Electricity mediated [3+2]-cycloaddition of N-sulfonylcyclopropanes with olefins via N-centered radical intermediates: access to cyclopentane analogs†
Debarshi
Saha
,
Irshad Maajid
Taily
,
Nakshatra
Banerjee
and
Prabal
Banerjee
 *
*
Lab no. 406, Department of Chemistry, Indian Institute of Technology (IIT), Ropar, Rupnagar, Punjab-140001, India. E-mail: prabal@iitrpr.ac.in
First published on 30th March 2022
Abstract
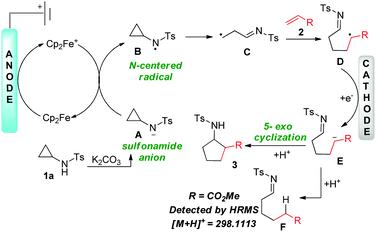
An external oxidant free electrochemical strategy is designed towards the β-scission of strained C–C bonds in cyclopropylamine. Moreover, the mechanistic studies ascertained that the methodology encompasses the N-center radical (NCRs) route and provides access to di- or tri-substituted cyclopentane analogs.
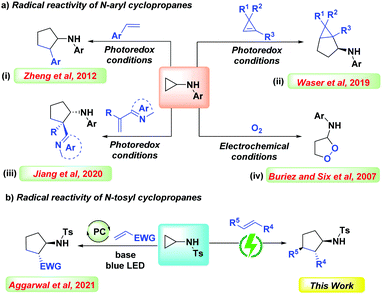
Over the past two decades, the scientific community has exploited the strain embodied cyclopropanes for diverse reactions towards the construction of complex molecular architectures.1 Despite the ring strain and inefficient orbital overlap, the cyclopropane ring is kinetically inert and requires activation.2a The advent of donor–acceptor cyclopropanes (DACs) has augmented the exploitation of cyclopropanes and has paved the way for several notable transformations.2b However, the activation of DACs, mostly requires Lewis or Brønsted acids.3 On the contrary, commercially available cyclopropylamines comprise N-substituents which act as a handle for stimulating strained C–C bond cleavage.4 Recently there has been an enormous surge in protocols promoting the generation of N-centered radicals (NCRs) by employing modern methodologies like photoredox catalysis and electroorganic synthesis.5 Being a versatile reaction intermediate, NCRs can react with various π-systems or undergo hydrogen atom transfer (HAT), providing access to several nitrogen-containing molecular frameworks.6 In this context, several notable transformations have been documented, wherein the N–H precursor undergoes proton/electron or electron/proton transfer to generate the NCRs.7,8 In 2012, Zheng et al. disclosed a photoredox mediated intermolecular [3+2] cycloaddition of cyclopropylamines with olefins (Scheme 1a, i).9 Later, in 2014, the same group further extended the protocol to alkynes affording cyclic allylic amines.9b Following this, in 2019, Waser et al. demonstrated the annulation of cyclopropylamines with cyclopropenes paving the way to bicyclohexanes (Scheme 1a, ii).10 In continuation of this endeavor, Jiang and Huang et al. revealed the photoredox based generation of all-carbon quaternary stereocenters via radical-based asymmetric reaction of cyclopropylamines with alkenes (Scheme 1a, iii).11
Electroorganic protocols are gaining influence due to their ability to encompass sustainable transformations.12 The recent resurgence in this area has led to the evolution of several unique synthetic methodologies.12 Numerous processes like decarboxylation,12a rearrangements,12b alkene bifunctionalization,12c and annulations12d have been established. In the same line of thought, recently, our group also disclosed some C–N,13a C–O,13b and C–C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13c bond forming electrochemical protocols. In this context, several electrochemical protocols have been revealed for the electrochemical activation of cyclopropylamines. In 2007, Buriez and Six et al. reported an electrochemical strategy to access endoperoxide rings via aerobic oxidation (Scheme 1a, iv).14 Very recently, Chen, Zare, and Zheng et al. demonstrated an electrocatalytic redox neutral annulation protocol for the synthesis of cyclopentane analogs using N-cyclopropylanilines.15 From the literature, it is evinced that most of the reported protocols highlight the utility of N-arylcyclopropanes certainly due to the low oxidation potential of the amino group (Ep/2 = 0.92 V vs. SCE).16 On substituting the aryl motif with the tosyl group, a substantial increase in the oxidation potential of the amino group (Ep/2 = 2.24 V vs. SCE) was observed, which makes it tedious for a facile oxidation process. Hence, the generation of N-radical cation becomes more challenging. However, the advantage of incorporating tosyl involves the easy deprotonation of the sulfonamide, making it readily available for oxidation.16 In the same line of thought, in 2021, Aggarwal et al. demonstrated a diastereoselective photoredox protocol for [3+2] cycloaddition of N-tosylcyclopropylamines with electron-deficient olefins via an NCRs pathway (Scheme 1b).16 Motivated by our constructive results in the electroorganic synthesis and our optimistic experience in the area of strained rings,13b we were encouraged to employ electricity as our major tool for performing the unprecedented [3+2] cycloaddition of N-sulfonylcyclopropylamines with olefins.
