Open Access Article
Open Access ArticleConvenient synthesis of tricyclic N(1)–C(2)-fused oxazino-indolones via [Au(I)] catalyzed hydrocarboxylation of allenes†
Riccardo
Pedrazzani
 ab,
Emanuele
Pinosa
ab,
Giulio
Bertuzzi
ab,
Magda
Monari
ab,
Emanuele
Pinosa
ab,
Giulio
Bertuzzi
ab,
Magda
Monari
 *ab,
Samuel
Lauzon
*ab,
Samuel
Lauzon
 c,
Thierry
Ollevier
c,
Thierry
Ollevier
 *c and
Marco
Bandini
*c and
Marco
Bandini
 *ab
*ab
aDipartimento di Chimica “Giacomo Ciamician”, Alma Mater Studiorum – Università di Bologna, via Selmi 2, Bologna, 4016, Italy. E-mail: magda.monari@unibo.it; marco.bandini@unibo.it
bCenter for Chemical Catalysis - C3, Alma Mater Studiorum, via Selmi 2, Bologna, 4016, Italy
cDépartement de chimie, 1045 avenue de la Médecine, Université Laval, Québec, G1V 0A6, Qc, Canada. E-mail: Thierry.Ollevier@chm.ulaval.ca
First published on 28th June 2022
Abstract
A new [Au(I)] catalyzed intramolecular hydrocarboxylation of allenes is presented as a valuable synthetic route to oxazino-indolones. The use of 3,5-(CF3)2–C6H3–ImPyAuSbF6 as the optimal catalyst (5 mol%) was necessary to guarantee (i) wide tolerance of functional groups, (ii) mild reaction conditions (r.t., 16 h), and (iii) high yields (up to 90%). Preliminary attempts towards an enantioselective version (81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 er) are also documented by means of a new family of chiral C1-symmetric ImPyAuCl complexes.
19 er) are also documented by means of a new family of chiral C1-symmetric ImPyAuCl complexes.
The development of sustainable synthetic methodologies for the realization of an N(1)–C(2)-polycyclic fused indolyl scaffold is currently receiving growing credit in the chemical community.1 In particular, 3,4-dihydro-1H-[1,4]oxazino[4,3-a]indol-1-ones (i.e., oxazino-indol-1-one scaffold, A) keep stimulating progress in organic synthesis due to their wide presence in bioactive compounds and naturally occurring species, and as precursors of pharmacologically active ingredients (Fig. 1a).2 Nowadays, the available synthetic routes to the titled scaffold commonly require harsh reaction conditions (i.e., high temperatures)3 and/or stoichiometric additives (i.e., halogens, AgNO3)2e,4 and lead to moderately functionalizable polycyclic-fused indolyl scaffolds (Fig. 1b). On the contrary, the use of a catalytic approach has never been adopted for building up oxazino-indolone cores, to date.5 Additionally, catalytic asymmetric variants are unprecedented so far.
Aiming at addressing the afore-described gap in the literature, we envisioned the development of an intramolecular condensation of readily available indole-2-carboxylic acids featuring N-tethered allenyl units 1 (Fig. 1c).6 This approach would lead to direct access to 3,4-dihydro-1H-[1,4]oxazino[4,3-a]indol-1-ones 2 possessing a tertiary stereogenic center and carrying a synthetically versatile vinyl unit.
