Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An iodine-containing probe as a tool for molecular detection in secondary ion mass spectrometry†
Selda
Kabatas Glowacki‡
 ab,
Paola
Agüi-Gonzalez‡
ab,
Paola
Agüi-Gonzalez‡
 ab,
Shama
Sograte-Idrissi
ab,
Shama
Sograte-Idrissi
 ab,
Sebastian
Jähne
ab,
Sebastian
Jähne
 b,
Felipe
Opazo
b,
Felipe
Opazo
 a,
Nhu T. N.
Phan§
a,
Nhu T. N.
Phan§
 ab and
Silvio O.
Rizzoli
ab and
Silvio O.
Rizzoli
 *ab
*ab
aCenter for Biostructural Imaging of Neurodegeneration, University Medical Center Göttingen, Von-Siebold-Straße 3a, 37075 Göttingen, Germany. E-mail: srizzol@gwdg.de
bDepartment of Neuro and Sensory Physiology, University Medical Center, Göttingen, Humboldtalee 23, 37073 Göttingen, Germany
First published on 7th June 2022
Abstract
We developed here an iodine-containing probe that can be used to identify the molecules of interest in secondary ion mass spectrometry (SIMS) by simple immunolabelling procedures. The immunolabelled iodine probe was readily combined with previously-developed SIMS probes carrying fluorine, to generate dual-channel SIMS data. This probe should provide a useful complement to the currently available SIMS probes, thus expanding the scope of this technology.
Mass spectrometry imaging (MSI) was introduced to biology a few decades ago, as an imaging technique able to reveal the chemical composition of tissues and cells, and has improved steadily, reaching very high levels of precision.1–3 To analyse the chemical species on a sample and estimate their abundance, the primary ion beam of a secondary ion mass spectrometer is focused on the surface of the sample, causing the ejection of secondary particles by the impact of the primary ions.4 A broad spectrum of such instruments is available, with the highest lateral resolutions being provided by the NanoSIMS implementation, at 50 nm or lower.5 However, the intrinsic nature of such an instrument implies that only mono- or diatomic molecules are detected. Further chemical information is lost, due to the extensive fragmentation induced by the highly reactive primary ion sources (Cs+/O−).6 This problem also affects other SIMS implementations, such as time-of-flight-SIMS (TOF-SIMS), which can reveal molecules only up to ∼2.5 kDa.3
Thus, labelling particular molecules using SIMS-compatible probes is crucial to detect and visualize larger species, such as proteins. To make this possible, the probes must contain stable elements that are easily ionized, and thus provide a good secondary ion yield. A few different probes fulfilling these requirements have been successfully developed in the last few years. The first investigations employed antibodies linked to heavy metal particles such as gold7 or lanthanides.8 Further efforts were then focused on reducing the size of the probes, to go along with the continuous improvements in the spatial resolution of SIMS. Click-chemistry tags containing fluorine or 15N,9,10 as well as gold nanoparticles- or fluorine-conjugated nanobodies11,12 have also been successfully applied. Boron-containing probes, developed for the positive secondary ion mode of SIMS, have been recently produced and applied.13
However, few multi-targeted proteins SIMS imaging experiments have been performed in the last decades. Due to their low ionization energy,14 lanthanides are suitable for their detection in the positive ion mode, and enable multi-protein analysis,8 albeit the large size of their particles limits labelling efficiency. Fluorine or gold are better candidates for the negative ion mode and could be used, in principle, to simultaneously reveal the localization of two proteins of interest with SIMS, but such experiments have not yet been performed. Iodine is the element with the fourth highest electron affinity, after fluorine, chlorine and bromine.15 Thus, this element represents an excellent choice as SIMS reporter for the negative ion mode and would enable a straightforward strategy for dual-targeted proteins SIMS imaging in the negative secondary ion mode, when combined with another compatible probe. We therefore decided here to develop iodine-conjugated probes for SIMS imaging.
