Open Access Article
Open Access ArticleCarbon nano-onion induced organization of polyacrylonitrile-derived block star polymers to obtain mesoporous carbon materials†
Gabriela
Siemiaszko
 *a,
Agnieszka
Hryniewicka
a,
Joanna
Breczko
*a,
Agnieszka
Hryniewicka
a,
Joanna
Breczko
 ab,
Krzysztof
Brzezinski
ab,
Krzysztof
Brzezinski
 c and
Marta E.
Plonska-Brzezinska
c and
Marta E.
Plonska-Brzezinska
 *a
*a
aDepartment of Organic Chemistry, Faculty of Pharmacy with the Division of Laboratory Medicine, Medical University of Bialystok, Mickiewicza 2A, 15-222 Bialystok, Poland. E-mail: gsiemiaszko@gmail.com; marta.plonska-brzezinska@umb.edu.pl
bFaculty of Chemistry, University of Bialystok, Ciolkowskiego 1K, 15-245 Bialystok, Poland
cDepartment of Structural Biology of Prokaryotic Organisms, Institute of Bioorganic Chemistry, Polish Academy of Sciences, Noskowskiego 12/14, 61-074, Poznan, Poland
First published on 26th May 2022
Abstract
Herein, we report the synthesis of mesoporous carbon materials from diblock star copolymers derived from polyacrylonitrile. The size of the pores was controlled by manipulating the length of the polymer blocks. Furthermore, the organization of polymers on the carbon nano-onion's surface resulted in materials of higher surface area and superficial electrochemical performance.
Porous carbon materials are abundantly applied in materials science, especially in electrocatalysis or supercapacitors. Regular pore organization and narrow pore size distribution are crucial for effective porous structures. Carbon materials find different applications depending on the pore size (micro, meso, or macro).1–4 Targeted morphology can be obtained by applying an appropriate synthetic strategy. One of them is the so-called copolymer soft-templating method involving well-defined copolymers consisting of sacrificial polymer blocks that undergo depolymerization during pyrolysis (e.g., polymethyl acrylate (PMA)) and carbon skeleton-forming polymer blocks, which transform into three-dimensional (3D) networks in the chemical or thermal processes, protecting them from degradation in pyrolysis. The linear copolymers derived from polyacrylonitrile (PAN) are used for the carbon skeleton formation as they form N-doped nanographene due to annealing.5,6 Polymers of defined length are typically synthesized by controlled radical polymerization methods, including Reversible Addition–Fragmentation Chain Transfer (RAFT) and Atom Transfer Radical (ATR) polymerizations.7,8
Porosity and electrochemical performance can be enhanced by incorporating preformed carbon nanostructures into the polymer matrix.9,10 Carbon nano-onions (CNOs) themselves are attractive materials as electrodes in electric double-layer capacitors (EDLCs) due to their multiple advantages, including high conductivity and thermal stability.11,12 To the best of our knowledge, the CNO-based nanostructural matrix leading to the organization of PAN-based polymers has not been reported so far. Furthermore, using star-shaped PAN nanostructures to synthesize porous carbon materials is an original approach.
This study reports the preparation of a family of mesoporous carbon materials derived from well-defined star copolymers synthesized using the RAFT radical polymerization method. At first, a series of novel six-arms star copolymers 6-star-(polymethyl acrylate-b-polyacrylonitrile), (6-star-(PMAx-b-PAN200), x = 25, 50, 100, 150, series from P1 to P4) with an increasing length of PMA chains, were synthesized (Fig. 1A). Their structure was confirmed by Nuclear Magnetic Resonance Spectroscopy (1H NMR) and Fourier Transform Infrared Spectroscopy (FTIR) (Fig. S1–S4 and S5a–d, ESI†). Size-exclusion chromatography (SEC) confirmed synthesis a series of star polymers with growing molecular masses (Mn,SEC) in the range from 87 000 to 127 000 g mol−1 (Fig. 1B and Fig. S6, ESI†) similar to those estimated theoretically and by 1H NMR (Table S1, ESI†). The mass fraction of PMA chains (wt% PMA) in the molecules was determined based on the integration of the –CH3 signal of ester groups and the proton adjacent to nitrile (–CHCN) in the 1H NMR spectra, proving the increase of this value from 15 to 55% in the series from P1 to P4. The wt% PMA and the Mn,SEC values were used to estimate the molecular masses of PMA in polymers (Mn,PMA). The polymers were then subjected to annealing for 4 h at 250 °C under airflow to stabilize PAN domains, providing products from P1-S to P4-S. Subsequent pyrolysis for 2 h at 800 °C under an Ar atmosphere afforded carbon samples from P1-C to P4-C. The weight loss of the samples during thermal treatment increased in direct proportion to the PMA content indicating pores forming in the carbon materials (Table S2, ESI†). FTIR measurements confirmed both thermal steps.
