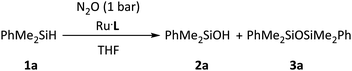
Open Access Article
Open Access ArticleReduction of N2O with hydrosilanes catalysed by RuSNS nanoparticles†
Pablo
Molinillo
a,
Bertrand
Lacroix
 bc,
Florencia
Vattier
d,
Nuria
Rendón
bc,
Florencia
Vattier
d,
Nuria
Rendón
 *a,
Andrés
Suárez
*a,
Andrés
Suárez
 *a and
Patricia
Lara
*a and
Patricia
Lara
 *a
*a
aInstituto de Investigaciones Químicas (IIQ), Departamento de Química Inorgánica, and Centro de Innovación en Química Avanzada (ORFEO-CINQA), CSIC and Universidad de Sevilla. Avda. Américo Vespucio 49, 41092 Sevilla, Spain. E-mail: patricia@iiq.csic.es
bDepartment of Material Science and Metallurgic Engineering, and Inorganic Chemistry, University of Cádiz, Spain
cIMEYMAT: Institute of Research on Electron Microscopy and Materials of the University of Cádiz, Spain
dInstituto de Ciencia de Materiales de Sevilla, CSIC-Universidad de Sevilla. Avda. Américo Vespucio 49, 41092 Sevilla, Spain
First published on 30th May 2022
Abstract
A series of RuSNS nanoparticles, prepared by decomposition of Ru(COD)(COT) with H2 in the presence of an SNS ligand, have been found to catalyse the reduction of the greenhouse gas N2O to N2 employing different hydrosilanes.
Nitrous oxide (N2O) is a potent ozone-depleting, greenhouse gas, with a climate warming impact three hundred times that of carbon dioxide.1,2 The increasing concentration of this gas in the Earth's atmosphere is attributed to human activities involving the use of fertilizers, the large-scale combustion of fossil fuels and biomass, and industrial chemical processes that produce it as a by-product.3 Therefore, there is a significant interest in the development of chemical transformations for the degradation of N2O into non-harmful species,4 as well as for its revalorization as a chemical feedstock in the context of a circular economy.5 A procedure for N2O mitigation consists of the hydrogenation of this molecule to innocuous nitrogen gas (N2) and water, which can be carried out using heterogeneous catalysts operating under relatively harsh conditions.6 Moreover, transition metal complexes have also been recently demonstrated to catalyse the hydrogenation7,8 and the hydroboration9 of N2O.
Although lacking the future prospects of clean, large-scale production of H2 from renewable sources, hydrosilanes are commonly employed as reducing agents in both academic laboratories and industrial settings since reduction of a large diversity of compounds, including small gas molecules such as CO2,10 can be efficiently performed.11 Advantages associated with the use of silanes as reducing agents include their low cost, easy handling and for some derivatives, as in the case of poly(methylhydrosiloxane) (PMHS), their attributed low environmental impact. However, the reduction of N2O with silanes has only been briefly investigated. In 2017, Milstein et al. made use of a Ru-PNP pincer complex in the reduction of N2O with PhMe2SiH, Ph2MeSiH and tBuMe2SiH.7 Reactions were carried out using 1–2 mol% Ru at 65 °C under 3.4 bar of N2O for 36–72 h. Recently, Cantat et al. reported a metal-free reduction of N2O using disilanes.12 It is also worth noting that oxidation of hydrosilanes with N2O leads to the formation of technologically important Si–O containing derivatives, such as silanols and siloxanes, with applications in the synthesis of silicon-based polymeric materials as well as reagents in organic synthesis.13 This process could contribute to the use of waste N2O in the context of a circular economy, and complement current methods for silane oxidation based on the use of H2O, H2O2 and O2.14
Metal nanoparticles (NPs) have been widely employed in a diverse range of catalytic processes due to their particular electronic configurations and much larger surface areas when compared with bulk metals.15 The preparation of NPs through the decomposition of an organometallic precursor with H2 in the presence of substoichiometric amounts of a ligand, as pioneered by Chaudret, Philippot et al., provides materials possessing well-controlled size, shape and surface state.16 Herein, we report on the synthesis and characterization of a series of Ru NPs stabilized with readily available SNS ligands. More interestingly, based on the known reactivity of related NPs in hydrosilylation reactions,17 these nanocatalysts have been tested in the reduction of N2O with hydrosilanes.
