Open Access Article
Open Access ArticleDirect nitrogen interception from chitin/chitosan for imidazo[1,5-a]pyridines†
Kui
Zeng
a,
Ruhuai
Mei
a,
Xizhou Cecily
Zhang
b,
Loren B.
Andreas
 b and
Kai
Zhang
b and
Kai
Zhang
 *a
*a
aSustainable Materials and Chemistry, Dept. Wood Technology and Wood-based Composites, Georg-August-University of Göttingen, D-37077 Göttingen, Germany. E-mail: kai.zhang@uni-goettingen.de
bNMR-based Structural Biology, Max-Planck-Institute for Biophysical Chemistry, D-37077 Göttingen, Germany
First published on 8th April 2022
Abstract
A catalyst-free one-pot methodology that enables direct nitrogen interception of chitosan/chitin for imidazo[1,5-a]pyridines was developed. This strategy features direct synthesis of important deuterated imidazo[1,5-a]pyridines and tridentate ligands. In particular, a broad group of previously inaccessible products including saturated 1-alkylimidazo[1,5-a]pyridines are unprecedently synthesized by this protocol.
Chitin and chitosan are abundant native linear polymers composed of randomly distributed units, namely, N-acetyl-D-glucosamine (GlcNAc) and D-glucosamine (GlcN) linked by β-1,4-linkages.1 The quantities of GlcNAc units are generally more than 50% in chitin, while chitosan contains less than 50% GlcNAc and has thus a degree of deacetylation (DD) of more than 50%.2–4 Human processing of sustainable chitin-containing raw materials represents only a very minor fraction of all the chitin produced annually in nature,5–10 while the major fraction remains intact.11 On the other hand, low molecular weight nitrogen-containing chemicals (NCCs) play a pivotal role in modern life, from pharmaceutical, agriculture and food fields to material fields.12–16 The nitrogen source of prevailing industrial processes for NCCs is mainly from NH3, NO3− and NO2−etc., which are obtained from N2 fixation.17–25 The quantities of nitrogen element fixed annually in chitin via the biochemical process are much more than the N2 fixed in the Haber–Bosch process.26 Therefore, the transformation of renewable chitin containing fixed nitrogen into value-added NCCs has drawn much attention.14
Four types of strategies are currently known for the activation of amines in chitin/chitosan (Scheme 1a). The first strategy (i) is referred to as the direct modification of amines on the chitosan/chitin backbone without the cleavage of C–N bonds.5 The second strategy (ii) involves the cleavage of C–N bonds via strong oxidants or acidic conditions with the simultaneous release of N2, acetamide and ammonium salts.27–29 Recently, the third strategy (iii) has emerged in biorefineries by converting chitin/chitosan into a preliminary C6 backbone via a depolymerization and by further conversion of the C6 backbone into diversified products.14,26,30 Although various protocols have been established through enzymatic, catalytic and/or hydrothermal treatments, only more than 10 NCC examples (including sugar derivatives, amino alcohols and furanic amides) have been obtained with complicated conditions and a low efficiency. The fourth strategy (iv) involves the cleavage of C–N bonds of chitin for the assembly of pyrrole with a low yield of 4%, which was realized in alkali aqueous solution at 300 °C in 2016.31 In particular, it should be stressed that the synthesis of N-heterocycles from chitin/chitosan biomass is challenging and introducing an unsustainable nitrogen source such as NH3 is the main pathway for the construction of N-heterocycles from biomass.32–34 The C–N bond of chitin/chitosan that offers a potential reactive site for various versatile chemical diversifications generally remains intact. Therefore, a one-pot protocol enabling the targeted efficient incorporation of nitrogen from chitin/chitosan into diverse valuable NCCs like N-heterocycles is highly attractive, which will advance the existing methodologies while expanding the library of NCCs derived from renewable sources.