Initially we focused on a precursor molecule with a structure similar to the pentafluorobenzoyl groups in FluorLink, which we developed for NanoSIMS use in the past.11 However, approaches with pentaiodo-benzoic acid or with reduced iodine content using tetraiodophthalic anhydride and triiodobenzoic acid did not lead to the desired products when coupled to a short peptide at its N-terminus. We had determined iodine loss by ESI-HRMS, which can be explained simply by the lower bond dissociation energy of iodine–carbon bonds and their instability under non-inert, oxidative conditions. We therefore searched for iodinated compounds in natural systems and chose diiodo-L-tyrosine, the precursor compound for the synthesis of L-thyroxine, a thyroid hormone essential for vertebrates.16
The synthesis was similar to previously published methods,9–11 beginning with amino acid coupling on a solid support (ESI,† Section S3). The peptide was synthesized from the C- to the N-terminus on an acid-sensitive Sieber amide resin using standard fluorenylmethyloxycarbonyl solid-phase peptide synthesis (Fmoc-SPPS). After coupling of the last amino acid, Fmoc-Lys(Fmoc)-OH, the Fmoc groups were deprotected by basic treatment. Then the commercially available N,O-diacetyl-3,5-diiodo-L-tyrosine was linked to the two amino groups of the N-terminal lysine. The final peptide was cleaved from the solid support under acidic conditions. In the next step, the fluorophore Star635 maleimide was coupled to the cysteine side chain by a sulfhydryl-maleimide reaction. Finally, a maleimide linker was added to the lysine side chain to generate IodLink with 4x127I atoms per molecule (Fig. 1A). Each reaction after solid support was analyzed by HPLC and verified by HR-ESI-MS with no iodine loss observed (ESI,† Section S7). The stability of the iodinated peptide was also confirmed in MALDI-ToF measurements applying different laser powers (ESI,† Section S7). After identification, the products of each step were purified by HPLC to achieve purities of ≥95%. IodLink was then conjugated to a secondary nanobody directed against the kappa (κ) light chain of mouse immunoglobulins (κIg), enabling it to be used in immunocytochemistry experiments (ESI,† Section S4) (Fig. 1B). The selected nanobody has two ectopic cysteines that react with IodLink molecules in a sulfydryl-maleimide reaction, resulting in a doubling of the amount of 127I per detected target of interest.
The probe is thereby designed in a fashion that enables it to be detected both in SIMS and in fluorescence microscopy, to enable users to optimize their sample labelling using fluorescence imaging, before turning to the more laborious SIMS experiments.
After accomplishing the nanoprobe conjugation, we performed tests to optimize the labelling with IodLink-nanobody anti-mouse κlg. For this, we applied immunostainings on COS-7 cells with a mouse anti-α-tubulin as primary antibody and varying concentrations of IodLink-nanobody (ESI,† Fig. S2). We found that 20 nM IodLink-nanobody provides an adequate signal while maintaining a lower background level.
After demonstrating the nanoprobe's detectability and specificity by fluorescence microscopy, we turned to immunostaining on COS-7 cells, repeating the same approach as for α-tubulin but targeting the peroxisomal PMP70 protein, which is known to provide a very clear and easily recognized pattern in SIMS microscopy.11 To increase the signal intensity to the maximum possible, we employed a signal enhancement procedure in which we immunostained the cells with a rabbit polyclonal antibody for PMP70, followed by a secondary goat anti-rabbit antibody, and finally a mouse anti-goat antibody, to which the IodLink-nanobody anti-mouse κlg was bound (Fig. 1C). The samples were then embedded in resin, sliced to a thickness of 200 nm and imaged in NanoSIMS. To obtain negative secondary ions, we selected 133Cs+ as primary ion source and set several detectors to collect 12C14N−, 32S−, and 127I− respectively (Fig. 2). 12C14N− is the most common ion employed to visualize biological samples with NanoSIMS, providing an accurate image of nitrogen-rich cellular material such as proteins or nucleic acids.17
We also included 32S− to obtain additional information and facilitate the location of the cellular nuclei, due to its high abundance in structures such as the heterochromatin or nucleoli.18,19 Finally, we collected 127I− ion signal to locate and visualize our proteins of interest labelled with IodLink-nanobody anti-mouse κlg (Fig. 2).
