Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stereoregulation, molecular weight, and dispersity control of PMMA synthesized via free-radical polymerization supported by the external high electric field†
Katarzyna
Chat
 *ab,
Paulina
Maksym
*ab,
Paulina
Maksym
 bc,
Kamil
Kamiński
ab and
Karolina
Adrjanowicz
bc,
Kamil
Kamiński
ab and
Karolina
Adrjanowicz
 *ab
*ab
aInstitute of Physics, University of Silesia, 75 Pulku Piechoty 1, 41-500 Chorzow, Poland. E-mail: kadrjano@us.edu.pl; kchat@us.edu.pl
bSilesian Center for Education and Interdisciplinary Research (SMCEBI), 75 Pulku Piechoty 1a, 41-500 Chorzow, Poland
cInstitute of Materials Engineering, University of Silesia, 75 Pulku Piechoty 1a, 41-500 Chorzow, Poland
First published on 13th April 2022
Abstract
We show the remarkable effect of using static (DC) and alternating (AC) electric fields to control the free-radical polymerization of methyl methacrylate (MMA). The magnitude and/or frequency of the applied electric field (up to 154 kV cm−1) were found to control the molecular weight, dispersity, and stereochemistry of the produced polymers.
Almost three decades ago, controlled radical polymerization (CRP) methods had been developed, completely revolutionizing the field of polymer science. In fact, these strategies have become the most powerful tools for producing polymers of high functionality and almost infinite variety, significantly influencing the properties they provide. However, CRPs, unlike inherently uncontrolled free radical polymerization (FRP), have some limitations, one of the greatest being the necessity of adding additional often toxic catalyst/reagents, the requirement to conduct the reaction in the inert gas atmosphere or, finally, complicated and sometimes even impossible synthesis of polymers with very high molecular weight (Mn) and low dispersity (Đ).1,2 Fortunately, the key to overcoming the last CRP barrier was using external factors such as light,3 ultrasound,4 microwave irradiation,5 pressure,6 geometrical constraints,7 or electric8 and magnetic9 fields that, in most cases, successfully induced the production of higher Mn polymers with low Đ.
Remarkably, some of these stimuli, i.e., pressure and 2D confined spaces, were also very effective in less rigorous FRP (that does not require additional reagents), allowing the production of polymeric materials with very high Mn and low/moderate Đ.10,11 What is more, both these externally-supported FRPs allowed controlling polymer microstructure (tacticity).12 In fact, studying this aspect seems particularly important as differences in macromolecule stereospecificity may strongly influence their melting point, degree of crystallinity, viscosity, or mechanical strength. It is also interesting because, in general, unlike anionic/coordination polymerizations, in free radical processes, it is much more challenging to control the polymer tacticity. This difficulty is related to the nature of free radicals showing only the slight variation between their electrophilicity/nucleophilicity, which, in consequence, strongly limits their selectivity. Indeed, several FRP strategies based on chemical modification of polymerizing systems are actively being utilized to enhance our ability to control polymer stereoregularity. Interestingly, being alternatives to externally-regulated processes, these protocols require manipulating the conditions (temperature, monomer concentration) and implementing additional reagents as Lewis acids, solvents, or chiral auxiliaries.13,14 Moreover, keeping in mind that there is constant pressure from synthetic strategies that are very well suited to vinyl monomers polymerization, especially in the preparation of stereoregular polymers, searching for inexpensive, simple, and environmentally friendly methods is of great need.
Here, to meet the expectation of modern polymer chemistry, we proposed and developed a unique and innovative FRP strategy supported by the high electric field. Noteworthy, so far, polymerizations utilizing electric fields were implemented mainly for electrolytic processes, electropolymerization, electrografting in various electrolyte-containing solutions, or electrochemically mediated atom transfer radical polymerization (eATRP).8,15–17 Nevertheless, understanding the effect of the electric field on the polymerization kinetics and the properties of obtained materials has been generally overlooked. We envisage that using a static (DC) and alternating (AC) electric field as the additional external stimulus for thermally-induced FRP would enable us to control Mn, Đ, and, most excitingly, tacticity of produced polymers. We also believe that the proposed by us methodology could enrich the toolbox of free radical processes with relatively high stereoselectivity.
