Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Aggregation-induced emission by sequence-selective assembly of cyanolated distyrylbenzene in supramolecular DNA architectures†
Hülya
Ucar
and
Hans-Achim
Wagenknecht
 *
*
Institute of Organic Chemistry, Karlsruhe Institute of Technology (KIT), Fritz-Haber-Weg 6, 76131 Karlsruhe, Germany. E-mail: Wagenknecht@kit.edu
First published on 3rd May 2022
Abstract
Cyanolated distyrylbenzene conjugated to 2′-deoxyuridine is a new building block for supramolecular DNA architectures combining aggregation-induced emission and sequence-selective binding. A high number of binding sites at the DNA template are occupied by cyanolated distyrylbenzenes. Light can be harvested in this assembly and transferred to terminal Atto dyes. Mixed DNA architectures with perylene were programmed by the sequence of the DNA template.
The supramolecular assembly of chromophores into designed hierarchically ordered architectures gives materials with unique optical properties.1 DNA as a template for such architectures allows the sequence-specific assembly of chromophores via the encoded hydrogen bonding pattern.2 The important features of DNA, chirality and helicity, persist even in the solid state for optoelectronic applications.3 Organic fluorophores typically show aggregation-caused quenching of their fluorescence in DNA-templated assemblies, e.g. of naphthalenes and oligo(para-phenylene vinylenes),4 porphyrines,5,6 pyrenes,7–9 perylenes10 and nile reds.7,8 An enhanced fluorescence intensity, so-called “aggregation-induced emission” (AIE), is beneficial for optoelectronic applications and was described initially for 1,2,3,4,5-pentaphenylsilole.11 Since then, there have been more and more examples of differently structured organic chromophores with AIE.12–14 We recently applied tetraphenylethylene as the most prominent example for non-covalently assembled DNA architectures with AIE.15 However, only 10–11 out of 20 available binding sites of the DNA templates were occupied by the tetraphenylenes.16 The propeller-shaped conformation of this chromophore – although planarized in the DNA assembly – sterically hinders and prohibits the sequence-selective DNA assembly in combination with a second chromophore. Herein, we present a cyanolated distyrylbenzene17,18-conjugate with 2′-deoxyuridine (Cs-dU, Fig. 1(A)) allowing not only a higher occupancy of the DNA template due to its flatter conformation, but also a mixed assembly with a perylene-modified 2′-deoxynucleoside.
 | ||
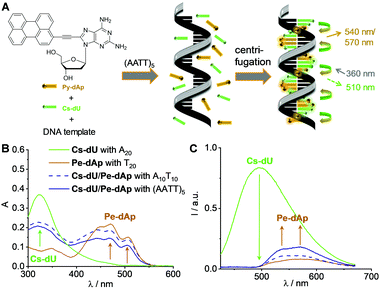
| Fig. 1 (A) Structure of Cs-dU and illustration of the supramolecular assembly of Cs-dU in the presence of A20 as a template. (B) Image of the solvatochromic fluorescence of Cs-dU using a UV-A handheld lamp. (C) Solvatochromic fluorescence of Cs-dU in different solvents; 37.5 μM Cs-dU, 1 h at r.t., λexc = 390 nm. (D) Circular dichroism (CD) of Cs-dU without and with A20; 1.25 μM DNA in H2O (1% DMSO), 25 μM Cs-dU, 250 mM NaCl, 1 h at r.t. (E) Fluorescence of Cs-dU without and with A20 (and T20 in comparison), image of the cuvettes using a handheld UV-A lamp (inset in A); 1.25 μM DNA in H2O (1% DMSO), 1.25 μM (n = 1) and 25 μM (n = 20) Cs-dU, 250 mM NaCl, 1 h at r.t, λexc = 360 nm; for UV/Vis absorption see Fig. S8 (ESI†). | ||
Cs-dU was synthesized by a Sonogashira coupling of the iodinated chromophore precursor to 5-ethynyl-2′-deoxyuridine (Scheme S1 and Fig. S1–S6, see ESI†). The ethynylene linker between the chromophore and 2′-deoxuridine is important because it allows coplanar orientation of the aromatic systems on both sides, which is a prerequisite for the successful DNA-templated assembly.19Cs-dU shows a strong solvatochromic fluorescence (Fig. 1(B) and (C), Table S1 and Fig. S7, ESI†), typical for this chromophore.20 Without the DNA template, Cs-dU shows a low fluorescence intensity (ΦF = 0.05, λexc = 389 nm) probably due to soluble aggregates. The Cs-dU obviously assemble in a coplanar