The iodine signal reproduces the typical distribution of the peroxisomes, showing a significantly higher signal in small dotted regions that are exclusively found in the cytoplasm, never penetrating the nucleus (Fig. 2). The unspecific signal obtained in negative control samples was negligible (Fig. 2 and ESI,† Fig. S3), suggesting that the IodLink-nanobody provides high specific labeling and low background signal for NanoSIMS imaging.
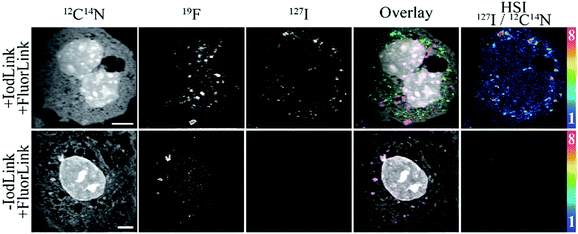
To further test the possibilities of this new nanoprobe, we performed an immunostaining for two proteins of interest, by combining IodLink-nanobody anti-mouse κlg with the FluorLink-nanobodies that we developed in the past.11 The mass dispersion range offered by the NanoSIMS 50L instrument allows the simultaneous detection of 127I− and other mass peaks, such as 12C14N− and 19F−, which enabled us to analyse the mitochondrial marker (TOM70) linked to a green fluorescent protein (GFP) on COS-7 cells, using FluorLink-nanobodies anti-GFP #1 and #2, while we employed IodLink-nanobody to label the peroxisomal marker PMP70 (Fig. 3). Both probes could be introduced simultaneously through a single immunolabelling incubation step. The resulting negative ion mode NanoSIMS images showed that this procedure determines with relative ease the distribution of two proteins of interest. The regions enriched with 127I and 19F indicated that there were no interferences between FluorLink and IodLink probes as they showed typical patterns for mitochondrial and peroxisomal markers, respectively (Fig. 3).
The probes seem to be highly specific, but one should still take into account the fact that IodLink contains 3 fluorine atoms delivered from the fluorophore Star635. The doubling of IodLink after coupling to the nanobody's two ectopic cysteines results in 6 fluorine atoms, which in principle could interfere with our capacity to distinguish the location of two proteins of interest if combined with FluorLink-nanobodies which deliver 52xF per target. To check this, we tested the correlation level between 127I− and 19F− within the cells, using the correlation between 32S− and 12C14N− as an internal reference (ESI,† Fig. S4). With this analysis, we observed the expected a strong correlation between 32S− and 12C14N−, and a very weak, not significant correlation between 127I− and 19F−. These results demonstrate that the signal obtained from IodLink is clearly distinguishable from the FluorLink. This is in line with our previous observations, when we noticed that NanoSIMS imaging cannot solely rely on the fluorine atoms of the fluorophore Star635 to reveal the localization of proteins of interest, due to its low number of F atoms.
These experiments imply that iodine-based SIMS imaging is feasible, and can also be employed in multi-targeted proteins imaging with NanoSIMS by combining it with other elements, detectable in negative ion mode, such as fluorine. In comparison to other elements, iodine offers a very sparse endogenous presence within biological samples. Fluorine, for example, is a prominent element in the production of detergents and plastics, including the common tubes and reagent holders used in biomedical sciences.20 This implies that the background level for iodine tends to be much lower than for fluorine, as we can appreciate in the double staining images, in which a low fluorine signal can be observed throughout the samples.
