Cp*Ir(iii) and Cp*Rh(iii)-catalyzed annulation of salicylaldehydes with fluorinated vinyl tosylates†
Abstract
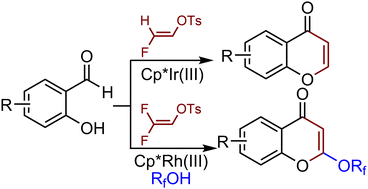
A mild, selective and redox-neutral Cp*Ir(III)- and Cp*Rh(III)-catalyzed C–H activation/annulation of salicylaldehydes with fluorovinyl tosylates is reported. The use of monofluorovinyl tosylate favors the synthesis of C2- and C3-substitution-free chromones via C–H activation/β-F elimination/annulation, whereas difluorovinyl tosylate leads to the construction of C2-fluoroalkoxy chromones. Mild reaction conditions and good functional-group tolerance were observed. Further functionalization of the resulting chromones via halogenation, alkynylation, alkylation and hydrocyanation was successfully realized.


 Please wait while we load your content...
Please wait while we load your content...