSM, as described before. The confocal images indicated that the cellular uptake was not efficient and showed predominantly endo lysosomal signals (Fig. S25, ESI†). However, upon quantification, we observed that the ratio was equal for both the diUbs based on the nuclear TAMRA intensity calculation.
In this study, we aimed to investigate the effect of two conformationally different (compact vs. extended) proteins that have the same amino acids (Lys48 & Lys63-linked diUb), the nature of the CPP (cR10 & cR10D), and the linkage between these proteins and CPP (disulfide & maleimide) on the cell delivery. Notably, the delivery of Lys48 or Lys63-linked diUbs was not possible without CPP. The combination of all these parameters and their corresponding results are summarized in Table 1.
Our results show that cR10D linked to the dimers via a cleavable disulfide bond has more influence on the delivery of Lys48-linked diUb than the Lys63 linkage. On the other hand, attaching the same CPP unit through maleimide linkage led to a similar delivery of the two diUb. Our results also show that when we employed the disulfide linkers with the unmodified cR10 the delivery of the two diUb became equal. However, when we employed the same CPP yet with the maleimide linker the ratio was 1·4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 towards Lys48-linked diUb. Gratifyingly, we observed an enhanced efficiency of Cys-cR10D compared to the unmodified Cys-cR10, which is in line with our previous reports.4d
1 towards Lys48-linked diUb. Gratifyingly, we observed an enhanced efficiency of Cys-cR10D compared to the unmodified Cys-cR10, which is in line with our previous reports.4d
The structural differences of the Lys48 and Lys63-linked diUbs and the resulting surface charge distribution, based on the adaptive Poisson–Boltzmann solver (APBS) of the diUb analogues (Fig. S26, ESI†) show that the Lys48-linked diUb have more intense positive charges yet buried between the two Ub units. On the other hand, the Lys63-linked diUb has a more hydrophobic surface and positive charges that are distributed over the dimer, despite being less intense compared to that of the Lys48 analogue.
In our system, the Lys63-linked diUb has more propensity for cell delivery when considering the hydrophobicity and positive charge distribution over the surface as reported for GFP.16 On the other hand, the compact structure of Lys48-linked diUb might favor its delivery, as conformational rigidity is an important factor in the delivery of cyclic peptides.17 Our results suggest that the conformational difference in the cell delivery of the two diUbs appears to be less dominant as in each diUb there are unique contributing factors to the delivery process. As the Lys48-linked diUb has been reported to change its conformational dynamics during DUB activity,18 it is reasonable to speculate that both the chains might adopt similar conformation during membrane internalization. Therefore, in such a system the CPP and the linker types have more influential roles as evident by the fluctuation of the delivery depending on the combination of these two parameters.
In nature, Ub's intricacy arises from chain elongation with different linkages,2,3 which makes this system ideal for further drawing more principles for cytosolic delivery of proteins. For example, how chain elongation does affect the delivery of the same type of chains (homo), or different ones (hetero/branched) can be similarly investigated. In terms of chain's degrees of freedom, one could also compare the same chain in its native and more rigid form that can be prepared via cyclization as has been demonstrated by Lys48-linked Ub chains.19 These studies are currently being performed in our laboratory.
S. M. did all the experimental work in this manuscript and assisted in writing the manuscript. A. B. supervised the work and wrote the manuscript.
A. B. holds The Jordan and Irene Tark Academic Chair. This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement no. [831783]).
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. Hershko and A. Ciechanover, Annu. Rev. Biochem., 1998, 67, 425–479 CrossRef CAS PubMed.
- C. M. Pickart and D. Fushman, Curr. Opin. Chem. Biol., 2004, 8, 610–616 CrossRef CAS PubMed.
- F. Ikeda and I. Dikic, EMBO Rep., 2008, 9, 536–542 CrossRef CAS PubMed.
- (a) M. P. C. Mulder, K. Witting, I. Berlin, J. N. Pruneda, K. P. Wu, J. G. Chang, R. Merkx, J. Bialas, M. Groettrup, A. C. O. Vertegaal, B. A. Schulman, D. Komander, J. Neefjes, F. El Oualid and H. Ovaa, Nat. Chem. Biol., 2016, 12, 523–530 CrossRef CAS PubMed; (b) W. Gui, C. A. Ott, K. Yang, J. S. Chung, S. Shen and Z. Zhuang, J. Am. Chem. Soc., 2018, 140, 12424–12433 CrossRef CAS PubMed; (c) G. Mann, G. Satish, R. Meledin, G. B. Vamisetti and A. Brik, Angew. Chem., Int. Ed., 2019, 58, 13540–13549 CrossRef CAS PubMed; (d) S. Mandal, G. Mann, G. Satish and A. Brik, Angew. Chem., Int. Ed., 2021, 60, 7333–7343 CrossRef CAS PubMed; (e) G. Mann, G. Satish, P. Sulkshane, S. Mandal, M. H. Glickman and A. Brik, Chem. Commun., 2021, 57, 9438–9441 RSC.