This work demonstrates the synthesis of IodLink, a non-radioactive, stable iodine-containing probe, which can be conjugated to different surfaces or proteins bearing an accessible thiol group. To show its applicability, we successfully conjugated IodLink to an anti-mouse-nanobody, indicating the feasibility of labelling specific proteins of interest, as well as the visualization and location of such proteins by both fluorescence microscopy and SIMS.
In our work, we also demonstrated that IodLink-nanobody was effective for specific protein imaging with NanoSIMS at subcellular resolution due to its high specificity and high signal-to-noise ratio, even in dual-protein targeted imaging. Future experiments combining additional probes, also detectable on the negative secondary ion mode of SIMS, such as gold-conjugated nanobodies, could further increase the number of proteins simultaneously visualized by SIMS. In the same manner, all of these probes are compatible with the use of other isotopes such as 13C, 15N or 18O, to study in parallel different cellular metabolic processes, such as the turnover of proteins or lipids.21–24 Importantly, IodLink could also be detected by other SIMS instruments such as ToF-SIMS. Likewise, IodLink could be tested on other imaging techniques such as X-ray scattering, implying that this probe, while only showcased here for its application to NanoSIMS, should enable substantially more applications in the future.
S. K. G. and S. O. R. conceptualized the project. S. K. G. performed all chemistry work. S. S. I. performed the immunolabelling experiments and fluorescence imaging. F. O provided materials and supervised nanobody conjugation and their applications in IF. S. J. performed the plastic embedding. P. A. G. performed the SIMS imaging and N. T. N. P. supervised all SIMS work. P. A. G. and S. O. R. analysed the data. S. K. G., P. A. G. and S. O. R. wrote the initial draft.
We thank Prof. Dr Ulf Diederichsen (Institute of Organic and Biomolecular Chemistry, University of Göttingen, Germany) for the generous support by using his infrastructure. We also acknowledge the service department of the faculty of chemistry (University Göttingen, Germany), especially Dr. Holm Frauendorf and his team for the mass spectrometric measurements. This work was supported by grants to S. O. R. from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG SFB1286/A03, RI 1967/7-3, RI 1967/11-1) and from the Nieders. Vorab (76251-12-6/19/ZN 3458). Also supported under Germany's Excellence Strategy-EXC 2067/1-390729940. Silvio O. Rizzoli and Felipe Opazo have received compensation as consultants of NanoTag Biotechnologies GmbH and own stocks in the company.
Conflicts of interest
The other authors declare no competing interests.Notes and references
- M. J. Taylor, J. K. Lukowski and C. R. Anderton, J. Am. Soc. Mass Spectrom., 2021, 32, 872–894 CrossRef CAS PubMed.
- A. P. Bowman, R. M. A. Heeren and S. R. Ellis, TrAC, Trends Anal. Chem., 2019, 120, 115197 CrossRef.
- P. Agüi-Gonzalez, S. Jähne and N. T. N. Phan, J. Anal. At. Spectrom., 2019, 34, 1355–1368 RSC.
- S. G. Boxer, M. L. Kraft and P. K. Weber, Annu. Rev. Biophys., 2009, 38, 53–74 CrossRef CAS PubMed.
- J. Malherbe, F. Penen, M.-P. Isaure, J. Frank, G. Hause, D. Dobritzsch, E. Gontier, F. Horréard, F. Hillion and D. Schaumlöffel, Anal. Chem., 2016, 88, 7130–7136 CrossRef CAS PubMed.
- B. L. Gorman and M. L. Kraft, Anal. Chem., 2020, 92, 1645–1652 CrossRef CAS PubMed.
- R. L. Wilson, J. F. Frisz, W. P. Hanafin, K. J. Carpenter, I. D. Hutcheon, P. K. Weber and M. L. Kraft, Bioconjug. Chem., 2012, 23, 450–460 CrossRef CAS PubMed.
