Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Accelerating quantitative 13C NMR spectra using an EXtended ACquisition Time (EXACT) method†
Zahra H.
Al-Aasmi
 a,
Alexandra
Shchukina
b and
Craig P.
Butts
a,
Alexandra
Shchukina
b and
Craig P.
Butts
 *a
*a
aSchool of Chemistry, University of Bristol, Cantocks Close, Bristol, BS8 1TS, UK. E-mail: craig.butts@bristol.ac.uk
bFaculty of Chemistry, University of Warsaw, Pastera 1, Warsaw, Poland
First published on 8th June 2022
Abstract
Accurate quantitative 13C NMR spectra can be accelerated by using EXACT (EXtended ACquisition Time) NMR methods which reduce Nuclear Overhauser Enhancement (NOE) during the FID. This allows 30–50% shorter experiment times to be used when achieving a given level of quantitative accuracy.
One of the key strengths of NMR spectroscopy is its inherent quantitation, as the intensity of a signal for a given nuclear environment in a molecule corresponds to the number of nuclei that occupy that chemical environment. This is widely exploited in 1H NMR spectroscopy for the characterisation or identification of organic molecules by comparison of the relative signal intensities for all proton environments in a sample. By extension, 1H NMR spectroscopy can also be used to quantify the composition of mixtures by comparing the signal intensities observed in the spectrum to those of some standard (either external or internal to the sample/spectrometer)1 with quantitative NMR informing research in numerous settings such as food analysis,2 authentication of natural products,3 and pharmaceutical analysis.4 A key limiting feature of these analyses is that the inter-scan relaxation delay must be sufficiently long to allow complete (or at least equal) relaxation of all signals of interest in a sample to equilibrium before the sample is excited by another radio frequency pulse in the next scan. This means that, commonly, quantitative 1H NMR spectra are run with ∼30 second relaxation delays corresponding to 5–7 times T1 (typical T1 values being <5 s) or with lower ‘flip angles’ which reduce the % of spin populations perturbed by the excitation pulse and thus lead to faster relaxation of the spins to equilibrium. The abundance and inherent sensitivity of the 1H nucleus means that practical access to quantitative NMR spectra can normally be achieved in a few scans, and thus only a few minutes of experiment time.
On the other hand, for non-1H techniques such as 13C, several hundred scans (and thus hours of experiment time) are often required to achieve adequate signal-to-noise data for accurate quantitation using such long relaxation delays. While reducing the flip angle of the excitation pulse, or ideally optimising it to the Ernst angle,5 can somewhat mitigate the relaxation time very long relaxation delays are still needed for such spectra (let alone the need to run multiple experiments if one were to establish the optimal flip angle). Despite this, 13C and similar NMR-active nuclei have several benefits such as a wide spectral range (about 200 ppm) and minimal peak overlap which can aid in quantitative analysis of complex mixtures, so there is value in achieving quantitative data in a minimum amount of time using such nuclei.
Quantitation in non-1H NMR methods is further complicated by the impact of differing levels of Nuclear Overhauser Enhancement (NOE) for each measured signal. The NOE arises from any (usually 1H) spin decoupling applied during the NMR experiment. This is commonly reduced by gating off the spin decoupling during the long relaxation delay, in an approach referred to as ‘inverse gated’ decoupling, Fig. 1a. Giraudeau and Baguet6 have further demonstrated that measurement of NOE contributions combined with longitudinal relaxation times can be used to optimise the time of quantitative 13C spectra for molecules containing carbons with different relaxation properties. However, this measurement is time-consuming in itself and in any case the NOE for each signal still builds up to some extent during the acquisition period of the FID where spin decoupling cannot be gated off. Indeed, in our experience, high accuracy quantitation in proton-decoupled 13C NMR spectra (and proton-decoupled spectra of other NMR-active nuclei) is often limited by the need to relax the NOE effect, more than the T1 relaxation of the heteronucleus itself. This can be imperfectly addressed by simply shortening the FID length (and thus reducing the amount of time that spin decoupling is applied). However, this results in substantially broader lines after Fourier Transform of the truncated time domain data, and in extreme cases, ‘sync wiggle’ truncation artifacts at the base of every peak in the spectrum, reducing the quantitative accuracy in the spectrum.