Certainly, the direct use of unprotected carboxylic acids as nucleophilic partners can introduce some constraints in terms of metal catalyst design; therefore, our attention moved to the use of poorly oxophilic but π-acidic metal species. In line with our research results on the development of “on-demand” Au(I)7 catalysts, some of us have recently documented the high performance of the CF3–aryl–ImPy-based gold complex Cat1 in the electrophilic manipulation of several π-systems (Fig. 1d).8 This peculiar catalytic activity was rationalized based on secondary interactions regarding the cationic alkenyl–[Au(I)]-type intermediates B.9
These findings, combined with our recent interest towards [Au(I)] assisted synthesis of polycyclic fused indolyl cores,10 prompted us to verify the efficiency of Cat1 in the model hydrocarboxylation reaction of 1a (Fig. 1c, R=H![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ). Interestingly, although gold catalyzed reactions of unactivated allenes, via C–C, C–N and C–O (mainly alcohols) bond forming protocols, have been extensively explored,11 [Au(I)] catalyzed hydrocarboxylations of cumulenes have faced far less success in the literature with applications merely related to the preparation of γ-butyrolactones.12
). Interestingly, although gold catalyzed reactions of unactivated allenes, via C–C, C–N and C–O (mainly alcohols) bond forming protocols, have been extensively explored,11 [Au(I)] catalyzed hydrocarboxylations of cumulenes have faced far less success in the literature with applications merely related to the preparation of γ-butyrolactones.12
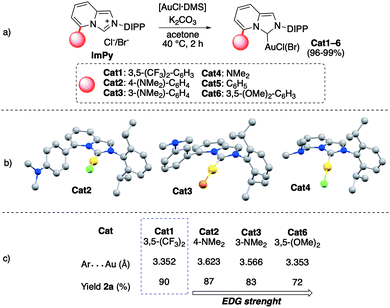
At the outset of the investigation, an extensive survey of reaction parameters was underpinned to determine the optimal conditions (see ESI† for details). Among the tested ligands, a family of ImPy13 nitrogen heterocyclic carbenes (NHCs),14 comprising diverse substitutions at the C(5)-position was considered (Fig. 2a). In this context, besides the already documented gold complexes Cat1,5,6, three new dimethyl amino-based ImPy scaffolds were targeted (Cat2–4) to assess potentially key hydrogen bond interactions during the ring-closure. Here, complexes Cat2,4,6 were accessible in high yields (96–99%) via direct condensation of the imidazopyridium salt precursors (ImPy2–4) with [Me2SAuCl] and K2CO3 in acetone.15 Furthermore, the new complexes Cat2–4 were fully characterized also via X-ray diffraction and the resulting molecular structures are reported in Fig. 2b. The arene⋯Au distance, that has been already proved to qualitatively predict the catalytic activity of the Au complexes in electrophilic activation of π-systems, was investigated for Cat2 and Cat3.8 These two pre-catalysts with electron-rich functionalization displayed longer arene⋯Au vs.Cat6 contact (3.352 Å) and a shortening of the distance was observed for meta-substituted arenes vs. para-ones (3.623 Å vs. 3.566 Å). Furthermore, both –NMe2 groups have a high degree of planarity, due to the conjugation with the phenyl ring (see ESI†). On the contrary, complex Cat4 showed a marked pyramidalization of the nitrogen atom of the –NMe2 group, revealing a tight interaction with the metal centre (N(3)⋯Au 3.112 Å) (see ESI†, Table S1).
| Entrya | Deviation from optimal | Yield 2a (%)b |
|---|---|---|
| a Reaction conditions: 1a (0.1 mmol, 0.1 M), under nitrogen atmosphere at r.t. b Determined after flash chromatography as an average of two runs. c Using 10 mol% of AgSbF6. NR: no reaction. picAuCl2: dichloro(2-pyridinecarboxylato)gold. | ||
| 1 | – | 90 |
| 2 | Cat2 | 87 |
| 3 | Cat3 | 83 |
| 4 | Cat4 | 82 |
| 5 | Cat5 | 83 |
| 6 | Cat6 | 72 |
| 7 | Cat7 (iPrAuCl) | 69 |
| 8 | Cat8 (PPh3AuCl) | 42 |
| 9 | Cat9 (JohnPhosAuCl) | <5 |
| 10c | Cat10 (picAuCl2) | <5 |
| 11 | Cat1/AgTFA | Traces |
| 12 | Cat1/NaBArF | Traces |
| 13 | Cat2/AgOTs | 27 |
| 14 | No AgSbF6 | NR |
| 15 | AgSbF6 without Cat1 | NR |
| 16 | Toluene | 36 |
| 17 | CH3CN | NR |
| 18 | CH2Cl2 | 63 |
Delightfully, Cat1 (5 mol%) exhibited high efficiency in the methodology by performing the chemo- and regioselective ring-closure of 1a leading exclusively to the six-membered product 2a in 90% isolated yield (5 mol% AgSbF6, [1a] = 0.1 M in THF, r.t. entry 1 Table 1). Variations on the electronic properties of the C(5)-aryl-pendants did not impact on the chemical outcome dramatically (82–90%, entries 1–5), with the only exception of 3,5-(MeO)2–C6H3–ImPy(Au)Cl complex Cat6 that produced 2a in a lower amount (72% yield, entry 6).