- M. Angelo, S. C. Bendall, R. Finck, M. B. Hale, C. Hitzman, A. D. Borowsky, R. M. Levenson, J. B. Lowe, S. D. Liu, S. Zhao, Y. Natkunam and G. P. Nolan, Nat. Med., 2014, 20, 436–442 CrossRef CAS PubMed.
- I. C. Vreja, S. Kabatas, S. K. Saka, K. Kröhnert, C. Höschen, F. Opazo, U. Diederichsen and S. O. Rizzoli, Angew. Chem., Int. Ed., 2015, 54, 5784–5788 CrossRef CAS PubMed.
- S. Kabatas, I. C. Vreja, S. K. Saka, C. Höschen, K. Kröhnert, F. Opazo, S. O. Rizzoli and U. Diederichsen, Chem. Commun., 2015, 51, 13221–13224 RSC.
- S. Kabatas, P. Agüi-Gonzalez, R. Hinrichs, S. Jähne, F. Opazo, U. Diederichsen, S. O. Rizzoli and N. T. N. Phan, J. Anal. At. Spectrom., 2019, 34, 1083–1087 RSC.
- P. Agüi-Gonzalez, T. M. Dankovich, S. O. Rizzoli and N. T. N. Phan, Nanomaterials, 2021, 11, 1797 CrossRef PubMed.
- S. Kabatas, P. Agüi-Gonzalez, K. Saal, S. Jähne, F. Opazo, S. O. Rizzoli and N. T. N. Phan, Angew. Chem., Int. Ed., 2019, 58, 3438–3443 CrossRef CAS PubMed.
- P. F. Lang and B. C. Smith, J. Chem. Educ., 2010, 87, 875–881 CrossRef CAS.
- T. Andersen, H. K. Haugen and H. Hotop, J. Phys. Chem. Ref. Data, 1999, 28, 1511–1533 CrossRef CAS.
- C. E. Citterio, H. M. Targovnik and P. Arvan, Nat. Rev. Endocrinol., 2019, 15, 323–338 CrossRef CAS PubMed.
- M. L. Steinhauser and C. P. Lechene, Semin. Cell Dev. Biol., 2013, 24, 661–667 CrossRef CAS PubMed.
- A. A. Legin, A. Schintlmeister, M. A. Jakupec, M. Galanski, I. Lichtscheidl, M. Wagner and B. K. Keppler, Chem. Sci., 2014, 5, 3135–3143 RSC.
- F. Lange, P. Agüi-Gonzalez, D. Riedel, N. T. N. Phan, S. Jakobs and S. O. Rizzoli, PLoS One, 2021, 16, e0240768 CrossRef CAS PubMed.
- S. Banerjee, Handbook of Specialty Fluorinated Polymers, Elsevier, 2015 Search PubMed.
- S. K. Saka, A. Vogts, K. Kröhnert, F. Hillion, S. O. Rizzoli and J. T. Wessels, Nat. Commun., 2014, 5, 3664 CrossRef PubMed.
- S. Jähne, F. Mikulasch, H. G. H. Heuer, S. Truckenbrodt, P. Agüi-Gonzalez, K. Grewe, A. Vogts, S. O. Rizzoli and V. Priesemann, Cell Rep., 2021, 34, 108841 CrossRef PubMed.
- M. L. Kraft and H. A. Klitzing, Biochim. Biophys. Acta, 2014, 1841, 1108–1119 CrossRef CAS PubMed.
- C. He, X. Hu, R. S. Jung, T. A. Weston, N. P. Sandoval, P. Tontonoz, M. R. Kilburn, L. G. Fong, S. G. Young and H. Jiang, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 2000–2005 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc02290g |
| ‡ Both authors contributed equally to this manuscript. |
| § Current address: Department of Chemistry and Molecular Biology, University of Gothenburg, Kemivägen 10, SE-412 96 Gothenburg, Sweden. |
| This journal is © The Royal Society of Chemistry 2022 |