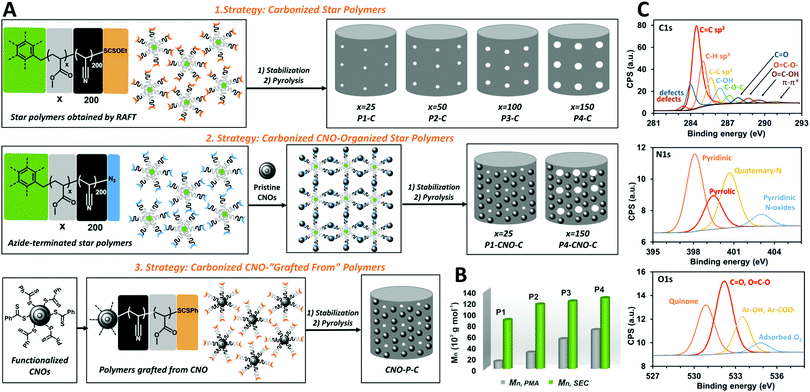 | ||
| Fig. 1 (A) Schematic representation of the synthesis steps of carbonaceous materials. Three different strategies have been used to synthesize of porous materials; please see ESI.† (B) Molecular masses of P1-P4 polymers determined by SEC. (C) XPS spectra of P4-CNO-C C 1s, N 1s, and O 1s spectral regions. The C 1s, O 1s, and N 1s high-resolution spectra were recorded at pass energy of 50 eV at room temperature. | ||
The characteristic signals of ester groups, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1730 cm−1), C–O (1150 cm−1) and nitriles (2240 cm−1) disappeared due to PMA depolymerization or cross-linking and cyclization reaction of –CN, respectively, resulting in C
O (1730 cm−1), C–O (1150 cm−1) and nitriles (2240 cm−1) disappeared due to PMA depolymerization or cross-linking and cyclization reaction of –CN, respectively, resulting in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1587 cm−1) and C–N (1363 cm−1) bonds formation (Fig. S5e and f, ESI†).13
C (1587 cm−1) and C–N (1363 cm−1) bonds formation (Fig. S5e and f, ESI†).13
Afterward, we attempted to hierarchically organize selected polymeric stars P1 and P4 on the CNO's surface (Fig. 1A). For this purpose, dithiocarbonate groups (–SCSOEt) at the ends of polymeric stars’ arms were transformed into azide groups (–N3), confirmed by the disappearance of –CH2– at ca. 4.60 ppm on the 1H NMR spectra (Fig. S7 and S8, ESI†). The products were subjected to reactions with CNOs to generate aziridine linkages between polymers and carbon nanoparticles.14 FTIR analysis supports that the resulting polymer-carbon hybrids possessed characteristic polymer (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and CN) signals on the CNO's surface (Fig. S5g and h, ESI†). Next, P1-CNO and P4-CNO were stabilized and pyrolyzed using general procedures to give carbon materials: P1CNO-C and P4-CNO-C. CNOs content of ca. 5–6% was estimated by dividing the mass of CNOs used in the synthesis by the weight of the pyrolyzed hybrid sample (Table S2, ESI†).