Interestingly, the family of ImPyAuCl catalysts proved more competent with respect to IPrAuCl/AgSbF6 (entry 7), and 2a was obtained in 69% yield in 16 h. Additionally, the catalytic performance of the present ImPyAuCl complexes was also compared to benchmark P-based gold(I) catalysts, such as PPh3AuCl (Cat8) and JohnPhosAuCl (Cat9).
Overall, phosphine-based ligands proved inefficient in the model reaction, providing 2a in 42% yield and trace amounts, respectively (entries 8 and 9). Similarly, a disappointing outcome was recorded using picAuCl2 (Cat10, entry 10).
The genuine cationic gold catalysis was demonstrated by running the model protocol in the absence of an Ag salt (entry 14) and an Au(I) complex (entry 15), resulting in no conversion in both cases. Finally, other parameters, such as a gold counterion and reaction media, were investigated but no improvements with respect to the optimal conditions were recorded (entries 11–18). The catalytic performance of the C(5)-aryl-containing complexes Cat1–3 and Cat6 was analysed in comparison with the relative Ar⋯Au distances recorded in the solid state (Fig. 2c). Interestingly, in the small series of electron-rich arene containing species (i.e., Cat2,3 and Cat6) a shortening of the arene/metal contact directly related to the strengths of the EDGs is observed, with the meta substitution predominating over the para-ones (see Cat2vs.Cat3). In particular, stronger interactions resulted in a lower catalytic performance (from 87% to 72%) and this output is ascribable to the stabilization effect of the EDG units on cationic organometallic intermediates formed upon Au-activation of the cumulene group of 1. On the contrary, the destabilizing role played by the meta-substituted ring (3,5-(CF3)2–C6H3) on cationic organogold intermediates sped up the ring-closing event resulting in 90% isolated yield of 2a.
Therefore, the generality of the protocol was verified by subjecting a range of readily accessible and diversely substituted N-allenyl-indole-2-carboxylic acids 1b–j to the optimal C–O ring-closure conditions (Scheme 1, see ESI† for synthetic details). Remarkably, electron-donating groups (Me and OMe) could be conveniently accommodated both at the C(3), C(4) and C(5) positions of the benzenoid ring by providing the desired compounds 2b–d,k in good to excellent yields (66–85%). Analogously, electron-withdrawing substituents at the indole core (i.e., F, Cl and Br) were adequately tolerated (i.e., C(5) and C(6) positions) providing the corresponding oxazino-indolones 2e–h in very high yields (75–98%). The use of trisubstituted allenyl units as starting materials 1i,j was assessed. Here, although the sterically congested cHex-substituted allenyl framework (1j) caused a significant drop in conversion (2j, 29% yield), the use of gem-Me2-substituted precursor 1i yielded the desired tricyclic scaffold 2i in a synthetically useful 69% yield.
On the other hand, the procedure also faced some limitations in substrate scope. As a matter of fact, attempts to extend the process to differently structured seven-membered rings (2l,m) or pyrrolyl-2-carboxylic acid 1n resulted in modest conversions (ca. 12–18% yield).16
Based on these promising results, we then turned our attention towards the development of an unprecedented catalytic enantioselective variant of the synthesis of 3,4-dihydro-1H-[1,4]oxazino[4,3-a]indol-1-ones. In this direction, we decided to preserve the ImPy ligand core in order to guarantee synthetically useful catalytic turnovers and we accommodated stereochemical information at the C(5)-position, that is known to be in close proximity with the reaction centre.