We demonstrate here that NOE contributions can instead be reduced during quantitative 13C NMR measurements by using our recently reported EXACT (EXtended ACquisition Time)7–9 NMR method where, after initial excitation, the FID is measured in short (∼10–100 ms) 1H-decoupled chunks of data separated by periods where both the receiver and decoupler are switched off. By switching off the decoupler at regular intervals during the FID, the NOE build-up is repeatedly interrupted, leading an FID with less contribution from the NOE. This allows shorter relaxation delays to be used and thus quantitative 13C spectra can be acquired around 30–50% faster for a given desired level of quantitative accuracy.
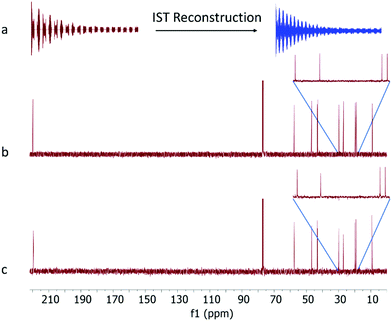
The EXACT NMR pulse sequence used to acquire quantitative 13C spectra, with receiver and decoupling gating in the acquisition block, is shown in Fig. 1b (the Varian-format pulse sequence used here is available in the ESI†). The initial excitation 90°x pulse is followed by a data chunk acquisition (for a time Δc) and then a ‘gap’ period (for a time Δg) where the receiver is switched off and zero intensity datapoints are acquired on the observed 13C channel. On the decoupler 1H channel, standard WALTZ-16 composite pulse decoupling (CPD) is applied during acquired data chunks (Δc, 24–100 ms were used here) but is gated off during the ‘gaps’ (Δg, 24–100 ms were used here) and replaced by a J-refocusing spin-echo (Δg/2 – 180° – Δg/2) period on the 1H channel to refocus the JCH-coupling evolution while allowing the 13C chemical shift to continue to evolve. This ‘acquisition/gap’ pattern is then repeated until the end of the FID. Phase cycling of the 180°-degree pulses (180y/180−y) between the spin-echo periods gives more robust decoupling. By restricting the 1H decoupling to the CPD periods only, the NOE will build up during the decoupled chunks and then decay again during the gated gaps between the CPD period. An example of a 50% regularly sampled EXACT FID (Δc = 48 ms, Δg = 48 ms) acquired on a sample of Camphor in CDCl3 is shown in Fig. 2a (left). As we have described previously7 the missing data in the gap periods of the FID can reliably be regenerated by Iterative Soft Thresholding (IST)10–12 reconstruction to give a complete FID (Fig. 2a, right). IST reconstruction is based on the assumption of spectrum sparsity, i.e. that peaks occupy only a minor proportion of the frequency range, hence it is especially applicable to sparse data such as 13C NMR spectra. It is an algorithm of a well-known compressed sensing (CS) family applied to conventional non-uniform sampling (NUS) in NMR. The peculiarity of EXACT as an NUS method is in the relative regularity of the sampling scheme, however the challenges that arise, and the applicability of IST to the reconstruction of EXACT data, have been highlighted before.13
 | ||
| Fig. 1 (a) The inverse gated pulse sequence, (b) the EXACT pulse sequence used to accelerate the acquisition of quantitative 13C spectra. | ||
Fourier transform of this reconstructed FID gives a frequency spectrum (Fig. 2c) which shows all the peaks expected for Camphor with negligible reconstruction artefacts visible above the baseline. This spectrum compares well with the equivalent standard inverse-gated 13C experiment run for the same number of scans (Fig. 2b). It is notable that the signal-to-noise ratio of both spectra are similar as IST reconstruction is generally a non-linear procedure, meaning that higher peaks are reproduced most precisely in a reconstructed spectrum, while lower ones can be decreased.13 Noise, however, being the lowest, is likely to be decreased most intensively (depending on many factors, such as the sampling type, the reconstruction algorithm convergence point and the input parameters of the reconstructed algorithm, e.g. sparsity vs. data agreement trade-off term). This can even lead to sensitivity enhancement for NUS spectra in favourable conditions (where relaxation matched sampling schemes are used) or leave the sensitivity similar in comparison to fully measured spectra.14–16 Here, we deal with the latter case, as the sampling scheme is evenly distributed across the FID. Similarly, the underlying spectral resolution is not changed by EXACT, as it is not by other types of NUS. There is a probability that the undersampling, and subsequent reconstruction, will improve it if we allow for extrapolation,12 but otherwise it has no effect.