O and CN) signals on the CNO's surface (Fig. S5g and h, ESI†). Next, P1-CNO and P4-CNO were stabilized and pyrolyzed using general procedures to give carbon materials: P1CNO-C and P4-CNO-C. CNOs content of ca. 5–6% was estimated by dividing the mass of CNOs used in the synthesis by the weight of the pyrolyzed hybrid sample (Table S2, ESI†).
Another approach to the CNO-induced 3D organization of polyacrylonitrile-derived block polymers was based on the formation of polymeric chains grafted from carbon nanostructures (Fig. 1A). For this purpose, the CNO-derived chain transfer agent dithiocarbonate (CNO-SCSPh) was used to carry out controlled polymerization of acrylonitrile and methyl acrylate consecutively, affording carbon nanoparticles decorated with PAN-b-PMA copolymers (CNO-P). The course of the synthetic steps was followed by FTIR analysis, proving the growth of the polymeric chains on the CNO's surface supported by increasing intensity of characteristic signals of CN, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–C (1430 cm−1), and C–O groups (Fig. S5i–k, ESI†). As a result of annealing, the CNO-P-C carbon material was obtained with a CNO's content of ca. 2% (Table S2, ESI†).
O, C–C (1430 cm−1), and C–O groups (Fig. S5i–k, ESI†). As a result of annealing, the CNO-P-C carbon material was obtained with a CNO's content of ca. 2% (Table S2, ESI†).
X-ray Photoelectron Spectroscopy (XPS) was applied to determine the elemental composition of the surface of the C1-C, C4-C, C1-CNO-C, C4-CNO-C, and CNO-P-C materials. The results indicate the presence of C (84–90%), N (4–8%), and O (4–9%) in all studied materials (Table S3, ESI†). The deconvolution of the high-resolution spectral regions C 1s, O 1s, and N 1s of C4-CNO-C indicate a pattern of distribution of elements (Fig. 1C). Carbon atom forms mainly C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C sp2, C–H sp3, and C–C sp3 bonds.
C sp2, C–H sp3, and C–C sp3 bonds.
Bonding with oxygen results in the presence of C–OH, C–O–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, O
O, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O, and O
C–O, and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH groups.15,16 Furthermore, the sample is characterized by defects in carbon structure and π–π* interactions.17,18 The N 1s spectrum can be resolved into four regions indicating the formation of mainly pyridinic and quaternary N, as well as pyrrolic and N-pyridinic oxides to a smaller extent.19,20 The O 1s analysis supports the content of dominantly C
C–OH groups.15,16 Furthermore, the sample is characterized by defects in carbon structure and π–π* interactions.17,18 The N 1s spectrum can be resolved into four regions indicating the formation of mainly pyridinic and quaternary N, as well as pyrrolic and N-pyridinic oxides to a smaller extent.19,20 The O 1s analysis supports the content of dominantly C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, O
O, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O, quinone, Ar–OH, and Ar–COO– moieties.21 The remaining analyzed samples have a very similar elemental distribution pattern (Table S4 and Fig. S9, ESI†). Except for the quinone to Ar–OH/Ar–COO– ratio, which is dependent on the quinone–quinol equilibrium.22 These functional groups are beneficial for surface redox reactions related to the material's pseudocapacitance.
C–O, quinone, Ar–OH, and Ar–COO– moieties.21 The remaining analyzed samples have a very similar elemental distribution pattern (Table S4 and Fig. S9, ESI†). Except for the quinone to Ar–OH/Ar–COO– ratio, which is dependent on the quinone–quinol equilibrium.22 These functional groups are beneficial for surface redox reactions related to the material's pseudocapacitance.
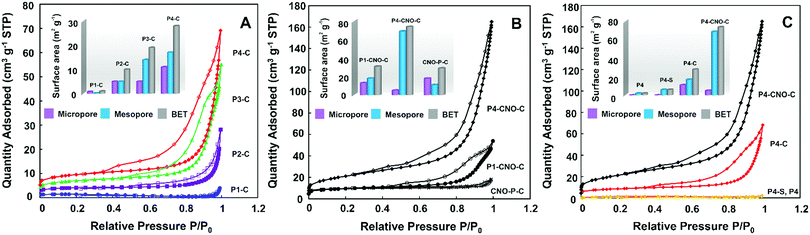
The study of the porosity of pyrolyzed 6-star-(PMAx-b-PAN200) polymers (Fig. 2A) clearly showed the significant influence of the PMA chain length on the amount of N2 adsorbed on the material's surface. Namely, the longer the polymer chain, the higher the quantity of adsorbed gas observed, which corresponds to the greater specific surface area (SBET) of the studied materials.
