 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
SHARPER-enhanced benchtop NMR: improving SNR by removing couplings and approaching natural linewidths†
Claire L.
Dickson
 a,
George
Peat
a,
Matheus
Rossetto
b,
Meghan E.
Halse
a,
George
Peat
a,
Matheus
Rossetto
b,
Meghan E.
Halse
 b and
Dušan
Uhrín
b and
Dušan
Uhrín
 *a
*a
aEaStCHEM School of Chemistry, University of Edinburgh, Edinburgh, UK. E-mail: dusan.uhrin@ed.ac.uk
bDepartment of Chemistry, University of York, York, UK
First published on 5th April 2022
Abstract
We present a signal enhancement strategy for benchtop NMR that produces SNR increases on the order of 10 to 30 fold by collapsing the target resonance into an extremely narrow singlet. Importantly, the resultant signal is amenable to quantitative interpretation and therefore can be applied to analytical applications such as reaction monitoring.
NMR spectroscopy is a well-established technique for reaction monitoring on high field NMR spectrometers, and more recently also on benchtop instruments.1–3 In principle, the reaction rate can be determined by monitoring the integral intensity of a single signal of a reactant. In this context, any splitting of the observed signal by scalar couplings decreases the signal-to-noise ratio (SNR) and therefore the removal of such splitting is desirable. The SHARPER (Sensitive, Homogeneous And Resolved PEaks in Real time) experiment4 achieves this by the means of data acquisition interrupted by 180° refocussing pulses.5–8 When non-selective pulses are used, all heteronuclear couplings are removed, while in selSHARPER, where selective 180° pulses are used, the homonuclear couplings also vanish; in both instances no X-channel pulses are required. As the SHARPER signal acquisition is embedded within a CPMG pulse sequence,9,10 it also eliminates the effects of magnetic field inhomogeneity, generating extremely narrow singlets with linewidths of a fraction of 1 Hz.
The SHARPER technique has been applied to a range of nuclei (1H, 19F and 31P) and tested on monitoring a variety of chemical reactions under challenging conditions, such as gas sparging. Significant SNR gains (up to 20 fold for reasonably well shimmed samples, much higher when the B0 homogeneity is poor) by SHARPER experiments substantially reduce the required sample quantities and/or experimental times, making reaction monitoring more efficient and allowing monitoring of faster reactions.4
Fluorinated organic molecules account for 20 and 60%, respectively, of pharmaceuticals11 and agrochemicals12 produced today. To support their production, development of efficient and environmentally safe fluorination methods13 positioned fluorine chemistry among the most active fields of organic chemistry, with efficient reaction monitoring making important contributions.14 Fortuitously, due to its large chemical shift dispersion, 19F NMR can take full advantage of SHARPER sequences, where even at low magnetic fields, the 19F resonances often are sufficiently isolated to allow for their selectively manipulation required by selSHARPER sequences.
Due to the close Larmor frequencies of 19F and 1H at 1-2T, the same coil can be used for both nuclei, hence most benchtop systems are typically capable of operating at both frequencies. Nevertheless, there are challenges for 19F NMR at benchtop spectrometers. The probes are usually tuned to favour the sensitivity of 1H detection at the expense of 19F. The SNRs of 19F spectra is further compromised by splittings caused by numerous JHF and JFF couplings, complex peak shapes and lower field homogeneity compared to high-field spectrometers. In addition, efficient 1H decoupling to remove the JHF couplings is very challenging when the same coil is used for 1H and 19F.
SHARPER presents a potential solution to all these problems, however, the existing versions require pulsed field gradients (PFGs) for both spin selection (in selSHARPER) and during the acquisition but PFGs are not standard on many benchtop NMR spectrometers15 and shaped selective pulses are currently only supported on certain models. Because of these hardware limitations, SHARPER sequences have yet to be implemented on benchtop instruments.
In this Communication we present several variations of the SHARPER technique that address these issues, including removal of PFGs from the acquisition loops and/or from the initial resonance selection step. We also introduce protocols for processing of spectra that further improve SNRs and generate high quality quantitative data required for reaction monitoring. Nevertheless, it should be noted that the developed acquisition and processing protocols are equally valuable for experiments performed on high-field instruments.