The introduction of an enantiomerically pure secondary alcohol at the C(5)-site was addressed, enabling electronic as well as steric fine-tuning at the stereogenic centre.17 In this direction, chiral ImPyAuCl complexes Cat11–14 were prepared (87–99%) by considering tBu and adamantyl substituents at the carbinol site and different oxygenated moieties (i.e., OH, OMe, and OAc groups) at the alcoholic site. Firstly, structural insights were obtained from X-ray diffraction analysis (Fig. 3). All the complexes Cat11–14 showed orthogonal orientation of the alkyl substituent with respect to the ImPy plane (dihedral angle range 92.36–94.04°) with no O⋯Au contacts. This general spatial arrangement minimizes steric congestions that would result in alternative pseudo-eclipsed conformations. Moreover, a solvated molecule of THF engaging a strong H-bonding interaction with the OH group (OTHF⋯H–O 1.864 Å) was localized in the Cat11 unit cell. Aiming at verifying the efficiency of the enantiomerically pure carbene complexes Cat11–14 in the present enantioselective hydrocarboxylation of allenes, the corresponding in situ formed cationic Au(I) complexes (5 mol% of AgSbF6) were tested in the ring-closure of 1a. In all cases, very high isolated yields of 2a were obtained at r.t. in THF and 16 h reaction time (80–90% yield). Interestingly, a marked effect of the carbinol functionalization on the stereochemical outcome of the process was recorded. As a matter of fact, while (R)-Cat11 featuring an unprotected OH group afforded (−)-2a in 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 er, the corresponding methyl ether (R)-Cat12 led to a significantly higher stereoinduction (75
33 er, the corresponding methyl ether (R)-Cat12 led to a significantly higher stereoinduction (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 er). Similar behaviour was also obtained using the OAc analogous (R)-Cat13 (72
25 er). Similar behaviour was also obtained using the OAc analogous (R)-Cat13 (72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 er of (−)-2a). Finally, the employment of the 1-Ad containing complex (S)-Cat14 led to a slight improvement in stereoselectivity, yielding (+)-2a in 81
28 er of (−)-2a). Finally, the employment of the 1-Ad containing complex (S)-Cat14 led to a slight improvement in stereoselectivity, yielding (+)-2a in 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 er.
19 er.
 | ||
| Fig. 3 New chiral ImPyAuCl complexes for the enantioselective synthesis of the 3,4-dihydro-1H-[1,4]oxazino[4,3-a]indol-1-ones 2a. (a) Chemical/stereochemical outomes; (b) X-ray structures. | ||
In summary, a new gold catalyzed intramolecular hydrocarboxylation of allenes is described as a direct synthetic route to densely functionalized 3,4-dihydro-[1,4]oxazinoindol-1-ones. The main advantages of the protocol rely on the ready availability of the starting material, the functional group tolerance, and the mild reaction conditions. Fine-tunable NHC-ImPy ligands, featuring electronically modulable aryl units, afforded high yields (up to 98%) to be obtained together with a high level of chemo- and regioselectivity. Preliminary attempts towards an unprecedented enantioselective variant of the protocol were also undertaken by means of modulable ImPy complexes Cat11–14. A moderate level of enantiomeric control (up to 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 er) was documented.
19 er) was documented.
Conflicts of interest
There are no conflicts to declare.Notes and references
- For a general review see: (a) S. Dong and J. Yanxing, Chin. J. Org. Chem., 2013, 33, 1144 CrossRef; (b) P. M. Barbour, L. J. Marholz, L. Chang, W. Xu and X. Wang, Chem. Lett., 2014, 43, 572 CrossRef CAS; (c) J. Song, D.-F. Chen and L.-Z. Gong, Nat. Sci. Rev., 2017, 4, 381 CrossRef CAS; (d) N. R. Connon and P. J. Guiry, Tetrahedron Lett., 2020, 61, 151696 CrossRef; (e) A. J. Rago and G. Dong, Green Synth. Catal., 2021, 2, 216 CrossRef.
- (a) S. Inaba, K. Ishizumi, M. Akatsu, R. Kume, K. Mori and H. Yamamoto, Jpn. Tokkyo Koho, JP49004238B, 1974 Search PubMed; (b) W. T. Zimmerman, PCT Int. Appl., WO9110668A1, 1991 Search PubMed; (c) W. T. Zimmerman, US5356862A, 1994; (d) S. Ayral-Kaloustian, N. Zhang, A. M. Venkatesan, T. S. Mansour, T. H. Nguyen and J. T. Anderson, U. S. Pat. Appl. Publ., US20090192147A1, 2009 Search PubMed; (e) W. G. Devine, R. Diaz-Gonzalez, G. Ceballos-Perez, D. Rohas, T. Satoh, W. Tear, R. M. Ranade, X. Barros-Alvarez, W. G. J. Hol, F. S. Buckner, M. Navarro and M. P. Pollastrini, ACS Infect. Dis., 2017, 3, 225 CrossRef CAS PubMed; (f) C. Maaliki, J. Fu, S. Villaume, A. Viljoen, C. Raynaud, S. Hammoud, J. Thibonnet, L. Kremer, S. P. Vincent and E. Thiery, Bioorg. Med. Chem., 2020, 28, 115579 CrossRef CAS PubMed.