The presence of CNOs in the P1-CNO-C, P4-CNO-C, and CNO-P-C structures also resulted in significant growth in the porosity (Fig. 2B) compared to the corresponding pristine polymers. The course of all recorded curves (Fig. 2) was a combination of type I and IV isotherms, indicating the coexistence of micro- and mesopores in the structure of the pyrolyzed carbon materials.23 Analysis of isotherms based on the Brunauer–Emmett–Teller (BET) theory,24 allowed us to determine SBET values (insert of Fig. 2, Fig. S10 and Table S5, ESI†), which increased linearly with an increase of the length of the polymeric chain (Fig. 2A and B and Table S5, ESI†). The t-plot analysis demonstrated that a significant part of the SBET values calculated for the studied materials derives from mesopores (Table S5, ESI†). However, it is of note that the increase in the surface area of mesopores is more distinct for the longer polymeric chains (P4-C) (insert of Fig. 2A and Table S5, ESI†). Although the same tendency is also apparent for materials containing the CNOs, the difference between the surface area of micro- and mesopores is even more pronounced for P1-CNO-C and P4-CNO-C (insert of Fig. 2B and Table S5, ESI†). The successive increase in the pore diameter controlled at each synthesis step is shown in Fig. 2C. The graph confirms the growth in the surface area of mesopores due to the stabilization and pyrolysis processes in the presence of CNOs.
The morphology of all the pyrolyzed star 6-star-(PMA-b-PAN) polymers (from P1-C to P4-C) and those containing the CNOs (P1-CNO-C, P4-CNO-C, CNO-P-C) was studied using scanning electron microscopy (SEM) (Fig. 3). The images obtained for P1-C and P4-C (Fig. 3A and B, respectively) show that copolymers form a granular morphology with a different diameter of the aggregates. The copolymers with the shorter polymeric chains tend to aggregate with various sizes, while the longer ones form aggregates with approx. 1–2 μm (Fig. S11, ESI†). The structure of the carbon materials containing CNOs (Fig. 3C–E and Fig. S12, ESI†) appears more spongy and 3D than the pyrolyzed pristine polymers (Fig. 3A and B), indicating an increase in the number of channels and cavities, and thus the porosity enhancement. These observations agree with experimental data obtained by the N2 adsorption/desorption method. SEM observations are carried out at the microscale level, while the pores are only visible at the nano-level. Therefore, high-resolution transmission electron microscopic (HRTEM) studies were performed to analyze the carbonaceous materials (Fig. 3F and Fig. S13, ESI†). All samples derived from copolymers and CNOs have uniformly distributed spherical cavities in the structure, which may be assigned to pores (Fig. S13b, ESI†). The addition of CNOs to the polymeric materials causes an increase in crystallinity. These studies of the materials containing CNOs (P4-CNO-C) clearly show the presence of multi-layered fullerenes in their structure.
 | ||
| Fig. 3 SEM images of (A) P1-C, (B) P4-C, (C) CNO-P-C, (D) P1-CNO-C, and (E) P4-CNO-C. (F) HRTEM image of P4-CNO-C. | ||
These observations are also confirmed by X-ray diffraction study (XRD). For all materials, the most substantial asymmetric peak located around the 2θ angle between 22–25° corresponds to the graphite's (002) plane,23,24 that suggests the presence of sp2-bonded carbon atoms (Fig. S15, ESI†). The addition of CNOs to the polymer sample increases the crystallinity of these hybrid materials. The (002) reflection is sharper for P1-CNO-C and P4-CNO-C materials compared to polymer reference samples (P1-C and P4-C), suggesting that the materials containing CNO's moiety are the graphite-like structures with a higher-order spacial arrangement (additional information please see ESI†).