To demonstrate our concept, we have selected first methyl methacrylate (MMA) as a model monomer and 2,2′-azobis(2-methylpropionitrile) (AIBN) as a thermoinitiator and performed the bulk MMA FRP (at 333 K, i.e. 60 °C)) in the presence of the electric field. The electric field magnitudes applied in this study are up to 154 kV cm−1, significantly beyond any previous investigation. Our studies were supported by real-time tracking of the reaction kinetics provided by dielectric spectroscopy (DS). We also performed throughout characterization of resulting poly(methyl methacrylate)s (PMMAs) using 1H NMR spectroscopy, DSC and SEC-LALLS. Experimental details and results of control experiments are provided in the ESI.†
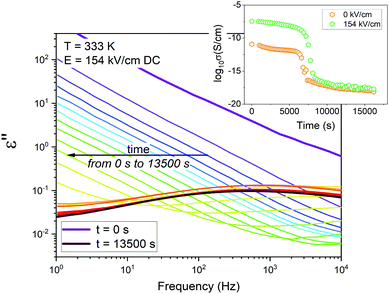
Fig. 1 shows the representative time evolution of the imaginary part of the complex dielectric permittivity ε′′, recorded upon MMA/AIBN polymerization in the presence of the DC electric field of E = 154 kV cm−1. At the initial stages, the dc-conductivity associated with the charge transport dominates in the dielectric loss spectra. As time passes, dc conductivity shifts towards lower frequencies. This effect is related to increased viscosity due to growing polymer chains. Then, at some point, we also start to observe a broad relaxation process that enters the experimentally accessible frequency window. It is due to overlapped segmental and secondary relaxations.18–24 The reaction was assumed to be completed when no further changes in the dielectric spectra were observed.
In the inset of Fig. 1, we have plotted the corresponding evolution of the conductivity, σ. Data were collected at zero-field and in the presence of the DC field (E = 154 kV cm−1). As shown, σ values decrease and reach the minimum value once the polymerization process is completed. A drop of the dielectric signal upon polymerization comes from replacing polar monomer molecules with less polar or non-polar polymer fragments. In this way, polymers are generally characterized by much lower dipole moment and conductivity values compared to their parent monomers. By looking at the results, we see that polymerization rates for DC fields of 0 kV cm−1 and 154 kV cm−1 are comparable. A similar finding, we have also observed in the presence of AC fields.
After completing the reaction, obtained materials were purified and isolated by multiple-step precipitations. Then, all polymers were characterized by NMR spectroscopy and SEC-LALLS. These results are summarized in Table 1 (SEC chromatograms and NMR data are in ESI†). A high degree of monomer conversion (>95%) was achieved for all MMA/AIBN polymerization experiments carried out at 333 K in the presence of a high electric field. Moreover, conversion is almost the same as for the zero-field case. We have also confirmed no degradation of the reagents at high fields based on NMR results. Thus, the high-field-assisted FRP could be an effective, safe, and clean alternative for other synthetic methods.
| Field magnitude (kV cm−1) | Conversiona (%) | M nSEC-LALLS (g mol−1) | Đ | |
|---|---|---|---|---|
| a Determined by 1H NMR spectroscopy (CDCl3). b Determined by SEC-LALLS (DMF + 10 mmol LiBr), estimated dn/dc = 0.0608. | ||||
| DC field | ||||
| 1 | 0 | >99 | 916![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.90 |
| 2 | 26 | 95 | 759![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.55 |
| 3 | 77 | >99 | 573![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.65 |
| 4 | 104 | 98 | 497![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
1.56 |
| 5 | 154 | 97 | 402![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 388 388 |
1.65 |
| AC field | ||||
| 1 | 77 (50 Hz) | >99 | 574![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.90 |
| 2 | 77 (1 Hz) | >99 | 425![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2.45 |
| 3 | 77 (0.01 Hz) | >99 | 410![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.78 |
The results summarized in Table 1 demonstrate that applying an electric field during polymerization generally yields polymers with lower molecular weights compared to the sample synthesized without using DC bias. The molecular weight of produced PMMA decreases with increasing the field magnitude, from Mn = 916![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 g mol−1 at 0 kV cm−1 to Mn = 402
900 g mol−1 at 0 kV cm−1 to Mn = 402![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 388 g mol−1 at 154 kV cm−1. So, by changing the field amplitude, it is possible to control the length of the polymer chains. We have also noticed that using the external electric field during MMA/AIBN polymerization affects the dispersities of produced polymers. In the case of the static electric field, all PMMA samples are characterized by lower dispersities (Đ = 1.55–1.65) compared to the zero-field reference (Đ = 1.90). In contrast to the molecular weight, its distribution does not show any clear trend with increasing the field amplitude.