- (a) R. Melnický, M. Grepl, A. Lycka, V. Bertolasi, L. Kvapil, B. Dvoráková and P. Hradil, Synthesis, 2013, 2447 Search PubMed; (b) J. K. Vandavasi, W.-P. Hu, G. C. Senadi, S. S. Kumar Boominathan, H.-Y. Chen and J.-J. Wang, Eur. J. Org. Chem., 2014, 6219 CrossRef CAS.
- (a) A. Putey, G. Fournet and B. Joseph, Synlett, 2006, 2755 CAS; (b) A. Putey, G. Fournet, O. Lozach, L. Perrin, L. Meijer and B. Joseph, Eur. J. Med. Chem., 2014, 83, 617 CrossRef CAS PubMed; (c) S. Hammoud, E. Anselmi, K. Cherry, J.-C. Kizirian and J. Thibonnet, Eur. J. Org. Chem., 2018, 6314–6327 CrossRef CAS; (d) A. Mayooufi, M. Romdhani-Younes, Y. Carcenac and J. Thibonnet, Synth. Commun., 2019, 49, 2168–2179 CrossRef CAS.
- For a gold(III) catalyzed synthesis of 3-methylene-oxazino-indol-1-ones see: S. Taskaya, N. Menges and M. Balci, Beilstein J. Org. Chem., 2015, 11, 897 CrossRef CAS PubMed.
- For a recent review on catalytic synthetic manipulation of indoles with allenes see: J. M. Alonso and M. P. Muñoz, Eur. J. Org. Chem., 2020, 7197 CrossRef CAS.
- “On-demand” is a commonly accepted terminology to describe synthetically flexible and catalytically adaptive ligands to fine-tune the overall catalytic properties of the resulting metal complexes towards organic transformations.
- R. Pedrazzani, A. Pintus, R. De Ventura, M. Marchini, P. Ceroni, C. Silva López, M. Monari and M. Bandini, ACS Org. Inorg., 2022, 2, 229–235 CrossRef CAS.
- For a related study on P-based ligand see: H. Darmandeh, J. Löffler, N. V. Tzouras, B. Dereli, T. Scherpf, K. S. Feichtner, S. V. Broeck, K. Van Hecke, M. Saab, C. S. J. Cazin, L. Cavallo, S. P. Nolan and V. H. Gessner, Angew. Chem., Int. Ed., 2019, 60, 21014 CrossRef PubMed.
- (a) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9533 CrossRef CAS PubMed; (b) M. Bandini, A. Eichholzer, A. Gualandi, T. Quinto and D. Savoia, ChemCatChem, 2010, 2, 661 CrossRef CAS; (c) M. Bandini, A. Gualandi, M. Monari, M. Tragni and D. Savoia, J. Organomet. Chem., 2011, 696, 338 CrossRef CAS; (d) G. Cera, P. Crispino, M. Monari and M. Bandini, Chem. Commun., 2011, 47, 7803 RSC; (e) G. Cera, M. Chiarucci, A. Mazzanti, M. Mancinelli and M. Bandini, Org. Lett., 2012, 14, 1350 CrossRef CAS PubMed; (f) G. Cera, S. Piscitelli, M. Chiarucci, G. Fabrizi, A. Goggiamani, R. S. Ramón, S. P. Nolan and M. Bandini, Angew. Chem., Int. Ed., 2012, 51, 9891 CrossRef CAS PubMed; (g) M. Bandini, A. Bottoni, M. Chiarucci, G. Cera and G. Miscione, J. Am. Chem. Soc., 2012, 134, 20690 CrossRef CAS PubMed; (h) M. Chiarucci, E. Matteucci, G. Cera, G. Fabrizi and M. Bandini, Chem. – Asian J., 2013, 8, 1776 CrossRef CAS PubMed; (i) M. Chiarucci, R. Mocci, L.-D. Syntrivanis, G. Cera, A. Mazzanti and M. Bandini, Angew. Chem., Int. Ed., 2013, 52, 10850 CrossRef CAS PubMed; (j) M. Jia, G. Cera, D. Perrotta and M. Bandini, Chem. – Eur. J., 2014, 20, 9875 CrossRef CAS PubMed; (k) M. Jia, M. Monari, Q.-Q. Yang and M. Bandini, Chem. Commun., 2015, 51, 2320 RSC; (l) P. Giacinto, G. Cera, A. Bottoni, M. Bandini and G. P. Miscione, ChemCatChem, 2015, 7, 2480 CrossRef CAS; (m) R. Ocello, A. De Nisi, M. Jia, Q.-Q. Yang, P. Giacinto, A. Bottoni, G. P. Miscione and M. Bandini, Chem. – Eur. J., 2015, 21, 18445 CrossRef CAS PubMed.