order that gives excitonic coupling and helicity according to the observed Cotton effect in the CD spectra (vide infra). Based on our experience with other chromophore–nucleoside conjugates (pyrene, perylene, nile red),7–10 these aggregates must be small, because they cannot be removed by centrifugation. If Cs-dU was assembled with A20 as a complementary DNA template, there is a strong AIE with a maximum at 550 nm (ΦF = 0.14). The AIE is significantly smaller in the non-templated assemblies (without any DNA) and with the non-complementary (wrong) DNA template T20, which shows that Cs-dU binds preferably and selectively to the dA moieties in the right template A20 (Fig. 1(E)), like all our previous chromophore-ethynylene-dU conjugates.7–9,19 Notably, the AIE with T20 shows nearly the same maximum at 544 nm as the AIE with A20 at 546 nm and differs from the emission of pure Cs-dU in water with a maximum at 558 nm, indicating the effect of hydrogen-bonding to the templates, mismatched with T20 and matched with A20. Notably, Cs-dU is a modified T and provides hydrogen-bonding to another Cs-dU, but the AIE is small compared to the templated assemblies. The combination of the intrinsic stacking of Cs-dU in water and the recognition to the template A20 that controls further stacking of Cs-dU into larger assemblies yields the strongest AIE. In the presence of 15–16 equivalents Cs-dU (with respect to the amount of the template A20) the templated fluorescence intensity increase changes to the more shallow linear increase that is also observed in water without any DNA template (see analysis in Fig. S9 and S10, ESI†). This clearly shows that there is additional AIE by the stacking of Cs-dU in the assembly along the template A20 which cannot be induced in the small aggregates of Cs-dU formed without the DNA template. That means additionally that approximately 15–16 out of 20 possible binding sites available on A20 get occupied by the Cs-dU monomers. This is a significantly higher occupancy degree and thus an improvement compared to the tetraphenylethylene conjugate.16 These results do not provide any evidence for cooperativity in the DNA architectures. The CD spectra of both the non-templated and the templated samples of Cs-dU in water show a positive couplet between 290 and 350 nm with zero crossing at 318 nm (Fig. 1(D)). This reveals that the non-templated, smaller aggregates (as mentioned above) of stacked Cs-dU in water exhibit already excitonic coupling and an intrinsic right-handed helicity induced by the 2′-deoxyribofuranoside, which fits then perfectly to the chirality of the DNA template A20. The template extends the size of the Cs-dU assemblies, which cannot be seen in the CD spectrum because there is no long-range excitonic coupling. Taken together with the strong fluorescence intensity increase observed with the template A20, this provides further evidence that stacking of Cs-dU in this assembly causes the AIE.
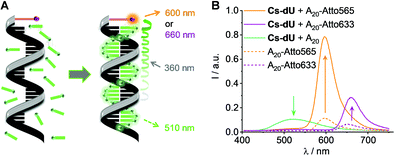
DNA templates A20-Atto565 and A20-Atto633 with the Atto dyes attached to their 5′-termini were applied to prepare DNA-based light harvesting systems with Cs-dU (Fig. 2(A) and Fig. S11, ESI†). We assume that the AIE of Cs-dU in the assembly along A20 is a prerequisite for the energy transfer because it provides significant overlap with the absorbance of the Atto dyes according to Förster. The fluorescence intensity increase that is observed as AIE of Cs-dU in the assembly along A20 shows that competing non-radiative processes are suppressed which opens the possibility for an energy transfer to appropriate acceptors. Using these supramolecular DNA architectures, the excitation energy is transferred from the planarized and thus emissive Cs-dU within the assembly to the terminal Atto dyes as energy acceptors. When Cs-dU as an energy donor in the DNA assemblies is excited at 389 nm, the fluorescence of the Cs-dU units in both assemblies, with A20-Atto565 and