388 g mol−1 at 154 kV cm−1. So, by changing the field amplitude, it is possible to control the length of the polymer chains. We have also noticed that using the external electric field during MMA/AIBN polymerization affects the dispersities of produced polymers. In the case of the static electric field, all PMMA samples are characterized by lower dispersities (Đ = 1.55–1.65) compared to the zero-field reference (Đ = 1.90). In contrast to the molecular weight, its distribution does not show any clear trend with increasing the field amplitude.
In addition to static fields, we have also tried polymerization of MMA/AIBN using an AC field (E = 77 kV cm−1) of different frequencies. The properties of PMMA samples obtained in this way are also listed in Table 1. As can be seen, an alternating electric field, just as a static field, induces changes in the properties of the produced polymers. The molecular weight of PMMA obtained at 77 kV cm−1 with ν = 50 Hz is almost twice lower than that produced in the absence of an external electric field. Moreover, it depends on the frequency of the applied AC field. The polymer material generated using exceedingly low frequencies of AC field is characterized by lower molecular weight. For the PMMA sample obtained at 77 kV cm−1 and ν = 50 Hz, the molecular weight is Mn = 574![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1. This is almost the same as for static fields of the same field magnitude. When the AC field frequency (77 kV cm−1) is decreased to ν = 0.01 Hz, we get polymer with Mn = 410
800 g mol−1. This is almost the same as for static fields of the same field magnitude. When the AC field frequency (77 kV cm−1) is decreased to ν = 0.01 Hz, we get polymer with Mn = 410![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1. The samples synthesized using AC bias fields have relatively higher dispersities than for dc bias (Đ = 1.78–2.45). Hence, the length of the growing PMMA polymer chain can be additionally controlled by the frequency of the applied AC field, while DC fields provide better control of dispersity.
500 g mol−1. The samples synthesized using AC bias fields have relatively higher dispersities than for dc bias (Đ = 1.78–2.45). Hence, the length of the growing PMMA polymer chain can be additionally controlled by the frequency of the applied AC field, while DC fields provide better control of dispersity.
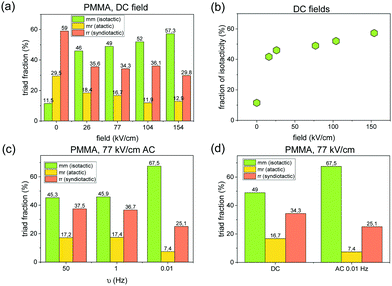
To gain more insight into the properties of PMMA samples produced at high electric fields, we have analyzed their stereoregularity. For this purpose, we use collected NMR data shown in ESI.† Surprisingly, we found that the presence of an electric field upon MMA/AIBM polymerization affects the tacticity of the produced polymers. Fig. 2(a) evaluates the content of the isotactic, syndiotactic, and atactic triads for all considered samples. Results for the zero-field sample are also shown as a reference. In the absence of a high electric field, syndiotactic rich PMMA polymer is preferred. However, isotactic alignment becomes more preferential when using the DC electric field. The data presented in Fig. 2(b) show a pronounced increase in the proportion of isotactic triads with increasing the DC field magnitude. In parallel, a significant decrease of a syndiotactic and atactic fraction is observed. The tacticity of PMMA samples obtained in the presence of an AC electric field was also analyzed. Fig. 2(c) demonstrates changes in the isotactic, syndiotactic, and atactic triad as a function of the field frequency. As shown, AC bias with ν = 0.01 Hz results in a significant isotacticity increase. On the other hand, the tacticity of PMMA samples generated using electric fields of 77 kV cm−1 and frequencies 1 Hz or 50 Hz are almost the same. In Fig. 2(d), we have compared the content of particular triads in the samples polymerized at static and alternating (ν = 0.01 Hz) fields of the same field amplitude (E = 77 kV cm−1). PMMA produced at low-frequency conditions is characterized by a relatively higher content of isotactic structures than that synthesized in the presence of the DC field. In this case, increased isotacticity is accompanied by well-controlled molecular weights and lower dispersities. This is the first evidence that the tacticity of the polymer could be finely tuned by the amplitude and/or frequency of the applied electric field.
As a final point, we have also investigated the glass transition dynamics of produced PMMA samples. Dielectric relaxation and calorimetric measurements were carried out for three samples obtained at high DC fields: 0 kV cm−1, 77 kV cm−1, and 154 kV cm−1. The representative dielectric loss spectra for PMMA produced in the presence of a DC electric field can be found in ESI.† These results demonstrate that the segmental dynamics of PMMA polymers obtained at high fields are faster compared to the sample synthesized at no electric field. In line with that, for the samples obtained in the DC field with 77 kV cm−1 and 154 kV cm−1, the calorimetric glass transition temperature is 394 K and 386 K, respectively. Thus, increasing the field amplitude upon high-field polymerization decreases the glass transition temperature of the obtained polymer. For PMMA with Mn > 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Tg does not depend on the molecular weight. In this way, the spatial configuration, not the molecular weight effect, is the main factor influencing the glassy dynamics of produced PMMA samples (for details, please look at ESI†).
