AI and computational chemistry-accelerated development of an alotaketal analogue with conventional PKC selectivity†
Abstract
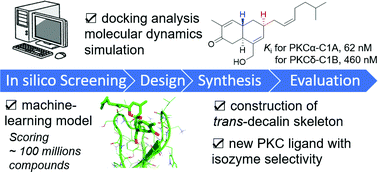
The protein kinase C (PKC) family consists of ten isozymes and is a potential target for treating cancer, Alzheimer's disease, and HIV infection. Since known natural PKC agonists have little selectivity among the PKC isozymes, a new scaffold is needed to develop PKC ligands with remarkable isozyme selectivity. Taking advantage of machine-learning and computational chemistry approaches, we screened the PubChem database to select sesterterpenoids alotaketals as potential PKC ligands, then designed and synthesized alotaketal analogues with a different ring system and stereochemistry from the natural products. The analogue exhibited a one-order higher affinity for PKCα-C1A than for the PKCδ-C1B domain. Thus, this compound is expected to serve as the basis for developing PKC ligands with isozyme selectivity.
- This article is part of the themed collection: 2022 Pioneering Investigators


 Please wait while we load your content...
Please wait while we load your content...