A20-Atto633, at 550 nm is quenched compared to the aggregation-induced emission of the Cs-dU assembly with A20. Concomitantly, the fluorescence intensity of the acceptor at 598 nm (with A20-Atto565) and at 660 nm (with A20-Atto633) is strongly enhanced (Fig. 2(B)). The quantum yield of Cs-dU (fluorescence range 400–550 nm) is ΦD = 0.03 with A20-Atto565 (and ΦD = 0.04 with A20-Atto633) and the quantum yield of the Atto dyes (fluorescence range 550–675 nm) is ΦDA = 0.16 with A20-Atto565 (and ΦA = 0.14 with A20-Atto633). The energy transfer efficiencies were determined according to 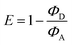 , which gives values of E = 81% for the DNA assemblies with A20-Atto565 and E = 72% with A20-Atto633 (Table S2, ESI†). The energy transfer is more efficient than in light-harvesting DNA architectures with 18 pyrenes as the conventional fluorophore for which 56% energy transfer efficiency was observed.21 Notably, the latter example was based on the pyrene excimer fluorescence in their systems, which complements the use of AIE in our DNA architectures, because excimers are formed only in the excited state and bathochromically shift the pyrene fluorescence. It can be assumed that the energy transfer between the Cs-dU units and the Atto dyes in these supramolecular DNA architectures is even more efficient because the fluorescence ranges of Cs-dU and the Atto dyes overlay to a significant extent between 550 nm and 650 nm and thus cannot be completely separated for the calculation of E. Based on the calculated E values, at least 11–13 out of 15–16 DNA-bound Cs-dU units are involved and their fluorescence is quenched. Using a regular stacking distance of 3.4 Å, the farest Cs-dU involved in energy transfer is located between 51 Å and 68 Å away from the Atto dye at the 5′-terminus. We assume, therefore, that the efficient fluorescence quenching over such distances occurs via a step-wise homo-energy transport between the Cs-dU units preceeding the final energy transfer to the Atto dyes. This energy transfer mechanism was previously validated for similar supramolecular DNA architectures with tetraphenylethylene-dU covalently incorporated into oligonucleotides complementary to A20.16
, which gives values of E = 81% for the DNA assemblies with A20-Atto565 and E = 72% with A20-Atto633 (Table S2, ESI†). The energy transfer is more efficient than in light-harvesting DNA architectures with 18 pyrenes as the conventional fluorophore for which 56% energy transfer efficiency was observed.21 Notably, the latter example was based on the pyrene excimer fluorescence in their systems, which complements the use of AIE in our DNA architectures, because excimers are formed only in the excited state and bathochromically shift the pyrene fluorescence. It can be assumed that the energy transfer between the Cs-dU units and the Atto dyes in these supramolecular DNA architectures is even more efficient because the fluorescence ranges of Cs-dU and the Atto dyes overlay to a significant extent between 550 nm and 650 nm and thus cannot be completely separated for the calculation of E. Based on the calculated E values, at least 11–13 out of 15–16 DNA-bound Cs-dU units are involved and their fluorescence is quenched. Using a regular stacking distance of 3.4 Å, the farest Cs-dU involved in energy transfer is located between 51 Å and 68 Å away from the Atto dye at the 5′-terminus. We assume, therefore, that the efficient fluorescence quenching over such distances occurs via a step-wise homo-energy transport between the Cs-dU units preceeding the final energy transfer to the Atto dyes. This energy transfer mechanism was previously validated for similar supramolecular DNA architectures with tetraphenylethylene-dU covalently incorporated into oligonucleotides complementary to A20.16
 | ||
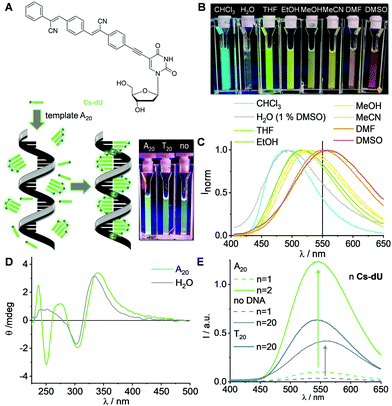