000 g mol−1, Tg does not depend on the molecular weight. In this way, the spatial configuration, not the molecular weight effect, is the main factor influencing the glassy dynamics of produced PMMA samples (for details, please look at ESI†).
As already noted, literature data show that radical polymerization allows obtaining polymers with narrow dispersities, defined topography, and molecular weight.25–27 However, controlling tacticity in the free radical processes remains a challenge.12 One strategy is to use metal cation catalysts, which is more practical and efficient than anionic and coordination polymerizations.12 Attempts have been made to control the tacticity of PMMA by combining ATRP with Lewis acid and fluoroalcohol.13,14,28 Reversible addition–fragmentation chain transfer (RAFT) polymerization in the presence of Lewis acid is also one of the strategies. Nevertheless, these methods are quite time- and energy-consuming.29 Some of these approaches often use metallic catalysts or complex mediators; thus, they are expensive to implement. The use of high-electric field stimuli is straightforward and does not require multi-step reactions; it requires only a monomer, an initiator.
This new concept requires further studies to elucidate the exact role of the electric field. Nevertheless, it is reasonable to suppose that a high electric field favors a particular chain alignment upon polymerization, especially when monomer units are highly polar. Just like, it drives the conversion from the ring- to chain-type hydrogen-bonded structures in monohydroxy alcohols.30 Our finding opens up new possibilities for field-induced stereocontrol that can be easily established for other polymer systems and synthesis routes.
To sum up, in this work, we have successfully performed free radical polymerization of methyl methacrylate in the presence of high electric fields. The reaction conditions were very simple, and the process did not require catalysts or solvents (AIBN was only used as the initiator). We found that applying a high electric field, both AC and DC, significantly affects the properties of the obtained polymer. Namely, molecular weight decreases with increasing the amplitude of the DC field or either decreasing the frequency of the AC field. In addition to that, PMMAs synthesized at high electric fields were characterized by lower dispersities. Interestingly, it is also possible to affect the tacticity of the synthesized polymers at high electric fields. Increasing the field magnitude or decreasing the frequency of the applied AC field stimulates changes in the tacticity. Isotactic rich structures seem to be more preferred. Using dielectric spectroscopy (DS) and differential scanning calorimetry (DSC), we have also characterized the glass transition dynamics of the obtained materials (see ESI† for further results). The molecular weights of polymers materials obtained in high electric fields are above the entanglement point, meaning their Tg values are not affected by the molecular weight. This proves that tacticity affects the segmental dynamics of produced polymers. Increased isotacticity leads to faster α-relaxation and lower glass transition temperature.
The present study demonstrates a straightforward and versatile approach that employs an external electric field to control the bulk polymerization of the modeled polymer system. This method ensures high monomer conversion and good control of the properties of produced polymers (molecular weight, dispersities, and tacticity). Given the “green chemistry” requirements and the difficulties in obtaining polymers with a specific architecture, molecular weight, or stereoregularity, it is also extremely promising. We envisage that an external electric field can be successfully implemented into other polymerization mechanisms and verified for more complex systems/problematic monomers. In addition to that, it can be easily extended for other systems, giving countless opportunities to design new methods and novel functionalized materials.
KC and KA are grateful for the financial support from the National Science Centre within the framework of the SONATA BIS project (Grant No. 2017/26/E/ST3/00077).
Conflicts of interest
There are no conflicts to declare.References
- A. C. Albertsson and I. K. Varma, Adv. Polym. Sci., 2002, 157, 1–40 CrossRef CAS.
- M. Labet and W. Thielemans, Chem. Soc. Rev., 2009, 38, 3484–3504 RSC.
- X. Pan, M. A. Tasdelen, J. Laun, T. Junkers, Y. Yagci and K. Matyjaszewski, Prog. Polym. Sci., 2016, 62, 73–125 CrossRef CAS.
- T. G. McKenzie, F. Karimi, M. Ashokkumar and G. G. Qiao, Chem. – Eur. J., 2019, 25, 5372–5388 CrossRef CAS PubMed.
- K. Kempe, C. R. Becer and U. S. Schubert, Macromolecules, 2011, 44, 5825–5842 CrossRef CAS.
- K. Kaminski, M. Paluch, R. Wrzalik, J. Ziolo, R. Bogoslovov and C. Michael Roland, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3795–3801 CrossRef CAS.
- P. Maksym, M. Tarnacka, K. Wolnica, A. Dzienia, K. Erfurt, A. Chrobok, A. Zieba, R. Bielas, K. Kaminski and M. Paluch, Polym. Chem., 2018, 9, 335–345 RSC.
- J. Chen, L. Gao, X. Han, T. Chen, J. Luo, K. Liu, Z. Gao and W. Zhang, Mater. Chem. Phys., 2016, 169, 105–112 CrossRef CAS.
- L. Lv, W. Wu, G. Zou and Q. Zhang, Polym. Chem., 2013, 4, 908 RSC.
- P. Maksym, M. Tarnacka, A. Dzienia, K. Wolnica, M. Dulski, K. Erfurt, A. Chrobok, A. Zięba, A. Brzózka, G. Sulka, R. Bielas, K. Kaminski and M. Paluch, RSC Adv., 2019, 9, 6396–6408 RSC.
- T. Uemura, Y. Ono, K. Kitagawa and S. Kitagawa, Macromolecules, 2008, 41, 87–94 CrossRef CAS.
- K. Satoh and M. Kamigaito, Chem. Rev., 2009, 109, 5120–5156 CrossRef CAS PubMed.
- J. F. Lutz, B. Kirci and K. Matyjaszewski, Macromolecules, 2003, 36, 3136–3145 CrossRef CAS.
- W. Wang, Z. Zhang, J. Zhu, N. Zhou and X. Zhu, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 6316–6327 CrossRef CAS.
- Z.-F. Cai, G. Zhan, L. Daukiya, S. Eyley, W. Thielemans, K. Severin and S. De Feyter, J. Am. Chem. Soc., 2019, 141, 11404–11408 CrossRef CAS PubMed.
- F. M. McFarland, X. Liu, S. Zhang, K. Tang, N. K. Kreis, X. Gu and S. Guo, Polymer, 2018, 151, 56–64 CrossRef CAS.
- H. J. Kreuzer, Surf. Interface Anal., 2004, 36, 372–379 CrossRef CAS.
- K. L. L. Ngai, T. R. R. Gopalakrishnan and M. Beiner, Polymer, 2006, 47, 7222–7230 CrossRef CAS.
- K. L. Ngai, J. Chem. Phys., 1998, 109, 6982–6994 CrossRef CAS.
- K. L. Ngai and M. Paluch, J. Chem. Phys., 2004, 120, 857–873 CrossRef CAS PubMed.
- R. Bergman, F. Alvarez, A. Alegría and J. Colmenero, J. Non-Cryst. Solids, 1998, 235–237, 580–583 CrossRef CAS.
- R. Casalini, A. W. Snow and C. M. Roland, Macromolecules, 2013, 46, 330–334 CrossRef CAS.
- K. Schmidt-Rohr, A. S. Kulik, H. W. Beckham, A. Ohlemacher, U. Pawelzik, C. Boeffel and H. W. Spiess, Macromolecules, 1994, 27, 4733–4745 CrossRef CAS.
- K. Chat, W. Tu, A. Beena Unni and K. Adrjanowicz, Macromolecules, 2021, 54, 8526–8537 CrossRef CAS.
- J. Nicolas, Y. Guillaneuf, C. Lefay, D. Bertin, D. Gigmes and B. Charleux, Prog. Polym. Sci., 2013, 38, 63–235 CrossRef CAS.
- G. Moad, E. Rizzardo, S. H. Thang, G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2012, 65, 985–1076 CrossRef CAS.
- K. Matyjaszewski, Macromolecules, 2012, 45, 4015–4039 CrossRef CAS.
- Y. Miura, T. Satoh, A. Narumi, O. Nishizawa, Y. Okamoto and T. Kakuchi, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 1436–1446 CrossRef CAS.
- Y. K. Chong, G. Moad, E. Rizzardo, M. A. Skidmore and S. H. Thang, Macromolecules, 2007, 40, 9262–9271 CrossRef CAS.
- L. P. Singh and R. Richert, Phys. Rev. Lett., 2012, 109, 167802 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR spectra, SEC chromatograms, additional dielectric results with further discussion. See DOI: https://doi.org/10.1039/d2cc01186g |
| This journal is © The Royal Society of Chemistry 2022 |