The first modification of the original SHARPER sequences involves removing PFGs from the acquisition loops, which was made possible by adjusting the phase cycle of the train of the 180° pulses (Fig. 1a). This allows for more time to be spent on signal acquisition, reducing undesirable relaxation and diffusion effects and producing narrower and more intense signals. Higher SNRs compared to the original experiments are obtained, improving the precision of integrals. A comparison of the two approaches is presented in the ESI,† Fig. S1. This modification is applied to all pulse sequences introduced here.
A complete removal of PFGs is possible by replacing the initial single pulsed field gradient spin echo (SPFGSE, Fig. 1b) by other means of signal selection. Two alternative selSHARPER sequences were tested. The first, SE selSHARPER (Fig. 1c), uses two scans to cancel off-resonance signals without requiring PFGs, while the second, 270 G selSHARPER (Fig. 1d), uses a 270° Gaussian pulse.16 The latter is more suited for faster chemical reactions, where acquisition of a single scan per time point is required. It should be noted that some benchtop systems have non-linear amplifiers and calibration of power levels using standard procedures and concentrated samples is required; this can be fine-tuned efficiently by the selSHARPER pulse sequence on real, diluted samples as detailed in the ESI.†
A less general, but effective application of the pulse sequence in Fig. 1a achieves signal selection when only two resonances are present in the spectrum. This could be the case when a reaction of a mono-fluorinated compound is monitored, with the reactant and the product producing one 19F signal each. Referred to here as rectangular selSHARPER, this pulse sequence uses lower power rectangular pulses adjusted in duration so that the first zero point of their excitation (inversion) profile falls at the frequency of the off-resonance signal. The length of such 90° and 180° pulses is calculated from the frequency difference between the two signals, Δ in Hz, as  and
and  , respectively.17 Alternatively, the pw180 is set to 2pw90 calculated by the first equation and the power level is kept constant for both pulses. This positions the second zero point of the sinc lobes of the 180° rectangular pulse at the frequency of the off-resonance signal.
, respectively.17 Alternatively, the pw180 is set to 2pw90 calculated by the first equation and the power level is kept constant for both pulses. This positions the second zero point of the sinc lobes of the 180° rectangular pulse at the frequency of the off-resonance signal.
The implementation of SHARPER experiments for benchtop NMR spectrometers is illustrated using fluorobenzene, 1, pentafluorobenzene, 2, and 2,2,3,3,3-pentafluoropropanol, 3, as model compounds. Their 1H and 19F spectra are presented in Fig. S2 (ESI†). Parameters of SHARPER experiments can also be found in the ESI.† Fluorobenzene, 1, which contains a single 19F atom and thus is suitable for testing both the non-selective and selective SHARPER pulse sequences was used initially. The spectra (Fig. 2) were acquired on a 60 MHz Spinsolve Ultra spectrometer without a dedicated gradient channel and the PFGs required for the SPFGSE selSHARPER experiments were generated by a shim coil. With the exception of the SE selSHARPER pulse sequence (Fig. 1c), which requires two scans, all other spectra can be acquired using a single scan. Additional phase cycling of the 180° pulses (Fig. 1a, φ2 = y, −y) applicable to all sequences improves the quality of the spectra (Fig. S3, ESI†).
All spectra were acquired using 4 scans by keeping identical repetition time ∼5T1 of 19F in 1 and processed following the guidelines presented later in the paper. Significant SNR improvements relative to the standard 1D 19F spectrum were achieved: 20.8× for the non-selective SHARPER and up to 12.8× for the selective pulse sequences, despite some losses in observed integral intensities, particularly when selective pulses were used in the acquisition loops. These losses are attributed to the B1 inhomogeneity affecting selective pulses. Even larger improvements per unit of time in SNR are obtained for 1-scan spectra as illustrated in the ESI.† The use of 5 ms selective 180° pulses in selSHARPER caused a ∼20% increase in signal line widths (Δ1/2 = 0.18 Hz), compared to the non-selective SHARPER (Δ1/2 = 0.14 Hz). This can be attributed to the increased apparent relaxation rates caused by the use of selective pulses.
The SNR improvements described here are larger than the 8-fold gains observed for 1 on a 400 MHz instrument.4 This improvement goes beyond the gain expected by removing PFGs from the acquisition loops and using data processing described in the forthcoming paragraphs. Such additional enhancement is due to fact that the 19F multiplet of 1 at 56.46 MHz is largely unresolved. This is caused in part by lower magnetic field homogeneity, but also because at 56.5 MHz, 19F is coupled to a higher order AA′BCC′ 1H spin system, while at 376.5 MHz the proton spins are less strongly coupled (Fig. S4, ESI†).