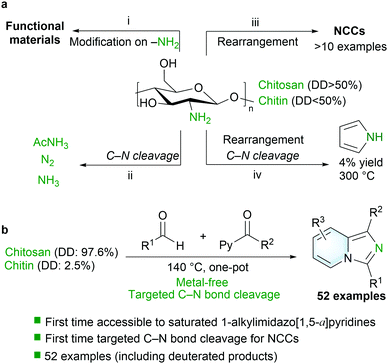 | ||
| Scheme 1 (a) General strategies for the activation of amines in chitin/chitosan. (b) This work: direct incorporation of nitrogen from chitosan/chitin for imidazo[1,5-a]pyridines. | ||
Herein, we developed an efficient catalyst-free one-pot protocol for the direct integration of nitrogen from the renewable feedstock chitosan/chitin that yields various valuable imidazo[1,5-a]pyridines (52 examples with yields up to 92%) under mild conditions (Scheme 1b), which provides ready access to 1-alkylimidazo[1,5-a]pyridines and 1-arylimidazo[1,5-a]pyridines.
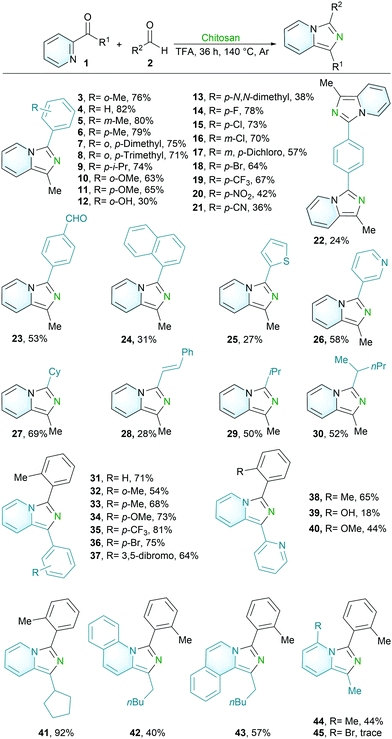
We initiated our studies by using 2-acetylpyridine (1a) and 2-methylbenzaldehyde (2a) as substrates to evaluate the envisioned nitrogen interception from chitosan for desired imidazo[1,5-a]pyridine 3 employing metal-free conditions. At the outset, product 3 was detected by 1H NMR analysis under conditions with a solvent mixture of AcOH/H2O (0.9/0.1 mL) (Table S1, entries 1 and 2, †). After that, several silver salt additives were investigated. Interestingly, the yield of 3 was increased obviously from 3% to 13% when silver trifluoroacetate (AgTFA) was exploited, while silver acetate (AgOAc) was not beneficial for the efficacy (Table S1, entries 3 and 4, †). These results indicated that the anion −OCOCF3 in AgTFA might facilitate the transformation. Therefore, a solvent mixture of CF3COOH/H2O (0.9/0.1 mL) was employed as a substitute of the previous solvent AcOH/H2O (0.9/0.1 mL). To our delight, the yield was improved to 29% when CF3COOH/H2O was used as reaction media (Table S1, entry 5, †). Then, a higher yield (61%) was achieved with CF3COOH (1.0 mL) as the solvent (Table S1, entry 6, †). Besides, diverse reaction temperatures, ranging from 90 °C to 140 °C, were examined (Table S1, entries 7 and 8, †). Thus, the optimal temperature was determined to be 140 °C and a 78% yield was obtained. Moreover, the reaction was conducted at 12 h, 24 h and 36 h with a yield of 31%, 59% and 78%, respectively. The lower equivalent of chitosan leads to inferior yield. Combining all factors regarding the equivalent of chitosan, reaction temperature and time, the optimal reaction conditions were identified as shown in entry 8: 140 °C for 36 h (Table S1, †).
With the optimized reaction conditions in hand, we next explored the versatility of metal-free nitrogen interception from chitosan with various aldehydes for NCCs (Scheme 2). A series of aromatic aldehydes, including those bearing electron-donating or electron-withdrawing groups at different positions (ortho, meta or para) were subjected to the optimized conditions. As a result, these aldehydes could be efficiently transformed into the corresponding products 3–23. Aldehydes with electron-donating groups, such as methyl, isopropyl and methoxy groups (3–11), are well compatible with the transformations, while lower yields were obtained for substrates with a hydroxyl group and dimethylamino group (12 and 13). The reason could be that the dimethylamino and hydroxyl groups with high nucleophilicity are much more sensitive to carbonyl groups. Surprisingly, a larger scale (1a, 0.5 mmol) with a lower load of chitosan (2.0 equiv.) in a smaller volume of TFA (0.5 M) was also well compatible for the desired product 4 (Fig. S4, †). In addition, electron-withdrawing substituents, including halogen and trifluoromethyl groups, were well accommodated (14–19). Moderate yields were achieved for aldehydes with nitro and nitrile groups (20 and 21). It is noteworthy that the challenging para-dialdehyde also delivered the corresponding products with diverse conditions (22 and 23). With the controllable reaction conditions, products 22 and 23 can be obtained separately, but it is not excluded that there is a tiny amount of 23 in 22, and a small amount of 22 in 23 (the amount can be ignored). Furthermore, 1-naphthaldehyde (24), thiophene-2-carbaldehyde (25) and nicotinaldehyde (26) were also amenable in our protocol. In addition to aromatic aldehydes, those aliphatic aldehydes (27–30) were well compatible with the protocol.