- For comprehensive articles, see: (a) N. Krause and C. Winter, Chem. Rev., 2011, 111, 1994 CrossRef CAS PubMed; (b) J. Erdsack and N. Krause, Synthesis, 2013, 3741 Search PubMed; (c) H. C. Shen, Tetrahedron, 2008, 64, 3885 CrossRef CAS; (d) M. P. Muñoz, Org. Biomol. Chem., 2012, 10, 3584 RSC; (e) N. Huguent and M. A. Echavarren, Top. Organomet. Chem., 2013, 43, 291 CrossRef; (f) W. Yang and A. S. K. Hashmi, Chem. Soc. Rev., 2014, 43, 2941 RSC; (g) D. Campeau, D. F. León Rayo, A. Mansour, K. Muratov and F. Gagosz, Chem. Rev., 2021, 121, 8756 CrossRef CAS PubMed and references therein; see also: G. L. Hamilton, E. J. Kang, M. Mba and F. D. Toste, Science, 2007, 317, 496 Search PubMed; S. Handa, D. J. Lippincott, D. H. Aue and B. H. Lipshutz, Angew. Chem., Int. Ed., 2014, 53, 10658 Search PubMed; S. Handa, S. S. Subramanium, A. A. Ruch, J. M. Tanski and L. M. Slaughter, Org. Biomol. Chem., 2015, 13, 3936 Search PubMed; J. Zhou, C. Fu and S. Ma, Nat. Commun., 2014, 9, 1654 Search PubMed; R. Ye, A. V. Zhukhovitskiy, R. V. Kazantsev, S. C. Fakra, B. B. Wickermeyer, F. D. Toste and G. A. Somorjai, J. Am. Chem. Soc., 2018, 140, 4144 Search PubMed.
- (a) R. Blieck, M. Taillefer and F. Monnier, Chem. Rev., 2020, 120, 13545 CrossRef CAS PubMed; (b) A. Cadierno, Catalysts, 2020, 10, 1026 CrossRef.
- (a) C. Burstein, C. W. Lehmann and F. Glorius, Tetrahedron, 2005, 61, 6207 CrossRef CAS; (b) M. Alcarazo, S. J. Roseblade, A. R. Cowley, R. Fernández, J. M. Brown and J. M. Lassaletta, J. Am. Chem. Soc., 2005, 127, 3290 CrossRef CAS PubMed; (c) R. J. Roseblade, A. Ros, D. Monge, M. Alcarazo, E. Álvarez, J. M. Lassaletta and R. Fernández, Organometallics, 2007, 26, 2570 CrossRef.
- (a) S. Díez-González, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612 CrossRef PubMed; (b) M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485 CrossRef CAS PubMed; (c) D. Zhang and G. Zi, Chem. Soc. Rev., 2015, 44, 1898 RSC.
- (a) A. Collado, A. Gómez-Suárez, A. R. Martin, A. M. Z. Slawin and S. P. Nolan, Chem. Commun., 2013, 49, 5541 RSC; (b) R. Visbal, A. Laguna and M. C. Gimeno, Chem. Commun., 2013, 49, 5642 RSC.
- Limitation of the reaction scope.
- S. Lauzon, L. Schouwey and T. Ollevier, Org. Lett., 2022, 24, 1116 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2167546–2167553. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc02303b |
| This journal is © The Royal Society of Chemistry 2022 |