- (a) M. J. Eddins, R. Varadan, D. Fushman, C. M. Pickart and C. Wolberger, J. Mol. Biol., 2007, 367, 204–211 CrossRef CAS PubMed; (b) R. Varadan, M. Assfalg, A. Haririnia, S. Raasi, C. Pickart and D. Fushman, J. Biol. Chem., 2004, 279, 7055–7063 CrossRef CAS PubMed; (c) A. B. Datta, G. L. Hura and C. Wolberger, J. Mol. Biol., 2009, 392, 1117–1124 CrossRef CAS PubMed.
- N. Haj-Yahya, M. Haj-Yahya, C. A. Castañeda, L. Spasser, H. P. Hemantha, M. Jbara, M. Penner, A. Ciechanover, D. Fushman and A. Brik, Angew. Chem., Int. Ed., 2013, 52, 11149–11153 CrossRef CAS PubMed.
- P. E. Dawson, T. W. Muir, L. C. Lewis and S. B. H. Kent, Science, 1994, 266, 776–779 CrossRef CAS PubMed.
- R. Meledin, S. M. Mali, O. Kleifeld and A. Brik, Angew. Chem., Int. Ed., 2018, 57, 5645–5649 CrossRef CAS PubMed.
- J. B. Blanco-Canosa, B. Nardone, F. Albericio and P. E. Dawson, J. Am. Chem. Soc., 2015, 137, 7197–7209 CrossRef CAS PubMed.
- T. Moyal, H. P. Hemantha, P. Siman, M. Refua and A. Brik, Chem. Sci., 2013, 4, 2496–2501 RSC.
- S. K. Maity, M. Jbara, S. Laps and A. Brik, Angew. Chem., Int. Ed., 2016, 55, 8108–8112 CrossRef CAS PubMed.
- D. C. Luther, T. Jeon, R. Goswami, H. Nagaraj, D. Kim, Y. W. Lee and V. M. Rotello, Bioconjugate Chem., 2021, 32, 891–896 CrossRef CAS PubMed.
- A. F. L. Schneider, A. L. D. Wallabregue, L. Franz and C. P. R. Hackenberger, Bioconjugate Chem., 2019, 30, 400–404 CrossRef CAS PubMed.
- P. Tompa, P. Buzder-Lantos, A. Tantos, A. Farkas, A. Szilágyi, Z. Bánóczi, F. Hudecz and P. Friedrich, J. Biol. Chem., 2004, 279, 20775–20785 CrossRef CAS PubMed.
- N. Nischan, H. D. Herce, F. Natale, N. Bohlke, N. Budisa, M. C. Cardoso and C. P. R. Hackenberger, Angew. Chem., Int. Ed., 2015, 54, 1950–1953 CrossRef CAS PubMed.
- (a) B. R. McNaughton, J. J. Cronican, D. B. Thompson and D. R. Liu, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 6111–6116 CrossRef CAS PubMed; (b) K. A. Mix, J. E. Lomax and R. T. Raines, J. Am. Chem. Soc., 2017, 139, 14396–14398 CrossRef CAS PubMed.
- D. S. Nielsen, R. J. Lohman, H. N. Hoang, T. A. Hill, A. Jones, A. J. Lucke and D. P. Fairlie, ChemBioChem, 2015, 16, 2289–2293 CrossRef CAS PubMed.
- Y. Ye, G. Blaser, M. H. Horrocks, M. J. Ruedas-Rama, S. Ibrahim, A. A. Zhukov, A. Orte, D. Klenerman, S. E. Jackson and D. Komander, Nature, 2012, 492, 266–270 CrossRef CAS PubMed.
- (a) T. Moyal, S. N. Bavikar, S. V. Karthikeyan, H. P. Hemantha and A. Brik, J. Am. Chem. Soc., 2012, 134, 16085–16092 CrossRef CAS PubMed; (b) T. Yao and R. E. Cohen, J. Biol. Chem., 2000, 275, 36862–36868 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthetic procedure of Ub monomers, diUbs, CPPs, diUb-CPP conjugates, HPLC-MS analysis, confocal images and data quantification for all the data sets. See DOI: https://doi.org/10.1039/d2cc02476d |
| This journal is © The Royal Society of Chemistry 2022 |