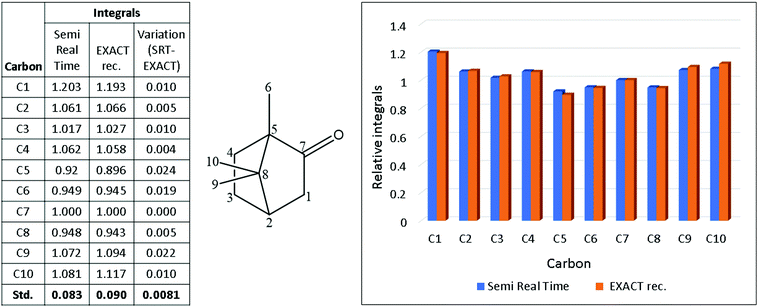
To explore the quantitative reliability of the reconstruction method, the reconstructed EXACT data were compared to those acquired with a semi-real time (SRT) approach.17 In the semi-real time method, the gaps in the EXACT FID are filled with data sampled in a second complementary EXACT experiment (Fig. 3a and b). Summing these two complementary EXACT datasets gives rise to a complete 13C FID (Fig. 3c) which does not require algorithmic reconstruction but arises from the same underlying pulse sequence as the reconstructed EXACT spectrum. Comparison of the integrals for each peak in the reconstructed EXACT and SRT spectra (Fig. 4), relative to a standardised 1.00 integral for C7, shows that the standard deviation between the two datasets is 0.8%, suggesting that the reconstruction method is, at the least, reliable to <1% accuracy when applied to this 50% regularly sampled FID with a 40 s relaxation delay (rd). Indeed, in this relatively weak spectrum, the 0.8% variation is likely dominated by noise contributions to the measured integrals rather than reconstruction artefacts.
 | ||
| Fig. 3 (a and b) Complimentary 50% regularly sampled EXACT FIDs. (c) The FID resulting from adding (a) and (b). | ||
Next, the quantitative value of reconstructed EXACT 13C NMR spectra was compared directly to inverse-gated data. 50% Regularly sampled EXACT experiments were acquired with relaxation delays of 10 s, 20 s 30 s, 40 s, and 60 s (Fig. 5). In every case, the EXACT spectra achieve lower standard deviations for their integrals than the inverse-gated experiment using the same relaxation delay (a standard deviation of 0 suggests every peak has an identical integral value). For example, standard deviations of <0.1 in normalised integrals were obtained with 50% sampled EXACT acquisition using a 40 second relaxation delay (Fig. 5, orange line, 14 minute total experiment time) but required a 60 second relaxation delay in an inverse-gated experiment (Fig. 5, blue line, 20 minute total experiment time) leading to a significant time-saving when a large number of scans are needed for the low sensitivity 13C nucleus. These data suggest that gating the decoupling off during gaps in the EXACT FID does indeed lead to a reduction in the NOE build-up during the FID and thus allows shorter relaxation delays to be used in quantitative 13C NMR experiments. Further data were acquired for DEET (diethyl meta toluamide) and strychnine in CDCl3 using the same methods (see ESI,† Fig. S1 and S2) and in both cases, spectra of similar quantitative value were obtained with EXACT 13C NMR spectra using shorter relaxation delays than the inverse-gated spectra.
In principle, the contribution of the NOE can be further reduced by extending the length of the gaps (where decoupling is gated off) relative to the acquired chunks (where decoupling must be applied). However, this reduces the sampling density of the FID which makes it more challenging to accurately reconstruct the NMR spectrum with the IST algorithm. In practice we found that reducing sampling density from 50% to 33.3% did indeed slightly reduce the NOE contribution to the integrals when using relatively short (10 s and 20 s) relaxation delays (Fig. 5, grey).
However, the accuracy achievable with the 33% sampling density appears to be limited with longer relaxation delays (30 s, 40 s and 60 s), as the reliability of the IST reconstruction becomes more of a factor. Consequently, with the reconstruction algorithms to hand we suggest that 50% EXACT sampling densities are optimal for acquiring quantitative 13C spectra while minimising relaxation delays and thus overall experiment times.