We further investigated the viable scope of differently substituted pyridine ketone 1 as the general coupling partners for this transformation (Scheme 2). The phenyl(pyridin-2-yl)methanones were well compatible with the protocol (31). Pyridine ketones with electron-donating groups (32–34) or electron-withdrawing groups (35–37) displayed good reactivity and delivered the corresponding products efficiently. These results indicated that the current protocol was not sensitive to the electronic or stereoscopic properties of pyridine ketones. N-Heterocyclic compounds play an important role in the materials and organic synthesis fields, especially as ligands in chemical transformations. Thus, the utilization of a sustainable protocol for the assembling of N-heterocyclic tridentate ligands is highly desirable. Herein, these di/tri-dentate ligands, 1-(pyridin-2-yl)-3-(o-tolyl)imidazo[1,5-a]pyridine (38), 2-(1-(pyridin-2-yl)imidazo[1,5-a]pyridin-3-yl)phenol (39) and 3-(2-methoxyphenyl)-1-(pyridin-2-yl)imidazo[1,5-a]pyridine (40), were accessed concisely through the interception of nitrogen from chitosan, which further demonstrates the practical synthetic use of our method. Moreover, cyclopentyl(pyridin-2-yl)methanone (41) and 2-quinoline/isoquinoline ketone compounds (42, 43) were also transformed efficiently. Three pyridine ketones with substituents in the pyridine ring were investigated in our protocol. As a result, a methyl group in the sixth position of pyridine was also well compatible for the desired product (44), while an electron-withdrawing group at the same position failed to deliver the corresponding product (45).
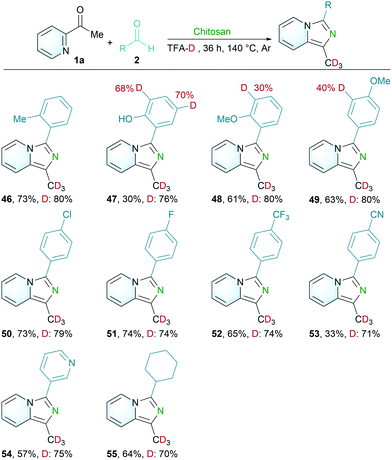
Isotope labeling showed important applications in drug absorption, distribution, metabolism and excretion.35–37 Inspired by the H–D exchange between α-C–H of the pyridine ketone and CF3COOD solvent, a variety of C(sp3)–H deuterated imidazo[1,5-a]pyridines derivatives were synthesized in a one-pot procedure (Scheme 3). Those aromatic aldehydes, pyridine aldehyde and cyclic aliphatic aldehyde were synthesized with deuterated incorporation of products (46–55). In addition, it is worth noting that the proton of the aromatic aldehyde with a hydroxy group and methoxy group could also be deuterated under this condition (47–49).
Furthermore, sustainable biomass chitin was proven to be a viable nitrogen source as well in this transformation, which could further bypass the deacylation process from chitin to chitosan and make the reaction more useful (Scheme 4). As a result, a group of imidazo[1,5-a]pyridines was obtained under the standard conditions, including 3 (71%), 14 (71%), 16 (53%), 24 (29%), 28 (17%), 38 (26%), 40 (16%) and 41 (89%). However, using chitosan as a nitrogen source in Fig. S2–S5 (†), these products can be obtained with higher yields, including 3 (76%), 14 (78%), 16 (70%), 24 (31%), 28 (28%), 38 (65%), 40 (44%) and 41 (92%). Although it is more convenient to utilize chitin rather than chitosan, chitosan shows better reaction efficiency to deliver NCCs with higher yields.