13c bond forming electrochemical protocols. In this context, several electrochemical protocols have been revealed for the electrochemical activation of cyclopropylamines. In 2007, Buriez and Six et al. reported an electrochemical strategy to access endoperoxide rings via aerobic oxidation (Scheme 1a, iv).14 Very recently, Chen, Zare, and Zheng et al. demonstrated an electrocatalytic redox neutral annulation protocol for the synthesis of cyclopentane analogs using N-cyclopropylanilines.15 From the literature, it is evinced that most of the reported protocols highlight the utility of N-arylcyclopropanes certainly due to the low oxidation potential of the amino group (Ep/2 = 0.92 V vs. SCE).16 On substituting the aryl motif with the tosyl group, a substantial increase in the oxidation potential of the amino group (Ep/2 = 2.24 V vs. SCE) was observed, which makes it tedious for a facile oxidation process. Hence, the generation of N-radical cation becomes more challenging. However, the advantage of incorporating tosyl involves the easy deprotonation of the sulfonamide, making it readily available for oxidation.16 In the same line of thought, in 2021, Aggarwal et al. demonstrated a diastereoselective photoredox protocol for [3+2] cycloaddition of N-tosylcyclopropylamines with electron-deficient olefins via an NCRs pathway (Scheme 1b).16 Motivated by our constructive results in the electroorganic synthesis and our optimistic experience in the area of strained rings,13b we were encouraged to employ electricity as our major tool for performing the unprecedented [3+2] cycloaddition of N-sulfonylcyclopropylamines with olefins.
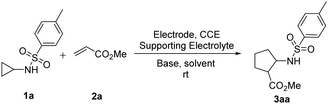
As illustrated in Table 1, our investigation commenced with an electrochemical reaction of N-tosylcyclopropylamine 1a and methyl acrylate 2a in an undivided cell equipped with carbon anode and nickel cathode in dichloroethane solvent. The reaction was performed under a constant current of 1.5 mA (2.8–3.4 V) with tetrabutylammonium tetrafluoroborate (Bu4NBF4) as the supporting electrolyte and ferrocene (Cp2Fe) as a mediator under ambient temperature for 5 h (entry 2). Delightfully, the protocol rendered the trans-product ethyl-2-((4-methylphenyl)sulfonamido)cyclopentane-1-carboxylate 3aa in 22% yield. The geometry of the compound was further supported by the NOE experiments (see ESI†). From the literature, we anticipated that a base could have remarkable impact on the generation of NCRs;17 as the base deprotonates the amine to generate the sulfonamide anion, which further oxidizes to active radical species. With this idea, organic bases like Et3N and DBU were employed; however, they failed to accomplish the desired transformation (entry 3). Further, performing the reaction with Na2CO3 resulted in an improved yield of up to 57% (entry 4). In contrast, the K2CO3 was found to be optimal with a 62% yield (entry 1). Next, solvents like DMSO, DMF, MeOH, and HFIP were found to be detrimental for the transformation (entry 5). A mixture of CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (4
H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) resulted in compromised yields (entry 6). At this juncture, we speculated that protic solvents might quench the sulfonamide anion leading to slower kinetics. Further examination regarding the choice of supporting electrolytes demonstrated Bu4NBF4 to be more suitable, while, Bu4NPF6, Et4NOTs or LiOTf proved incompatible (entry 7). The concentration of methyl acrylate proved to be a critical factor. A remarkable impact on the yield was observed when the reaction was attempted at higher concentrations of methyl acrylate (entry 8). Next, the investigation regarding the electrochemical conditions suggested that increasing or decreasing the current led to compromised results (entries 9 and 10). Subsequently, a series of electrode materials were varied; among them, the carbon anode/nickel cathode proved optimal (entries 11 and 12). Control experiments ascertained that electricity and ferrocene plays a crucial role in the transformation (entry 13).