In the following we outline the best practice for acquiring and processing of SHARPER spectra to yield the significant SNR improvements presented above. Digitising fully a slowly decaying SHARPER signals requires acquisition times on the order of seconds, potentially tens of seconds. Combined with the fact that spins are periodically inverted during acquisition and therefore the restoration of the Zeman polarisation takes place only during the inter-scan relaxation delays, SHARPER acquisition need to be carefully optimised. Optimization is also needed to minimise the time intervals between the sampling points of chemical reactions. In the following we address these issues and present optimal solutions.
In general, there is no benefit in continuing to record the free induction decay, FID, beyond  seconds, when the signals has decayed to 5% of the starting value and additional data points are dominated by noise.18 In this expression,
seconds, when the signals has decayed to 5% of the starting value and additional data points are dominated by noise.18 In this expression,  is an effective spin–spin relaxation time, which includes contributions from spin–spin relaxation, T2, chemical exchange (if present) and B0 inhomogeneity. SHARPER pulse sequences eliminate the last contribution (see Fig. S1d, ESI†) but introduce additional relaxation due to the inter-chunk refocusing pulses.
is an effective spin–spin relaxation time, which includes contributions from spin–spin relaxation, T2, chemical exchange (if present) and B0 inhomogeneity. SHARPER pulse sequences eliminate the last contribution (see Fig. S1d, ESI†) but introduce additional relaxation due to the inter-chunk refocusing pulses.
To quantify this effect, we have acquired non-selective and selective SHARPER spectra using compounds 1–3, measured the linewidths of the obtained Lorentzian SHARPER lines (ΔS1/2) and compared them with those derived from the T2 values obtained in separate CPMG experiments (Δ1/2 = 1/πT2). The results summarised in Table 1 show that the ΔS1/2 linewidths are larger (by <0.07 Hz) than the Δ1/2 linewidths, but considerably smaller than the linewidths observed in regular 1D spectra. We can therefore conclude that SHARPER singlets are indeed approaching the natural linewidths of resonances.
| No. | Solvent/atom(s) | 180° pulses | Pulse length/μs | T 2/s | Theor. Δ1/2/Hz | Exp. ΔS1/2/Hz |
|---|---|---|---|---|---|---|
| a τ = 20 ms. b Measured by CPMG. c Δ 1/2 = 1/πT2. d SHARPER linewidths. | ||||||
| 1 | Toluene-d8 | Hard | 264 | 2.99 | 0.11 | 0.14 |
| 2 | CDCl3, F1,5 | Gauss | 5000 | 3.00 | 0.11 | 0.12 |
| 2 | F3 | Gauss | 5000 | 3.44 | 0.09 | 0.14 |
| 2 | F2,4 | Gauss | 5000 | 2.63 | 0.12 | 0.16 |
| 3 | Neat, CF3 | Rect. | 786 | 1.23 | 0.26 | 0.30 |
| 3 | CF2 | Rect. | 786 | 1.31 | 0.24 | 0.31 |
| 3 | D2O, CF3 | Rect. | 786 | 4.77 | 0.07 | 0.07 |
| 3 | CF2 | Rect. | 786 | 5.84 | 0.05 | 0.08 |
When exponential line broadening matching the decaying FID in the form of exp (−πtΔ1/2) is used prior to FT, the linewidth of spectral lines doubles, but the SNR reaches maximum. This is a well-known property of matched filters.19 The parameters of SHARPER experiments that maximise the SNR therefore involve acquisition time of 3TS2, where TS2 is the effective T2 relaxation during the SHARPER acquisition, and application of exponential line broadening, LB = ΔS1/2 Hz. These dependent parameters (ΔS1/2 = 1/πTS2) can be easily determined by acquiring a preliminary SHARPER spectrum using a long acquisition time. This initial SHARPER experiment should also be used to direct the signal into a single (real) receiver channel by adjusting the receiver phase relative to that of the transmitter (see Fig. 3) for reasons explained later.