Naturally, compensating for NOE and relaxation within a molecule should also correct for differences in these behaviours between molecules in a mixture. To confirm this, a mixture of strychnine, camphor and DEET (0.162![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.000
1.000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.756 molar ratio) was analysed using EXACT acquisition with a 40 s relaxation delay. After IST reconstruction they provided an experimental ratio of 0.158
1.756 molar ratio) was analysed using EXACT acquisition with a 40 s relaxation delay. After IST reconstruction they provided an experimental ratio of 0.158![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.992
0.992![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.712 in signal intensities, which matched well with the expected values as well as those obtained with inverse-gated decoupling using a 60 s relaxation delay (0.159
1.712 in signal intensities, which matched well with the expected values as well as those obtained with inverse-gated decoupling using a 60 s relaxation delay (0.159![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.983
0.983![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.723) and SRT using a 40 s relaxation delay (see Section S5 of the ESI†).
1.723) and SRT using a 40 s relaxation delay (see Section S5 of the ESI†).
In summary, we have demonstrated that the NOE arising from heteronuclear spin decoupling in quantitative NMR spectra can be reduced by using an EXACT NMR approach i.e., introducing breaks in signal acquisition simultaneous to gating off heteronuclear decoupling during an FID, followed by IST reconstruction of the missing signal. This reduced NOE contribution in turn allows shorter relaxation delays, and thus experiment times, to be used while achieving similar spectrum quality and quantitative accuracy. We would note that the approach should also be applicable to other heteronuclear NMR nuclei where 1H-decoupling perturbs the intensities through NOE build-up e.g., 31P, 29Si, 15N, etc. From the data presented here, EXACT 13C spectra can provide a desired level of quantitative accuracy in just over half the experiment time of the corresponding inverse gated experiment.
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. K. Bharti and R. Roy, Trends Anal. Chem., 2012, 35, 5–26 CrossRef CAS.
- E. Hatzakis, Compr. Rev. Food Sci. Food Saf., 2019, 18, 189–220 CrossRef PubMed.
- Z. F. Wang, Y. L. You, F. F. Li, W. R. Kong and S. Q. Wang, Molecules, 2021, 26, 6308 CrossRef CAS PubMed.
- A. Khalil and M. Kashif, Crit. Rev. Anal. Chem., 2021, 9, 1–15 Search PubMed.
- R. R. Ernst and W. A. Anderson, Rev. Sci. Instrum., 1966, 37, 93–102 CrossRef CAS.
- P. Giraudeau and E. Baguet, J. Magn. Reson., 2006, 180, 110–117 CrossRef CAS PubMed.
- I. E. Ndukwe, A. Shchukina, K. Kazimierczuk, C. Cobas and C. P. Butts, Chem. Phys. Chem., 2016, 17, 2799–2803 CrossRef CAS PubMed.
- I. E. Ndukwe, A. Shchukina, K. Kazimierczuk, C. Cobas and C. P. Butts, Chem. Commun., 2016, 52, 12769–12772 RSC.
- I. E. Ndukwe, A. Shchukina, A. Zorin, C. Cobas, K. Kazimierczuk and C. P. Butts, Chem. Phys. Chem., 2017, 18, 2081–2087 CrossRef CAS PubMed.
- K. Kazimierczuk and V. Y. Orekhov, Angew. Chem., Int. Ed., 2011, 50, 5556–5559 CrossRef CAS PubMed.
- S. Foucart and H. Rauhut, A Mathematical Introduction to Compressive Sensing, Birkhäuser, New York, NY, 2013, pp. 1–39 Search PubMed.
- A. Shchukina, P. Kasprzak, R. Dass, M. Nowakowski and K. Kazimierczuk, J. Biomol. NMR, 2017, 68, 79–98 CrossRef CAS PubMed.
- A. Shchukina, M. Kaźmierczak, P. Kasprzak, M. Davy, G. R. Akien, C. P. Butts and K. Kazimierczuk, Chem. Commun., 2019, 55, 9563–9566 RSC.
- M. R. Palmer, C. L. Suiter, G. E. Henry, J. Rovnyak, J. C. Hoch, T. Polenova and D. Rovnyak, J. Phys. Chem. B, 2015, 119, 6502–6515 CrossRef CAS PubMed.
- M. R. Palmer, B. R. Wenrich, P. H. Stahlfeld and D. Rovnyak, J. Biomol. NMR, 2014, 58, 303–314 CrossRef CAS PubMed.
- K. Kazimierczuk, O. Lafon and P. H. Lesot, Analyst, 2014, 139, 2702–2713 RSC.
- P. Kiraly, M. Nilsson and G. A. Morris, J. Magn. Reson., 2018, 293, 19–27 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methodology, tabulated integral data, and pulse sequence code. See DOI: https://doi.org/10.1039/d2cc01768g |
| This journal is © The Royal Society of Chemistry 2022 |