Moreover, 11% of ammonium was trapped via1H-NMR analysis, which could provide support for the major release of nitrogen after condensation into an intermediate, such as imidazo[1,5-a]pyridine-2,4-diium or imidazo[1,5-a]pyridin-4-ium (Fig. S2, †). Besides, an imidazo[1,5-a]pyridin-4-ium 3′ was confirmed by ESI-HRMS analysis (calc. for C15H15N2+ [M]: 223.1230, found: 223.1232) (Fig. S3, †), which reveals a pathway for imidazo[1,5-a]pyridine 3via deprotonation of 3′. Based on the control experiment (Fig. S2 and S3, †), a plausible reaction pathway was proposed. First, deacetylation takes place which enables the transformation of chitin to chitosan.38 Then, an imine intermediate is generated via the dehydration between an aldehyde substrate and an amine group of chitosan. The imine intermediate attacks a pyridine ketone substrate to form an iminium intermediate. Next, an imidazo[1,5-a]pyridine-2,4-diium intermediate forms via the intramolecular nucleophilic addition between pyridine and iminium in the backbone of the iminium intermediate.39 With the help of TFA, the imidazo[1,5-a]pyridine-2,4-diium transforms into an imidazo[1,5-a]pyridin-4-ium (Fig. S3, †) via plausible pathways of dehydration and C–N bond cleavage. Finally, an imidazo[1,5-a]pyridine was generated via the deprotonation of the imidazo[1,5-a]pyridin-4-ium.
In summary, we have developed a new strategy for the rapid assembly of NCCs with ample scope through the direct use of native nitrogen sources from biomass. This strategy is achieved through a one-pot conversion approach of chitin/chitosan by cleaving the C–N bonds and simultaneously integrating the nitrogen in the synthesis of a broad range of imidazo[1,5-a]pyridines (52 examples) that show diverse potential applications. A broad group of previously inaccessible products including saturated 1-alkylimidazo[1,5-a]pyridines is unprecedently synthesized by this protocol. The amine groups of the chitin/chitosan backbone are intercepted via aldehyde and pyridine ketone assisted with CF3COOH under metal-free conditions. We believe that this approach will initiate research endeavors for the targeted efficient incorporation of nitrogen from biomass for high-value NCCs.
Kai Zhang thanks German Research Foundation (DFG) for the financial support for the project with the grant number ZH546/3-1 and University of Göttingen for the Department Start-up funding. Kui Zeng thanks China Scholarship Council for the PhD grant.
Conflicts of interest
The authors declare no competing financial interest.Notes and references
- N. K. Mathur and C. K. Narang, J. Chem. Educ., 1990, 67, 938 CrossRef CAS.
- F. Croisier and C. Jérôme, Eur. Polym. J., 2013, 49, 780 CrossRef CAS.
- M. Rinaudo, Prog. Polym. Sci., 2006, 31, 603 CrossRef CAS.
- K. Zeng, T. Groth and K. Zhang, ChemBioChem, 2019, 20, 737 CrossRef CAS PubMed.
- M. R. Kumar, R. A. Muzzarelli, C. Muzzarelli, H. Sashiwa and A. Domb, Chem. Rev., 2004, 104, 6017 CrossRef PubMed.
- R. Jayakumar, N. Nwe, S. Tokura and H. Tamura, Int. J. Biol. Macromol., 2007, 40, 175 CrossRef CAS PubMed.
- J. Ferrer, G. Paez, Z. Marmol, E. Ramones, H. Garcia and C. Forster, Bioresour. Technol., 1996, 57, 55 Search PubMed.
- G.-H. Jo, R.-D. Park and W.-J. Jung, Chitin, Chitosan, Oligosaccharides Their Deriv., 2010, 37 Search PubMed.
- R. Yang, H. Li, M. Huang, H. Yang and A. Li, Water Res., 2016, 95, 59 CrossRef CAS PubMed.
- P. Deng, Z. Xu and Y. Kuang, Food Chem., 2014, 157, 490 CrossRef CAS PubMed.
- D. L. Bertuzzi, T. B. Becher, N. M. Capreti, J. Amorim, I. D. Jurberg, J. D. Megiatto Jr and C. Ornelas, Global Challenge, 2018, 2, 1800046 CrossRef PubMed.
- J. R. Rostrup-Nielsen, Science, 2005, 308, 1421 CrossRef CAS PubMed.