Nevertheless, acquisition of SHARPER FIDs over a period shorter than the optimal 3TS2 is desirable when monitoring faster reactions and/or multi-scan accumulation is needed to improve the SNR. Not considering apodisation, it has been shown20 that the maximum SNR of NMR spectra is obtained for the acquisition time, AQ, of 1.26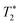 . At this point an FID has decayed only to 28% of its initial value and apodisation is necessary to obtain spectra free from truncation artefacts. It follows that exponential line broadening of 1.4ΔS1/2 will reduce the intensity of the last point of an FID acquired with AQ = 1.25TS2 to 5%. Using the 19F signals of 3 we demonstrate (see ESI†) that this treatment retains >95% SNR level of the maximum obtained for AQ = 3TS2 and a FID apodised with LB = ΔS1/2 Hz.18 This analysis also shows that even shorter SHARPER FIDs still provide significant SNR enhancements and in combination with the relaxation delay of 5T1 should be used for reaction monitoring of faster reactions. We note that when the SHARPER acquisition is used for a purpose different than reaction monitoring, the maximum SNR per unit of time is achieved by setting the inter scan relaxation delay to 1.3T1.21
. At this point an FID has decayed only to 28% of its initial value and apodisation is necessary to obtain spectra free from truncation artefacts. It follows that exponential line broadening of 1.4ΔS1/2 will reduce the intensity of the last point of an FID acquired with AQ = 1.25TS2 to 5%. Using the 19F signals of 3 we demonstrate (see ESI†) that this treatment retains >95% SNR level of the maximum obtained for AQ = 3TS2 and a FID apodised with LB = ΔS1/2 Hz.18 This analysis also shows that even shorter SHARPER FIDs still provide significant SNR enhancements and in combination with the relaxation delay of 5T1 should be used for reaction monitoring of faster reactions. We note that when the SHARPER acquisition is used for a purpose different than reaction monitoring, the maximum SNR per unit of time is achieved by setting the inter scan relaxation delay to 1.3T1.21
Next we describe how the artefacts associated with the SHARPER spectra can be reduced and the SNR increased by post-acquisition processing. When the signal is directed to a single channel (Fig. 3a and b), the FID produced by SHARPER experiments is a decaying exponential at zero frequency with small perturbations due to the evolution of scalar couplings during the acquisition chunks, τ, and a small downwards step caused by the relaxation during the pulsed intervals. These perturbations lead to the appearance of sidebands in the SHARPER spectra at frequencies of ±n/τ Hz. When the signal is directed into the real channel by adjusting the receiver phase, the imaginary channel collects only noise and the signals of the half chunk sidebands at ±n/2τ Hz that are 90° out of phase relative to the main signal (Fig. 3c). By removing the imaginary data, the spectra become symmetrical around the centre and contain sidebands with average intensity of their original values. Artefacts around the main SHARPER singlet are much reduced and the overall SNR is improved by a factor of  (Fig. 3c and d). A Python script and Bruker AU program for such processing are included in the ESI.†
(Fig. 3c and d). A Python script and Bruker AU program for such processing are included in the ESI.†
In SHARPER spectra, the integrals of the main signal remain precise throughout the reaction monitoring experiment only if the frequency of the monitored signal does not change. This may not always be the case. Without following such movement with the r.f. carrier frequency, the main signal will decrease, while the chunking sidebands will increase.4 Decreasing the length of the acquisition chunks helps, but especially for the selSHARPER, this broadens the acquired signal due to increased number of selective pulses interrupting signal acquisition.
A more general solution, which also addresses the appearance of sidebands caused by J coupling, is possible. The sideband intensity depends on the relationship between the J values of the monitored nucleus and the length of the acquisition chunk, τ. Typically, the inequality τ < 1/(4J) is used to set the value of τ; for larger J values the sidebands will be stronger. These considerations become important when integral intensities of multiple signals of nuclei with different J values acquired in separate SHARPER experiments need to be compared, e.g. those of a reactant and a product. A practical solution, widening of the integrated region to include the first chunking sidebands,22 will eliminate the effects of diverse J values and produce reliable integrals (see ESI†).
In conclusion, the pulse sequences presented in this Communication accommodate varied hardware capabilities of benchtop NMR spectrometers. Combined with the discussed acquisition and processing protocols, they surpass the sensitivity gains achieved by the original SHARPER experiments. They preserve the relative integral intensities and address the pitfalls (suboptimal tuning of coils, higher order effects) of 19F detection at 1–2 T magnetic fields typically used on benchtop instruments and increase by more than an order of magnitude the achievable SNRs. Their invariance to the magnetic field inhomogeneity makes them the method of choice for challenging environments and challenging reaction conditions.
This research was supported by EPSRC grants EP/S016139/1, EP/N509644/1, EP/R030065/1, EP/M020983/1 and EP/R028745/1. We thank Dr Stuart Kennedy (The Falcon Project Ltd and) for access to a Spinsolve 60 MHz NMR spectrometer and Dr Craig Eccles from Magritek Ltd for technical assistance. Spectra presented in Fig. 2 and 3 can be found at https://doi.org/10.7488/ds/3439.
Conflicts of interest
There are no conflicts to declare.References
- T. Castaing-Cordier, D. Bouillaud, J. Farjon and P. Giraudeau, in Annual Reports on NMR Spectroscopy, ed. G. A. Webb, Academic Press, 2021, vol. 103, pp. 191–258 Search PubMed.
- B. Blumich and K. Singh, Angew. Chem., Int. Ed., 2018, 57, 6996–7010 CrossRef PubMed.
- T. A. van Beek, Phytochem. Anal., 2021, 32, 24–37 CrossRef CAS PubMed.
- A. B. Jones, G. C. Lloyd-Jones and D. Uhrin, Anal. Chem., 2017, 89, 10013–10021 CrossRef CAS PubMed.
- R. Freeman and H. D. W. Hill, J. Chem. Phys., 1971, 54, 301–313 CrossRef CAS.
- J. Bocan, G. Pileio and M. H. Levitt, Phys. Chem. Chem. Phys., 2012, 14, 16032–16040 RSC.
- P. T. Callaghan, Translational Dynamics and Magnetic Resonance: Principles of Pulsed Gradient Spin Echo NMR, Oxford Scholarship Online, 2011 Search PubMed.
- G. A. Morris and A. Gibbs, J. Magn. Reson., 1988, 78, 594–596 CAS.
- H. Y. Carr and E. M. Purcell, Phys. Rev., 1954, 94, 630–638 CrossRef CAS.
- S. Meiboom and D. Gill, Rev. Sci. Instrum., 1958, 29, 688–691 CrossRef CAS.
- M. Inoue, Y. Sumii and N. Shibata, ACS Omega, 2020, 5, 10633–10640 CrossRef CAS PubMed.
- Y. Ogawa, E. Tokunaga, O. Kobayashi, K. Hirai and N. Shibata, iScience, 2020, 23, 101467 CrossRef CAS PubMed.
- T. Furuya, A. S. Kamlet and T. Ritter, Nature, 2011, 473, 470–477 CrossRef CAS PubMed.
- Y. Ben-Tal, P. J. Boaler, H. J. A. Dale, R. E. Dooley, N. A. Fohn, Y. Gao, A. García-Domínguez, K. M. Grant, A. M. R. Hall, H. L. D. Hayes, M. M. Kucharski, R. Wei and G. C. Lloyd-Jones, Prog. Nucl. Magn. Reson. Spectrosc., 2022, 129, 28–106 CrossRef CAS PubMed.
- B. Gouilleux, J. Farjon and P. Giraudeau, J. Magn. Reson., 2020, 319, 106810 CrossRef CAS PubMed.
- L. Emsley and G. Bodenhausen, J. Magn. Reson., 1989, 82, 211–221 CAS.
- G. S. Rule and T. K. Hitchens, Fundamentals of protein NMR spectroscopy, Springer Science & Business Media, 2006 Search PubMed.
- E. D. Becker, J. A. Ferretti and P. N. Gambhir, Anal. Chem., 1979, 51, 1413–1420 CrossRef CAS.
- R. G. Spencer, Concepts Magn. Reson., Part A, 2010, 36A, 255–265 CrossRef PubMed.
- S. G. Hyberts, S. A. Robson and G. Wagner, J. Biomol. NMR, 2013, 55, 167–178 CrossRef CAS PubMed.
- J. S. Waugh, J. Mol. Spectrosc., 1970, 35, 298–305 CrossRef CAS.
- M. Davy, C. L. Dickson, R. Wei, D. Uhrín and C. P. Butts, Analyst, 2022, 147, 1702–1708 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details, further experimental results, a pulse programme and processing scripts. See DOI: https://doi.org/10.1039/d2cc01325h |
| This journal is © The Royal Society of Chemistry 2022 |