1) resulted in compromised yields (entry 6). At this juncture, we speculated that protic solvents might quench the sulfonamide anion leading to slower kinetics. Further examination regarding the choice of supporting electrolytes demonstrated Bu4NBF4 to be more suitable, while, Bu4NPF6, Et4NOTs or LiOTf proved incompatible (entry 7). The concentration of methyl acrylate proved to be a critical factor. A remarkable impact on the yield was observed when the reaction was attempted at higher concentrations of methyl acrylate (entry 8). Next, the investigation regarding the electrochemical conditions suggested that increasing or decreasing the current led to compromised results (entries 9 and 10). Subsequently, a series of electrode materials were varied; among them, the carbon anode/nickel cathode proved optimal (entries 11 and 12). Control experiments ascertained that electricity and ferrocene plays a crucial role in the transformation (entry 13).
| S. no. | Variation from standard conditions | Yield (%) |
|---|---|---|
| a Standard conditions: 1a (0.236 mmol), 2a (0.708 mmol), C(+)/Ni(−), 1.5 mA (CCE), Cp2Fe (0.3 equiv.), K2CO3 (1.5 equiv.), Bu4NBF4 (2.0 equiv.), DCE (0.04 M). b Electrolysis performed for 5 h. c Reaction in CH3CN:H2O. d 2a (1.416 mmol). | ||
| 1. | None | 62 |
| 2.b | Absence of K2CO3 | 22 |
| 3. | Et3N, DBU instead of K2CO3 | N.R. |
| 4.c | Na2CO3 instead of K2CO3 | 57 |
| 5. | DMSO, DMF, MeOH or HFIP instead of DCE | N.R. |
| 6. | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (4 H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) instead of DCE 1) instead of DCE |
34 |
| 7. | Bu4NPF6, Et4NOTs, LiOTf instead of Bu4NBF4 | N.R. |
| 8.d | 1.416 mmol of 2a instead of 0.708 mmol | 43 |
| 9. | 2.5 mA, 3.0 mA instead of 1.5 mA | 47 |
| 10. | 0.5 mA instead of 1.5 mA | 38 |
| 11. | Pt or Cu cathode instead of Ni | N.R. |
| 12. | Pt anode instead of C | N.R. |
| 13. | Without Cp2Fe and electricity | N.R. |
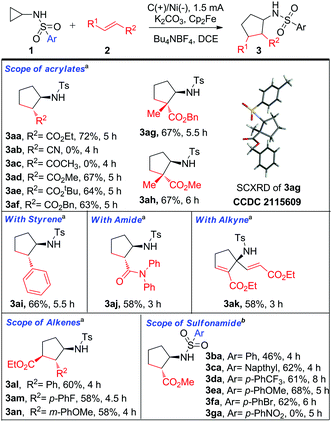
Having established the optimized conditions, an evaluation of the substrate scope was performed. As depicted in Scheme 2, initially, the ester substituted olefins were screened. Pleasingly, it was observed that ethyl 3aa, methyl 3ad, tert-butyl 3ae, and benzyl substituents 3af were well tolerated and delivered the desired annulated product with modest yields. However, replacing the acrylates with acrylonitrile 3ab or methyl vinyl ketone 3ac were found to be detrimental for the methodology. Presumably, the more electron-withdrawing nature of cyano (–CN) or ketone (–COCH3) hindered the generation of the N-center radical from its corresponding sulfonamide anion, as the latter underwent intermolecular trapping with these electron-deficient olefins and led to the formation of the Michael adduct (see ESI†). Subsequently, the replacement of acrylates with 1,1-disubstituted alkenes proceeded well and furnished the anticipated cyclopentane rings bearing the all-carbon quaternary stereocenters in moderate yields 3ag-3ah. Interestingly, under our optimized conditions, substituting the acrylates with styrene proved viable for the protocol 3ai. Further, the employment of acrylamides was found to be compatible with the above transformation 3aj. To our delight, the transformation was insensitive to substitution on the aryl ring of the cinnamates and rendered the trisubstituted cyclized product in modest yields 3al-3an. The regioselectivity of the product 3al was further confirmed by single-crystal X-ray crystallography (CCDC no. 2145541). Presumably, the reversal of regioselectivity obtained could be due to the higher stability of the benzyl radical formed in comparison to the electron-deficient olefin radical.