| Fig. 2 (A) Formation of supramolecular light-harvesting DNA architectures with Cs-dU and the templates A20-Atto565 and A20-Atto633. (B) Fluorescence, 1.25 μM DNA template in water, 25 μM Cs-dU, +1% DMSO, 250 mM NaCl, λexc = 360 nm. For the UV/Vis absorption see Fig. S12 (ESI†). | ||
The fact that 15–16 binding sites of the A20 templates can be occupied by Cs-dU indicates that the cyanolated distyrylbenzene chromophore is more planar and fits better into the DNA-based assembly compared to the tetraphenylethylene chromophore, where only 11 binding sites were occupied.16 This property allows us to use Cs-dU in combination with Pe-dAp as a second chromophore nucleoside conjugate in mixed assemblies (Fig. 3(A)). Such mixed assemblies could not be accomplished with the tetraphenylethylene chromophore. The chromophore sequence in these assemblies is encoded in the sequence of the DNA template, based on the canonical pairing of Cs-dU preferably to A20 (see above) and Pe-dAp selectively to T20.10,19 The mixed chromophore assemblies were prepared with A10T10 and (AATT)5 as DNA templates. Both templates have an identical number of binding sites for both Cs-dU and Pe-dAp, but different sequences, and identical length to the DNA templates A20 and T20. After incubation at r.t. for 1 h, excess of unbound monomers was removed by centrifugation (1 min, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g, Fig. S13, ESI†). The supernatant contains the DNA assembly with bound chromophores. The mixed DNA assemblies show the characteristic absorption of Cs-dU at 323 nm and of Pe-dAp at 470 nm and 506 nm (Fig. 3(B)). These absorbances of the mixed assemblies with the DNA templates A10T10 and (AATT)5 (each 1.25 μM) have to be compared with the pure assemblies of Cs-dU with A20 and Pe-dAp with T20 to obtain enough evidence that mixed assemblies were indeed formed. The absorption of the pure assembly of Pe-dAp along T20 is A = 0.151 at 506 nm. This wavelength is selective for Pe-dAp. If we assume that approximately 15 out of 20 available binding sites at T20 are occupied, the absorption A = 0.104 of the mixed assemblies along A10T10 and (AATT)5 as templates indicates full occupancy of their 10 available binding sites by Pe-dAp monomers. The wavelength 323 nm is not selective for Cs-dU because there is significant absorption overlap with Pe-dAp. The absorption of the pure assembly of Cs-dU along A20 at 323 nm is A = 0.369, and the mixed assembly along A10T10 has an absorption of A = 0.206. 10 Pe-dAp units account for an absorption of maximal ΔA = 0.05 (based on the 323 nm absorption A = 0.081 for the pure Pe-dAp assembly along T20). The remaining absorption ΔA = 0.156 at 323 nm clearly evidences that 6–7 out of 10 binding sites at the template A10T10 are occupied by Cs-dU monomers. Taken together, there is clear evidence for sequence selective assembly of both chromophores into the desired mixed assemblies given by the sequence of the DNA template. The similarity of the UV/Vis absorption of the DNA assemblies with A10T10 and (AATT)5 adds even more evidence for the formation of the sequence-selective assembly because both templates provide an equal number of binding sites for Cs-dU and Pe-dAp, but a different sequence. As a result, we observe an energy transfer between Cs-dU and Pe-dAp in these mixed DNA architectures. The AIE of the Cs-dU units in the pure assembly with A20 at 500 nm is completely quenched by the Pe-dAp units in the assembly with A10T10 and (AATT)5. Instead, an increase of the Pe-dAp fluorescence intensity at 538/570 nm as a result of the energy transfer is observed compared to the fluorescence of the pure assembly of Pe-dAp with T20 although the mixed assemblies with A10T10 and (AATT)5 contain a bisected amount of Pe-dAp monomers. Based on the sequence-selective assembly dictated by the template, the number of interfaces between Cs-dU and Pe-dAp is higher in the mixed assembly with (AATT)5 than with A10T10. More interfaces improve the energy transfer efficiency; thus, the fluorescence intensity is higher for the assembly with (AATT)5.