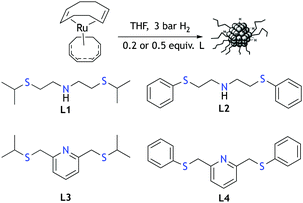
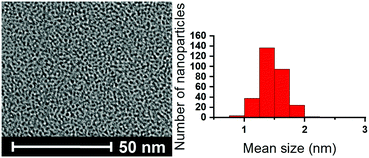
Ru·L NPs were easily synthesized by exposing THF solutions of Ru(COD)(COT), (1,5-cyclooctadiene)(1,3,5-cyclooctatriene)ruthenium(0), to 3 bar of H2 in the presence of the SNS ligands L1–L418 (Scheme 1). Different ligand/metal ratios were explored to obtain well-controlled metal nanoparticles. Thus, a series of colloids were prepared using 0.5 equiv of L1–L4. In all the cases, small and well-dispersed crystalline nanoparticles exhibiting mean sizes between 1.5 and 1.9 nm were obtained, as revealed by TEM (Fig. 1 and Fig. S1–S6, ESI†). The metal content (32–47% Ru) in the nanoparticles was determined by inductive coupled plasma (ICP) analysis of the purified materials. Attempts to obtain monodispersed nanoparticles using lower amounts of ligand, i.e. 0.2 equiv, yielded agglomerated metal, with the exception of L4 that led to the main formation of small and monodispersed nanoparticles (mean size: 2.3 (0.4) nm) along with slight metal agglomeration, as observed on the TEM grid (Fig. S7 and S8, ESI†). As observed previously for Ru NPs, the mean size of the particles slightly decreases upon using higher ligand/metal ratios.19
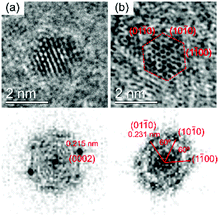
The crystalline character of the Ru·L NPs thus prepared is clearly demonstrated by HRTEM observations for Ru·L20.5 (Fig. S11, ESI†) and Ru·L40.5 (Fig. 2). The fast Fourier transform (FFT) analysis of the micrographs shows interplanar spacings and angles that correspond to a ruthenium hexagonal close-packed (hcp) structure. In addition, the observation of some NPs along the [10−10] and [0001] zone axis clearly reveals the presence of (0001), {01−11} and {01−10} crystal facets.
For samples Ru·L20.5 and Ru·L40.5, the Ru composition of the NPs was confirmed by the EDX spectra and elemental map (Fig. S12 and Fig. 3, respectively). EDX also reveals (more particularly for the Ru·L20.5 sample) the presence of S expected to come from the SNS ligands, and O that could indicate oxidation of the NP surface. Although mostly detected in the NP regions, S and O signals are also spotted in between the NPs which could be explained by decomposition under the electron beam.
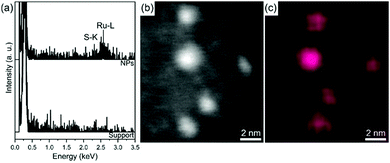 | ||
| Fig. 3 STEM-EDX results for the Ru·L40.5 NPs: (a) EDX spectra recorded over various NPs and over the support (for reference). (b) STEM-HAADF image. (c) Ru·L40.5 intensity map using the Ru–L line. | ||
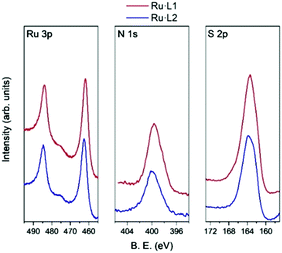
The nature and composition of the Ru·L nanoparticle surfaces were analysed using XPS (see ESI†). As is well known, the main photoemission peak for ruthenium atoms is the Ru3d signal, although this peak is very close and partially overlaps with the C1s peak. For this reason, the Ru3p photoemission signal is preferred for analysis.20Fig. 4 shows the high-resolution spectra for the Ru3p, N1s, and S2p regions for the Ru·L10.5 and Ru·L20.5 nanoparticles. For the Ru·L10.5 and Ru·L20.5 samples, high-resolution N1s peaks were found around 400 eV in binding energy (BE) and the S2p photoemission signal shows a wide peak centered at 163.5 eV. The Ru3p signal of Ru·L10.5 exhibits two peaks centered at 462.1 and 484.2 eV BE corresponding to 3p3/2 and 3p1/2 photoemission peaks, respectively. Ru·L20.5 shows these peaks at 462.5 and 484.6 eV BE. As shown in Fig. 5a, the high-resolution Ru 3p3/2 region for Ru·L10.5 is well-fitted with two components at 461.9 and 465.1 eV, corresponding to the Ru(0) and Ru(IV) oxidation states, respectively.20,21 Likewise, the Ru·L20.5 species shows the Ru(0) 3p3/2 signal at 462.3 eV, and at 465.7 eV for Ru(IV) (Fig. 5b). The surface oxidation ratio of the nanoparticles Ru·L10.5 and Ru·L20.5 was found to be very similar, i.e. 9% of Ru(IV). Finally, the separation between the major peaks due to spin–orbit splitting took a value of 22.3 eV, in both analyzed species. These results are in agreement with those previously observed in similar size Ru(0) nanoparticles.22
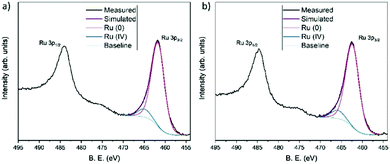 | ||
| Fig. 5 Experimental and fitted XPS spectra of the Ru3p3/2 regions for samples Ru·L10.5 (a) and Ru·L20.5 (b). | ||
The quantification of Ru, N, and S atoms on the surface of the nanoparticles was estimated by means of the relative intensities of the corresponding photoemission signals (Table 1). The N/S ratio is similar and very close to that expected for the stoichiometry of both ligands. This fact enables us to rule out adverse effects during the XPS analysis, such as fragmentation or decomposition of organic compounds. The value of the Ru/N ratio can be correlated to the degree of coverage of the nanoparticle surface by the N-coordinating ligands. Moreover, it could depend on the size of the Ru nanoparticles, although in these cases we have found very similar sizes as determined by TEM. Ru·L20.5 is less covered than Ru·L10.5, i.e. a higher Ru/N ratio, and this could explain its higher observed catalytic activity since it leaves more metal exposed to the reactants (vide infra).
| Ru·L | Ru (% At) | N (% At) | S (% At) | N/S ratio | Ru/N ratio |
|---|---|---|---|---|---|
| Ru·L10.5 | 31.6 | 21.8 | 45.3 | 0.48 | 1.5 |
| Ru·L20.5 | 37.8 | 19.7 | 42.5 | 0.46 | 1.9 |
The SNS-stabilized Ru nanoparticles were tested in the reduction of N2O with silanes. Initial experiments were performed using 1.0 mol% of Ru at 55 °C under 1 bar of N2O, employing PhMe2SiH (1a) (Table 2). While the nanoparticles synthetic precursor Ru(COD)(COT) provided a low silane conversion (entry 1), the reaction with Ru·L10.5 took place with 76% conversion (based on silane), leading to a mixture of the corresponding silanol (2a) and siloxane (3a) in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio, respectively (entry 2). Testing of other catalysts prepared using Ru/L ratios of 0.5 led to complete silane conversions with silanol:siloxane ratios ranging between 12
6 ratio, respectively (entry 2). Testing of other catalysts prepared using Ru/L ratios of 0.5 led to complete silane conversions with silanol:siloxane ratios ranging between 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 88 and 25
88 and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 (entries 3–5); meanwhile, the Ru·L40.2 nanoparticles showed a decreased conversion (entry 6). TEM analysis of the reaction with Ru·L40.5 reveals that the size of the Ru NPs remains practically constant after the catalytic reactions (mean size 1.5 (0.3) nm; Fig. S9 and S10, ESI†).
75 (entries 3–5); meanwhile, the Ru·L40.2 nanoparticles showed a decreased conversion (entry 6). TEM analysis of the reaction with Ru·L40.5 reveals that the size of the Ru NPs remains practically constant after the catalytic reactions (mean size 1.5 (0.3) nm; Fig. S9 and S10, ESI†).
| Entry | Ru cat. | SiH conv. [%] |
2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a ratio 3a ratio |
|---|---|---|---|
| Reaction conditions: 1.0 mol% Ru, 1 bar N2O, 55 °C, THF. Reaction time: 24 h. Conversion and selectivity were determined by 1H NMR spectroscopy using mesitylene as the internal standard. N2 formation was detected by GC-MS analysis of the headspace gas (see ESI). | |||
| 1 | Ru(COD)(COT) | <5 | — |
| 2 | Ru·L10.5 | 76 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
| 3 | Ru·L20.5 | >99 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 88 88 |
| 4 | Ru·L30.5 | >99 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
| 5 | Ru·L40.5 | >99 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
| 6 | Ru·L40.2 | 28 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
Next, other hydrosilanes were tested as reductants using Ru·L40.5 as a representative catalyst (Table 3). Complete silane conversion and a high selectivity towards the formation of the silanol 2b were observed in the reaction with Ph2MeSiH (1b) (entry 1). In marked contrast, the use of dimethylphenethylsilane (1c) led to the opposite product distribution, with the siloxane 3c formed with >99% selectivity. In addition, the reaction of N2O with tripropylsilane (1d) proceeded with a conversion of 95% with the formation of 2d and 3d in a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio, respectively; whereas no conversion was observed upon using bulky iPr3SiH (1e). Finally, the reaction with triethoxysilane (1f) took place with a high conversion leading to the corresponding silanol and siloxane derivatives in a 6
4 ratio, respectively; whereas no conversion was observed upon using bulky iPr3SiH (1e). Finally, the reaction with triethoxysilane (1f) took place with a high conversion leading to the corresponding silanol and siloxane derivatives in a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio (entry 5).
4 ratio (entry 5).
| Entry | Silane | SiH conv. [%] |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio 3 ratio |
|---|---|---|---|
| Reaction conditions, unless otherwise noted: 1.0 mol% Ru, 1 bar N2O, 65 °C, THF. Reaction time: 24 h. Conversion and selectivity were determined by 1H NMR spectroscopy using mesitylene as the internal standard.a Reaction time: 48 h. | |||
| 1 | Ph2MeSiH (1b) | >99 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2a | (PhCH2CH2)Me2SiH (1c) | 98 | >1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
| 3 | n Pr3SiH (1d) | 95 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
| 4 | i Pr3SiH (1e) | 0 | — |
| 5 | (EtO)3SiH (1f) | 98 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
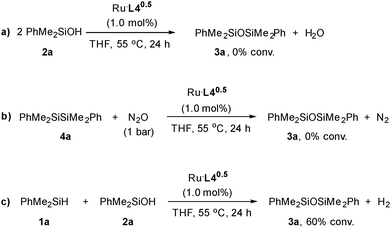
To get insight into the formation of the siloxane derivatives 3, a series of control experiments were performed (Scheme 2). We hypothesized that siloxane formation could take place through: (i) the ruthenium catalysed condensation of two silanol molecules (silanol dehydration),23 (ii) the oxidation of disilane formed through the dehydrogenative coupling of the hydrosilane,24,25 and/or (iii) the dehydrogenative coupling of silanol and silane.26 While no reaction was observed when a solution of PhMe2SiOH (2a) in THF was heated to 55 °C in the presence of Ru·L40.5 (Scheme 2a) or the disilane PhMe2SiSiMe2Ph (4a) was made to react with N2O (Scheme 2b), the reaction of 2a with silane 1a under these conditions proceeded with 60% conversion (Scheme 2c). These results are in agreement with the formation of 3a taking place through a Ru catalysed coupling of silanol and hydrosilane with concomitant H2 release.
In conclusion, a series of narrowly-dispersed Ru nanoparticles stabilized by tridentate SNS ligands have been prepared and characterized. These materials are able to catalyse the reduction of N2O, a relevant harmful greenhouse and ozone-depleting gas, with hydrosilanes under relatively mild reaction conditions (1 bar N2O, 55–65 °C) to yield innocuous N2 and potentially useful Si–O containing derivatives.
The financial support (FEDER contribution) from the Spanish Agencia Estatal de Investigación (PID2019-104159GB-I00/MCIN/AEI/10.13039/501100011033), Junta de Andalucía (P18-FR-3208 and US-1380604) and CSIC (COOPB20604) is gratefully acknowledged. The “Talent Attraction Program” of the University of Cádiz is acknowledged by supporting B. Lacroix contract code E-11-2019-0133241.
Conflicts of interest
There are no conflicts of interest to declare.Notes and references
- M. J. Prather, Science, 1998, 279, 1339 CrossRef CAS PubMed; A. R. Ravishankara, J. S. Daniel and R. W. Portmann, Science, 2009, 326, 123 CrossRef PubMed.
- J. Hansen and M. Sato, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 16109 CrossRef CAS PubMed; H. A. Rodhe, Science, 1990, 248, 1217 CrossRef PubMed; S. A. Montzka, E. J. Dlugokencky and J. H. Butler, Nature, 2011, 476, 43 CrossRef PubMed.
- E. A. Davidson and D. Kanter, Environ. Res. Lett., 2014, 9, 105012 CrossRef.
- M. Jabłońska and R. Palkovits, Catal. Sci. Technol., 2016, 6, 7671 RSC; M. Kjellberg, A. Ohleier, P. Thuéry, E. Nicolas, L. Anthore-Dalion and T. Cantat, Chem. Sci., 2021, 12, 10266 RSC; R. Deeba, F. Molton, S. Chardon-Noblat and C. Costentin, ACS Catal., 2021, 11, 6099 CrossRef CAS; M. Konsolakis, ACS Catal., 2015, 5, 639 Search PubMed; Ø. Nirisen, D. Waller and D. M. Brackenbury, Top. Catal., 2019, 62, 1113 CrossRef.
- For selected examples, see: K. O. Denisova, A. A. Ilyin, R. N. Rumyantsev, A. P. Ilyin and A. V. Volkova, Russ. J. Gen. Chem., 2019, 89, 1338 CrossRef CAS; K. A. Dubkov, G. I. Panov and V. N. Parmon, Russ. Chem. Rev., 2017, 86, 510 CrossRef; K. Severin, Chem. Soc. Rev., 2015, 44, 6375 RSC.
- A. Miyamoto, S. Baba, M. Mori and Y. Murakami, J. Phys. Chem., 1981, 85, 3117 CrossRef CAS; A. C. Gluhoi, M. A. P. Dekkers and B. E. Nieuwenhuys, J. Catal., 2003, 219, 197 CrossRef; G. Delahay, M. Mauvezin, A. Guzmán-Vargas and B. Coq, Catal. Commun., 2002, 3, 385 CrossRef; T. Nobukawa, M. Yoshida, K. Okumura, K. Tomishige and K. Kunimori, J. Catal., 2005, 229, 374 CrossRef; S. A. Carabineiro and B. E. Nieuwenhuys, Surf. Sci., 2001, 495, 1 CrossRef; L. Jacobs, C. Barroo, N. Gilis, S. V. Lambeets, E. Genty and T. Visart de Bocarmé, Appl. Surf. Sci., 2018, 435, 914 CrossRef; X. Huo, D. J. Van Hoomissen, J. Liu, S. Vyas and T. J. Strathmann, Appl. Catal., B, 2017, 211, 188 CrossRef.
- R. Zeng, M. Feller, Y. Ben-David and D. Milstein, J. Am. Chem. Soc., 2017, 139, 5720 CrossRef CAS PubMed.
- T. L. Gianetti, S. P. Annen, G. Santiso-Quinones, M. Reiher, M. Driess and H. Grützmacher, Angew. Chem., Int. Ed., 2016, 55, 1854 CrossRef CAS PubMed.
- X. Chen, H. Wang, S. Du, M. Driess and Z. Mo, Angew. Chem., Int. Ed., 2022, 61, e202114598 CAS.
- R. A. Pramudita and K. Motokura, ChemSusChem, 2021, 14, 281 CrossRef CAS PubMed.
- P. G. Andersson, I. J. Munslow, ed., Modern Reduction Methods, Wiley-VCH; Weinhein, 2008 Search PubMed.
- L. Anthore-Dalion, E. Nicolas and T. Cantat, ACS Catal., 2019, 9, 11563 CrossRef CAS.
- S. E. Denmark and C. S. Regens, Acc. Chem. Res., 2008, 41, 1486 CrossRef CAS PubMed; R. Murugavel, A. Voigt, M. G. Walawalkar and H. W. Roesky, Chem. Rev., 1996, 96, 2205 CrossRef PubMed; R. Murugavel, M. G. Walawalkar, M. Dan, H. W. Roesky and C. N. R. Rao, Acc. Chem. Res., 2004, 37, 763 CrossRef PubMed; F. Guida-Pietrasanta and B. Boutevin, Adv. Polym. Sci., 2005, 179, 1 CrossRef; Y. Abe and T. Gunji, Prog. Polym. Sci., 2004, 29, 149 CrossRef; H.-H. Moretto, M. Schulze and G. Wagner, “Silicones”. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH, 2005 CrossRef; Z. Ren and S. Yan, Prog. Mater. Sci., 2016, 83, 383 CrossRef.
- K. Kucinsky, H. Stachowiak-Dłużyńska and G. Hreczycho, Coord. Chem. Rev., 2022, 459, 214456 CrossRef.
- K. Philippot and A. Rocoux, Nanoparticles in Catalysis: Advances in Synthesis and Applications, Wiley-VCH; Weinheim, 2021 Search PubMed.
- K. Philippot and B. Chaudret, in Comprehensive Organometallic Chemistry III, R. H. Crabtree and M. P. Mingos, ed., Elsevier, 2007, Ch. 12–3, Vol. 12 Search PubMed.
- J. M. Asensio, D. Bouzouita, P. W. N. M. van Leeuwen and B. Chaudret, Chem. Rev., 2020, 120, 1042 CrossRef CAS PubMed.
- J. Schörgenhumer, A. Zimmermann and M. Waser, Org. Process Res. Dev., 2018, 22, 862 CrossRef.
- P. Lara, O. Rivada-Wheelaghan, S. Conejero, R. Poteau, K. Phlippot and B. Chaudret, Angew. Chem., Int. Ed., 2011, 50, 12080 CrossRef CAS PubMed.
- D. J. Morgan, Surf. Interface Anal., 2015, 47, 1072 CrossRef CAS.
- L. M. Martínez-Prieto, M. Puche, C. Cerezo-Navarrete and B. Chaudret, J. Catal., 2019, 337, 429 CrossRef.
- R. Nyholm and N. Maartensson, Solid State Phys., 1980, 13, L279 CrossRef CAS; K. W. Park, J.-H. Choi, B.-K. Kwon, S.-A. Lee, Y.-E. Sung, H.-Y. Ha, S.-A. Hong, H. Kim and A. Wieckowski, J. Phys. Chem. B, 2002, 106, 1869 CrossRef; N. Chakroune, G. Viau, S. Ammar, L. Poul, D. Veautier, M. M. Chemini, C. Mangeney, F. Villian and F. Fièvet, Langmuir, 2005, 21, 6788 CrossRef PubMed.
- E. A. Ison, R. A. Corbin and M. M. Abu-Omar, J. Am. Chem. Soc., 2005, 127, 11938 CrossRef CAS PubMed; A. Krüger and M. Albrecht, Chem. – Eur. J., 2012, 18, 652 CrossRef PubMed.
- M. Okazaki, S. Ohshitanai, H. Tobita and H. Ogino, Chem. Lett., 2001, 952 CrossRef CAS; M. Itazaki, K. Ueda and H. Nakazawa, Angew. Chem., Int. Ed., 2009, 48, 3313 CrossRef PubMed.
- C. Gryparis and M. Stratakis, Chem. Commun., 2012, 48, 10751 RSC.
- J. Kaźmierczak, K. Kuciński, D. Lewandowski and G. Hreczycho, Inorg. Chem., 2019, 58, 1201 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of Ru NPs. Catalytic procedures. NMR data of catalytic reaction products. See DOI: https://doi.org/10.1039/d2cc01470j |
| This journal is © The Royal Society of Chemistry 2022 |