Encouraged by the above results, we anticipated that the designed methodology might tolerate terminal alkynes to furnish cyclopentene rings. As expected, our methodology worked well; however, an interesting result was encountered. We reasoned that the N-tosylated cyclopentene obtained might undergo electrochemical oxidation to generate an N-center radical followed by a 1,2-HAT process leading to the formation of a C-center radical, which seized another molecule of alkyne and delivered 3ak in 58% yield (see ESI†). Motivated by the success of our cycloaddition strategy to alkenes, and the significance of sulfonamides in medicinal chemistry, we next turned our attention to the reactivity of sulfonyl groups. Notably, a wide array of substitutions, like aryl 3ba, naphthyl 3ca, and trifluoromethyl 3da successfully manifested the desired cyclized product. Among them, the aryl-substituted sulfonyl group proved less efficient in comparison to the tosyl group. Whereas the introduction of a more electron-rich aryl ring (para-methoxy) 3ea exhibited improvement in the yield. Notably, the presence of the para-nitro group failed to deliver the targeted cyclopentane ring 3ga indicating that the electron-withdrawing substitutions on the aryl ring were not efficient for the developed approach. This observation suggests that the efficiency of the protocol is directly proportional to the electronic factors associated with the substitution on the sulfonyl group. At last, the implementation of halide (para-bromo) on the sulfonyl moiety was well tolerated and provided scope for further derivatization 3fa.
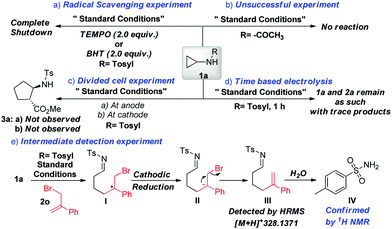
To gain insight into the mechanism of our designed electrochemical protocol, a series of experiments were performed (Scheme 3). Initially, to examine whether the reaction follows a radical pathway, the addition of radical scavengers like 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) or butylated hydroxytoluene (BHT) under the standard conditions led to the complete shutdown of the reaction emphasizing the involvement of a radical process (Scheme 3a). Further, to inspect the role of the tosyl group, the reaction was performed with N-cyclopropylacetamide 4 under standard conditions (Scheme 3b). Failure of this reaction highlights the importance of the tosyl group on the nitrogen, enabling the generation of aza-anion and facilitating the formation of active radical species. Next, divided cell experiments were performed to accentuate the cathodic reduction process (Scheme 3c). Performing the reaction for 1 h led to trace amounts of product formation with no further conversion in the absence of electricity, suggesting that a chain propagation mechanism is absent (Scheme 3d). Further, the reaction of N-tosylcyclopropane with (3-bromoprop-1-en-2-yl)benzene led to the formation of F, which is detected by HRMS, confirming the involvement of anions (Scheme 3e).16 Hydrolysis of F renders p-toluenesulfonamide which is isolated and confirmed by 1H NMR studies (see ESI†). Further, to ascertain the role of ferrocene and the base, cyclic voltammetry experiments were performed (see ESI†). As illustrated, the oxidation potential of 1a (i.e., Ep = 2.08 V vs. Ag/AgCl) experienced a slight shift of 2.24 V in the presence of ferrocene. However, a remarkable improvement in the catalytic current was observed in the presence of ferrocene (electrocatalytic effect). This result suggests that ferrocene makes the process more facile and delivers the anticipated product. A similar observation was noted when K2CO3 (base) was employed in the reaction mixture. Therefore, the inclusion of both base and ferrocene led to the enhancement