The electrochemical performance of all synthesized samples was investigated (Fig. S16–S18, ESI†). Considering that cyclic voltammetry (CV) allows us to easily show the relationship between the SBET and the specific capacitance (CS), we made measurements in a three-electrode system with the 1 M NaOH aqueous solution as a supporting electrolyte. The CVs recorded for the GCE electrode modified with the synthesized materials have an approximately rectangular shape, thus showing an EDL capacitor behavior. As expected, the obtained CS values were more significant, the higher the porosity and the longer the PMA chains in the structure of the tested material (Fig. S16 and Table S6, ESI†). The use of CNOs for the organization of the polymer network also contributed to a significant increase in the double layer capacitance (Fig. S17 and Table S6, ESI†). For shorter polymer chains (P1-C), the addition of CNOs to the polymer matrix (P1-CNO-C) causes a tenfold increase in the CS values, from 5 to 83 F g−1, respectively. As the length of the polymer chain increases, so does the value of the CS also increases. The P4-CNO-C (CS = 83 F g−1) exhibited the highest electrochemical capacitance. These observations agree with experimental data obtained by the N2 adsorption/desorption method. For this material, the largest pore volume of 0.2364 cm3 g−1 was also noted, with the majority in the micropore area (diameter below 2 nm) and in the mesopore area (diameter in the range of 8–20 nm) (Fig. S10, ESI†).
Electrochemical stability of P4-CNO-C in the wide potential range was also performed (Fig. S18, ESI†). Despite the functional groups containing N and O occurring in all materials, a contribution from a possible pseudocapacitance (faradaic reactions) is not detected in the CV curves in the studied potential window. Such observations have already been made in previous literature reports for porous carbon materials.25
In this study, we demonstrated the organization of star copolymers (6-star-(PMA-b-PAN)) on the CNO's surface, giving well-organized 3D porous structures with mesoporous characteristics. The selection of the appropriate polymerization method for the nanostructured matrix and the length of the polymer chain allowed us to synthesize a material with a homogeneous mesopore arrangement. The textural properties of this material, along with its high conductivity, make it possible to use it as an electrode material in supercapacitors and in the future in electrocatalysis.
We gratefully acknowledge the financial support of the National Science Centre, Poland, grant #2017/25/B/ST5/01414 to M.E.P-B. We gratefully acknowledge Prof. Luis Echegoyen from University of Texas at El Paso for providing the CNOs. Prof. Jacek Gebicki (Gdańsk University of Technology, Poland) is acknowledged for performing SEC analysis. The research was carried out with the equipment purchased thanks to the financial support of the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (POIG.02.01.00-06024/09, Maria Curie-Sklodowska University, Lublin, Poland) and the Operational Program Development of Eastern Poland 2007–2013 (POPW.01.03.00-20-034/09-00 and POPW.01.03.00-20-004/11, University of Bialystok, Poland).
Conflicts of interest
There are no conflicts to declare.References
- C. Xie, D. Yan, H. Li, S. Du, W. Chen, Y. Wang, Y. Zou, R. Chen and S. Wang, ACS Catal., 2020, 10, 11082–11098 CrossRef CAS.
- M. R. Benzigar, S. N. Talapaneni, S. Joseph, K. Ramadass, G. Singh, J. Scaranto, U. Ravon, K. Al-Bahily and A. Vinu, Chem. Soc. Rev., 2018, 47, 2680–2721 RSC.
- Q. Huang, Y. Xu, Y. Guo, L. Zhang, Y. Hu, J. Qian and S. Huang, Carbon, 2022, 188, 135–145 CrossRef CAS.
- Q. Huang, Y. Guo, D. Chen, L. Zhang, T.-T. Li, Y. Hu, J. Qian and S. Huang, Chem. Eng. J., 2021, 424, 130336 CrossRef CAS.
- J. P. McGann, M. Zhong, E. K. Kim, S. Natesakhawat, M. Jaroniec, J. F. Whitacre, K. Matyjaszewski and T. Kowalewski, Macromol. Chem. Phys., 2012, 213, 1078–1090 CrossRef CAS.