000 × g, Fig. S13, ESI†). The supernatant contains the DNA assembly with bound chromophores. The mixed DNA assemblies show the characteristic absorption of Cs-dU at 323 nm and of Pe-dAp at 470 nm and 506 nm (Fig. 3(B)). These absorbances of the mixed assemblies with the DNA templates A10T10 and (AATT)5 (each 1.25 μM) have to be compared with the pure assemblies of Cs-dU with A20 and Pe-dAp with T20 to obtain enough evidence that mixed assemblies were indeed formed. The absorption of the pure assembly of Pe-dAp along T20 is A = 0.151 at 506 nm. This wavelength is selective for Pe-dAp. If we assume that approximately 15 out of 20 available binding sites at T20 are occupied, the absorption A = 0.104 of the mixed assemblies along A10T10 and (AATT)5 as templates indicates full occupancy of their 10 available binding sites by Pe-dAp monomers. The wavelength 323 nm is not selective for Cs-dU because there is significant absorption overlap with Pe-dAp. The absorption of the pure assembly of Cs-dU along A20 at 323 nm is A = 0.369, and the mixed assembly along A10T10 has an absorption of A = 0.206. 10 Pe-dAp units account for an absorption of maximal ΔA = 0.05 (based on the 323 nm absorption A = 0.081 for the pure Pe-dAp assembly along T20). The remaining absorption ΔA = 0.156 at 323 nm clearly evidences that 6–7 out of 10 binding sites at the template A10T10 are occupied by Cs-dU monomers. Taken together, there is clear evidence for sequence selective assembly of both chromophores into the desired mixed assemblies given by the sequence of the DNA template. The similarity of the UV/Vis absorption of the DNA assemblies with A10T10 and (AATT)5 adds even more evidence for the formation of the sequence-selective assembly because both templates provide an equal number of binding sites for Cs-dU and Pe-dAp, but a different sequence. As a result, we observe an energy transfer between Cs-dU and Pe-dAp in these mixed DNA architectures. The AIE of the Cs-dU units in the pure assembly with A20 at 500 nm is completely quenched by the Pe-dAp units in the assembly with A10T10 and (AATT)5. Instead, an increase of the Pe-dAp fluorescence intensity at 538/570 nm as a result of the energy transfer is observed compared to the fluorescence of the pure assembly of Pe-dAp with T20 although the mixed assemblies with A10T10 and (AATT)5 contain a bisected amount of Pe-dAp monomers. Based on the sequence-selective assembly dictated by the template, the number of interfaces between Cs-dU and Pe-dAp is higher in the mixed assembly with (AATT)5 than with A10T10. More interfaces improve the energy transfer efficiency; thus, the fluorescence intensity is higher for the assembly with (AATT)5.
In conclusion, we demonstrated that Cs-dU is a unique building block for supramolecular DNA architectures. It follows the idea of non-covalent chromophore assembly along the DNA template by stacking interactions between the chromophores and specific recognition by hydrogen bonding to the DNA template, as evidenced earlier by Schenning et al.22,23 Our DNA architectures combine AIE in the non-covalent DNA assembly with energy transfer. The basic idea of our supramolecular DNA architectures with Cs-dU is not to simply state that AIE is needed for efficient energy transfer. However, the AIE provides the spectroscopic readout for the successful DNA-templated assembly of Cs-dU. The energy transfer pathways are then controlled by the stacked Cs-dUs in the assemblies with the terminal Atto dyes and to the Pe-dAps by their sequence-specific assembly. The rather planar conformation of Cs-dU yields a high degree of occupied binding sites at the DNA template achieved with Cs-dU, which cannot be realized with the propeller shaped tetraphenylethylenes. This paved the way for the preparation of mixed DNA assemblies with Pe-dAp. The sequence of the DNA template does not only program the sequence of the mixed assemblies, but modulates also their energy transfer and optical properties. So far, a precise arrangement of different chromophores in DNA architectures was only possible by covalent incorporation.24,25 Light harvesting through efficient energy transport within the DNA assemblies of the Cs-dU units to 5′-terminal Atto dyes was also realized with high efficiency. The AIE of these supramolecular DNA architectures and their energy transport properties represent new DNA-based materials for technological applications.
HU performed the experiments and wrote parts of the manuscript. HAW supervised the research and wrote the manuscript.
Financial support by the Deutsche Forschungsgemeinschaft (DFG, grant Wa 1386/20-1) and KIT is gratefully acknowledged.
Conflicts of interest
There are no conflicts to declare.Notes and references
- T. F.-A. De Greef, M. M.-J. Smulders, M. Wolffs, A. P.-H. J. Schenning, R. P. Sijbesma and E. W. Meijer, Chem. Rev., 2009, 109, 5687–5754 CrossRef CAS PubMed.