of the catalytic current, hence favoring the formation of cycloadduct. Based on the above experimental evidence and literature reports, we propose a plausible mechanism for the designed methodology (Scheme 4). The reaction gets initiated by the oxidation of Cp2Fe to generate Cp2Fe+, which oxidizes the sulfonamide anion A generated by the deprotonation of 1a to render the corresponding nitrogen centered radical B. Further, the NCR B triggers the β-scission of the cyclopropyl ring to deliver the radical species C. Next, the primary radical adds to the terminal carbon of the alkene to afford another radical species D, which subsequently undergoes cathodic reduction to carbanion intermediate E. Further, the 5-exo cyclization of the carbanion intermediate E followed by the protonation, renders the expected cyclopentane product 3. However, the carbanion intermediate E may be quenched by the proton to F (detected by HRMS), responsible for the compromised yields. Also, another mechanism could be operative where the radical D adds to the imine followed by electroreduction of the generated N-centered radical and subsequent protonation rendered product 3 (see ESI†).
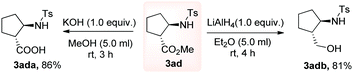
Delightfully, the protocol demonstrated scalability and furnished the desired product 3ad in a moderate yield of 38%. Further, to explore the synthetic utility, the derivatization of the cyclopentane products was carried out (Scheme 5). Initially, the cyclopentane product was subjected to saponification and rendered the corresponding acid derivative 3ada. Next, the reduction of ester functionality smoothly afforded the alcohol 3adb in moderate yields.
In summary, a novel route for the exploitation of cyclopropanes has been devised and involves electrochemical Csp3–Csp3 bond cleavage, enabling the [3+2] cycloaddition with olefins to render the di- and tri-substituted cyclopentane adducts. Additionally, the reaction features the synthesis of azaarene substituted derivatives and provides access to an all-carbon quaternary center. Moreover, the protocol circumvents the employment of harmful exogenous oxidants and utilizes the potential of traceless electrons. Further, mechanistic studies corroborate the significance of the N-center radical and also suggest that the sulfonyl group plays a paramount role in the designed methodology. Eventually, the cyclopentane products were subjected to the synthetically useful downstream modifications revealing the utility of the obtained product.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) H. U. Reissig and R. Zimmer, Chem. Rev., 2003, 103, 1151 CrossRef CAS PubMed; (b) K. B. Garve, P. G. Jones and D. B. Werz, Angew. Chem., Int. Ed., 2017, 56, 9226 CrossRef PubMed.
- (a) S. Das, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2017, 56, 11554 CrossRef CAS PubMed; (b) T. F. Schneider, J. Kaschel and D. B. Werz, Angew. Chem., Int. Ed., 2014, 53, 5504 CrossRef CAS PubMed.
- For reviews on donor–acceptor cyclopropanes: (a) H. K. Grover, M. R. Emmett and M. A. Kerr, Org. Biomol. Chem., 2015, 13, 655 RSC; (b) P. Singh, R. K. Varshnaya, R. Dey and P. Banerjee, Adv. Synth. Catal., 2020, 362, 1447 CrossRef CAS; (c) E. M. Budynina, K. L. Ivanov, I. D. Sorokin and M. Y. Melnikov, Synthesis, 2017, 3035 CrossRef CAS.
- O. O. Sokolova and J. F. Bower, Chem. Rev., 2021, 121, 80 CrossRef CAS PubMed.
- (a) M. D. Karkas, ACS Catal., 2017, 7, 4999 CrossRef; (b) P. Xiong and H. C. Xu, Acc. Chem. Res., 2019, 52, 3339 CrossRef CAS PubMed.
- (a) J. C. K. Chu and T. Rovis, Nature, 2016, 539, 272 CrossRef PubMed; (b) G. J. Choi, Q. Zhu, D. C. Miller, C. J. Gu and R. R. Knowles, Nature, 2016, 539, 268 CrossRef CAS.
- Few selected articles on N-centered radicals (NCRs): (a) G. J. Choi and R. R. Knowles, J. Am. Chem. Soc., 2015, 137, 9226 CrossRef CAS PubMed; (b) J. Jia, Y. A. Ho, R. F. Below and M. Rueping, Chem. – Eur. J., 2018, 24, 14054 CrossRef CAS PubMed; (c) H. B. Zhao, Z. W. Hou, Z. J. Liu, Z. F. Zhou, J. Song and H. C. Xu, Angew. Chem., Int. Ed., 2017, 56, 587 CrossRef CAS PubMed.
- (a) X. Hu, G. Zhang, L. Nie, T. Kong and A. Lei, Nat. Commun., 2019, 10, 5467 CrossRef; (b) Z. W. Hou, Z. Y. Mao, J. Song and H. C. Xu, ACS Catal., 2017, 7, 5810 CrossRef CAS.
- (a) S. Maity, M. Zhu, R. S. Shinabery and N. Zheng, Angew. Chem., Int. Ed., 2012, 51, 222 CrossRef CAS PubMed; (b) T. H. Nguyen, S. Maity and N. Zheng, Beilstein J. Org. Chem., 2014, 10, 975 CrossRef.
- B. Muriel, A. Gagnebin and J. Waser, Chem. Sci., 2019, 10, 10716 RSC.
- Y. Yin, Y. Li, T. P. Goncalves, Q. Zhan, G. Wang, X. Zhao, B. Qiao, K. W. Huang and Z. Jiang, J. Am. Chem. Soc., 2020, 142, 19451 CrossRef CAS PubMed.
- Selected review on electroorganic synthesis: (a) V. Ramadoss, Y. Zheng, X. Shao, L. Tian and Y. Wang, Chem. – Eur. J., 2021, 27, 3213 CrossRef CAS PubMed; (b) D. Saha, I. M. Taily, R. Kumar and P. Banerjee, Chem. Commun., 2021, 57, 2464 RSC; (c) H. Mei, Z. Yin, J. Liu, H. Sun and J. Han, Chin. J. Chem., 2019, 37, 292 CAS; (d) G. M. Martins, G. C. Zimmer, S. R. Mendesd and N. Ahmed, Green Chem., 2020, 22, 4849 RSC; (e) S. Kolb, M. Petzold, F. Brandt, P. G. Jones, C. R. Jacob and D. B. Werz, Angew. Chem., Int. Ed., 2021, 60, 15928 CrossRef CAS PubMed; (f) F. Lian, K. Xu and C. Zeng, Chem. Rec., 2021, 21, 2290 CrossRef CAS PubMed; (g) J. Li, S. Zhang and K. Xu, Chin. Chem. Lett., 2021, 32, 2729 CrossRef CAS; (h) F. Marken, A. J. Cresswell and S. D. Bull, Chem. Rec., 2021, 21, 2585 CrossRef CAS PubMed.
- (a) D. Saha, I. M. Taily, S. Naik and P. Banerjee, Chem. Commun., 2021, 57, 631 RSC; (b) D. Saha, I. M. Taily and P. Banerjee, Eur. J. Org. Chem., 2021, 5053 CrossRef CAS; (c) R. Kumar and P. Banerjee, J. Org. Chem., 2021, 86, 16104 CrossRef CAS PubMed.
- C. Madelaine, Y. Six and O. Buriez, Angew. Chem., Int. Ed., 2007, 46, 8046 CrossRef CAS PubMed.
- Q. Wang, Q. Wang, Y. Zhang, Y. M. Mohamed, C. Pacheco, N. Zheng, R. N. Zare and H. Chen, Chem. Sci., 2021, 12, 969 RSC.
- D. H. White, A. Noble, K. I. B. Milburn and V. K. Aggarwal, Org. Lett., 2021, 23, 3038 CrossRef CAS PubMed.
- N. Chen and H. C. Xu, Green Synth. Catal., 2021, 2, 165 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2115609 and 2145541. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d2cc00761d |
| This journal is © The Royal Society of Chemistry 2022 |