- M. Kopeć, M. Lamson, R. Yuan, C. Tang, M. Kruk, M. Zhong, K. Matyjaszewski and T. Kowalewski, Prog. Polym. Sci., 2019, 92, 89–134 CrossRef.
- Z. Zhou, T. Liu, A. U. Khan and G. Liu, Sci. Adv., 2019, 5, eaau6852 CrossRef PubMed.
- M. Kopeć, R. Yuan, E. Gottlieb, C. M.-R. Abreu, Y. Song, Z. Wang, J. F.-J. Coelho, K. Matyjaszewski and T. Kowalewski, Macromolecules, 2017, 50, 2759–2767 CrossRef.
- A. Cymann-Sachajdak, M. Graczyk-Zajac, G. Trykowski and M. Wilamowska-Zawłocka, Electrochim. Acta, 2021, 383, 138356 CrossRef CAS.
- M. Suleman, M. Deraman, S. A. Hashmi, M. A.-R. Othman, Y. Kumar, S. K. Rajouria and M. R.-M. Jasni, Electrochim. Acta, 2020, 333, 135547 CrossRef CAS.
- M. E. Plonska-Brzezinska and L. Echegoyen, J. Mater. Chem. A, 2013, 1, 13703 RSC.
- O. Mykhailiv, A. Lapinski, A. Molina-Ontoria, E. Regulska, L. Echegoyen, A. T. Dubis and M. E. Plonska-Brzezinska, Chem. Phys. Chem., 2015, 16, 2182–2191 CrossRef CAS.
- C. Tang, K. Qi, K. L. Wooley, K. Matyjaszewski and T. Kowalewski, Angew. Chem., Int. Ed., 2004, 43, 2783–2787 CrossRef CAS PubMed.
- J. Han and C. Gao, Nano-Micro Lett., 2010, 2, 213–226 CrossRef.
- M. Koinuma, H. Tateishi, K. Hatakeyama, S. Miyamoto, C. Ogata, A. Funatsu, T. Taniguchi and Y. Matsumoto, Chem. Lett., 2013, 42, 924–926 CrossRef CAS.
- G. Radaelli, J. A. Heredia-Guerrero, M. T. Masood, L. Ceseracciu, A. Davis, R. Carzino, M. Prato, I. S. Bayer and A. Athanassiou, Adv. Mater. Interfaces, 2016, 3, 1600069 CrossRef.
- H. Xu, B. Yang, J. Wang, S. Guang and C. Li, Macromolecules, 2005, 38, 10455–10460 CrossRef CAS.
- Y.-C. Chiang, Y.-J. Chen and C.-Y. Wu, Materials, 2017, 10, 1296 CrossRef.
- R. Arrigo, M. Hävecker, S. Wrabetz, R. Blume, M. Lerch, J. McGregor, E. P.-J. Parrott, J. A. Zeitler, L. F. Gladden, A. Knop-Gericke, R. Schlögl and D. S. Su, J. Am. Chem. Soc., 2010, 132, 9616–9630 CrossRef CAS.
- S. K. Ujjain, R. Bhatia and P. Ahuja, J. Saudi Chem. Soc., 2019, 23, 655–665 CrossRef.
- Y. J. Oh, J. J. Yoo, Y. I. Kim, J. K. Yoon, H. N. Yoon, J.-H. Kim and S. B. Park, Electrochim. Acta, 2014, 116, 118–128 CrossRef CAS.
- H. Berg and P. Zuman, J. Chem. Soc., Perkin Trans. 2, 2000, 1459–1464 RSC.
- K. S.-W. Sing, Pure Appl. Chem., 1982, 54, 2201–2218 CrossRef.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309–319 CrossRef CAS.
- J. Chen, Y. Han, X. Kong, X. Deng, H. J. Park, Y. Guo, S. Jin, Z. Qi, Z. Lee, Z. Qiao, R. S. Ruoff and H. Ji, Angew. Chem., Int. Ed., 2016, 55, 13822–13827 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc01452a |
| This journal is © The Royal Society of Chemistry 2022 |