- A. Ruiz-Carretero, P. G.-A. Janssen, A. Kaeser and A. P.-H. J. Schenning, Chem. Commun., 2011, 47, 4340–4347 RSC.
- S. Müller, F. Manger, L. G.-v Reventlow, A. Colsmann and H.-A. Wagenknecht, Front. Chem., 2021, 9, 645006 CrossRef PubMed.
- M. Surin, P. G.-A. Janssen, R. Lazzaroni, P. Leclère, E. W. Meijer and A. P.-H. J. Schenning, Adv. Mater., 2009, 21, 1126–1130 CrossRef CAS.
- G. Sargsyan, B. M. Leonard, J. Kubelka and M. Balaz, Chem. – Eur. J., 2014, 20, 1878–1892 CrossRef CAS PubMed.
- G. Sargsyan, A. A. Schatz, J. Kubelka and M. Balaz, Chem. Commun., 2013, 49, 1020–1022 RSC.
- R. Hofsäβ, S. Sinn, F. Biedermann and H. A. Wagenknecht, Chemistry, 2018, 24, 16257–16261 CrossRef.
- P. Ensslen, Y. Fritz and H.-A. Wagenknecht, Org. Biomol. Chem., 2015, 13, 487–492 RSC.
- S. Sezi and H.-A. Wagenknecht, Chem. Commun., 2013, 49, 9257–9259 RSC.
- S. Müller, Y. Fritz and H. A. Wagenknecht, ChemistryOpen, 2020, 9, 389–392 CrossRef PubMed.
- J. Luo, Z. Xie, J. W.-Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhuc and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- J. Li, J. Wang, H. Li, N. Song, D. Wang and B. Z. Tang, Chem. Soc. Rev., 2020, 49, 1144–1172 RSC.
- D. Wang and B. Z. Tang, Acc. Chem. Res., 2019, 52, 2559–2570 CrossRef CAS PubMed.
- K. Kokado and K. Sada, Angew. Chem., Int. Ed., 2019, 58, 8632–8639 CrossRef CAS PubMed.
- X. Gu, J. Yao, G. Zhang, Y. Yan, Y. Zhao and D. Zhang, Chem. – Asian J., 2013, 8, 2362–2369 CrossRef CAS.
- H. Ucar and H.-A. Wagenknecht, Chem. Sci., 2021, 12, 10048–10053 RSC.
- J. Han, J. You, X. Li, P. Duan and M. Liu, Adv. Mater., 2017, 29, 1606503 CrossRef.
- S. Yokoyama and N. Nishiwaki, J. Org. Chem., 2019, 84, 1192–1200 CrossRef CAS PubMed.
- Y. Fritz and H.-A. Wagenknecht, Front. Chem., 2019, 7, 659 CrossRef CAS PubMed.
- S. B. Noh, R. H. Kim, W. J. Kim, S. Kim, K.-S. Lee, N. S. Cho, H.-K. Shim, H. E. Pudavar and P. N. Prasad, J. Mater. Chem., 2010, 20, 7422–7429 RSC.
- O. O. Adeyemi, V. L. Malinovskii, S. M. Biner, G. Calzaferri and R. Häner, Chem. Commun., 2012, 48, 9589–9591 RSC.
- A. Ruiz-Carretero, P. G.-A. Janssen, A. L. Stevens, M. Surin, L. M. Herz and A. P.-H. J. Schenning, Chem. Commun., 2011, 47, 884–886 RSC.
- A. L. Stevens, P. G.-A. Janssen, A. Ruiz-Carretero, M. Surin, A. P.-H. J. Schenning and L. M. Herz, J. Phys. Chem. C, 2011, 115, 10550–10560 CrossRef CAS.
- P. Ensslen, F. Brandl, S. Sezi, R. Varghese, R.-J. Kutta, B. Dick and H.-A. Wagenknecht, Chem. – Eur. J., 2015, 21, 9349–9354 CrossRef CAS PubMed.
- P. Röthlisberger, V. Kaliginediand and C. J. Leumann, Chem. – Eur. J., 2017, 23, 2022–2025 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details about the synthesis of Cs-dU and additional optical spectroscopy data. See DOI: https://doi.org/10.1039/d2cc01161a |
| This journal is © The Royal Society of Chemistry 2022 |