- C. Somerville, H. Youngs, C. Taylor, S. C. Davis and S. P. Long, Science, 2010, 329, 790 CrossRef CAS PubMed.
- X. Chen, S. Song, H. Li, G. k. Gözaydın and N. Yan, Acc. Chem. Res., 2021, 54, 1711 CrossRef CAS PubMed.
- N. Huang, P. Wang and D. Jiang, Nat. Rev. Mater., 2016, 1, 1 Search PubMed.
- V. Smil, Nature, 1999, 400, 415 CrossRef CAS.
- E. Ferguson and W. Libby, Nature, 1971, 229, 37 CrossRef CAS PubMed.
- H. L. Rutledge and F. A. Tezcan, Chem. Rev., 2020, 120, 5158 CrossRef CAS PubMed.
- H. Chen, R. Cai, J. Patel, F. Dong, H. Chen and S. D. Minteer, J. Am. Chem. Soc., 2019, 141, 4963 CrossRef CAS PubMed.
- L. Li, Z. Wu, H. Zhu, G. H. Robinson, Y. Xie and H. F. Schaefer, J. Am. Chem. Soc., 2020, 142, 6244 CrossRef CAS PubMed.
- S.-L. Meng, X.-B. Li, C.-H. Tung and L.-Z. Wu, Chem, 2021, 7, 1431 CAS.
- C. Chen, X. Zhu, X. Wen, Y. Zhou, L. Zhou, H. Li, L. Tao, Q. Li, S. Du and T. Liu, Nat. Chem., 2020, 12, 717 CrossRef CAS PubMed.
- R. Murray and D. Smith, Coord. Chem. Rev., 1968, 3, 429 CrossRef CAS.
- W. J. Brill, Sci. Am., 1977, 236, 68 CrossRef CAS.
- D. R. MacFarlane, P. V. Cherepanov, J. Choi, B. H. Suryanto, R. Y. Hodgetts, J. M. Bakker, F. M. F. Vallana and A. N. Simonov, Joule, 2020, 4, 1186 CrossRef CAS.
- Y. Liao, S.-F. Koelewijn, G. Van den Bossche, J. Van Aelst, S. Van den Bosch, T. Renders, K. Navare, T. Nicolaï, K. Van Aelst and M. Maesen, Science, 2020, 367, 1385 CrossRef CAS PubMed.
- H. Zhao, D. Lu, J. Wang, W. Tu, D. Wu, S. W. Koh, P. Gao, Z. J. Xu, S. Deng and Y. Zhou, Nat. Commun., 2021, 12, 1 CrossRef PubMed.
- G. Allan, Chitin Chitosan, 1989, 443 Search PubMed.
- R. A. Muzzarelli, Chitin, Elsevier, 2013 Search PubMed.
- X. Ma, G. Gözaydın, H. Yang, W. Ning, X. Han, N. Y. Poon, H. Liang, N. Yan and K. Zhou, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 7719 CrossRef CAS PubMed.
- X. Gao, X. Chen, J. Zhang, W. Guo, F. Jin and N. Yan, ACS Sustainable Chem. Eng., 2016, 4, 3912 CrossRef CAS.
- H. Li, H. Guo, Z. Fang, T. M. Aida and R. L. Smith, Green Chem., 2020, 22, 582 RSC.
- H. Wu, H. Li and Z. Fang, Green Chem., 2021, 23, 6675 RSC.
- S. Song, V. Fung Kin Yuen, L. Di, Q. Sun, K. Zhou and N. Yan, Angew. Chem., 2020, 132, 20018 CrossRef.
- G. Büldt, H. Gally, A. Seelig, J. Seelig and G. Zaccai, Nature, 1978, 271, 182 CrossRef PubMed.
- M. I. Blake, H. L. Crespi and J. J. Katz, J. Pharm. Sci., 1975, 64, 367 CrossRef CAS PubMed.
- C. Schmidt, Nat. Biotechnol., 2017, 35, 493 CrossRef CAS PubMed.
- B. S. Butola, The impact and prospects of green chemistry for textile technology, Woodhead Publishing, 2018 Search PubMed.
- J. Wang, R. Mason, D. VanDerveer, K. Feng and X. R. Bu, J. Org. Chem., 2003, 68, 5415 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc01060g |
| This journal is © The Royal Society of Chemistry 2022 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: