 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The overlooked NIR luminescence of Cr(ppy)3†
Laura
Stein
a,
Pit
Boden
b,
Robert
Naumann
 a,
Christoph
Förster
a,
Gereon
Niedner-Schatteburg
a,
Christoph
Förster
a,
Gereon
Niedner-Schatteburg
 b and
Katja
Heinze
b and
Katja
Heinze
 *a
*a
aJohannes Gutenberg-University, Department of Chemistry, Duesbergweg 10-14, Mainz, Germany. E-mail: katja.heinze@uni-mainz.de; Tel: +49 6131 3925886
bDepartment of Chemistry and Research Center Optimas, TU Kaiserslautern, Erwin-Schrödinger-Straße 52, 67663 Kaiserslautern, Germany
First published on 21st February 2022
Abstract
Cr(ppy)3, a structural analog of the green phosphorescent Ir(ppy)3, emits even in solution at room temperature from a weakly distorted spin–flip state at 910 nm (Hppy = 2-phenylpyridine). The low energy arises from an enhanced covalence of the Cr–C bonds as compared to Cr–N bonds. Lower temperature reduces thermally activated decay increasing the emission intensity.
Cyclometalated complexes, in particular of d6 metal ions such as RuII,1,2 IrIII3,4 or FeII5–7 possessing intense metal-to-ligand charge transfer (MLCT) absorptions, have shaped the field of organometallic photochemistry. The prototypical complex fac-Ir(ppy)3 (Hppy = 2-phenylpyridine)8,9 with its intense green 3MLCT luminescence has paved the way to efficient luminescent materials for PHOLED applications,10,11 while cyclometalated RuII complexes are less luminescent.1,2 The first reported cyclometalated FeII counterparts are even non-luminescent5,6 and a phosphorescent iron(II) complex was reported only recently.7 In all d6 cases, 1/3MLCT states play key roles for absorption and emission, while highly distorted metal centered excited triplet/quintet states (3/5MC) provide non-radiative decay pathways in RuII and FeII complexes, which hamper efficient 3MLCT luminescence.1,10 Novel developments in the field of Earth-abundant complex chromophores beyond precious metal phosphors led to a paradigm change.12–16 Beside CT states, metal-centered spin–flip excited states can be highly luminescent between 709–775 nm as demonstrated with polypyridine CrIII complexes.17–22 A higher covalent bonding in a carbazolato pyridine CrIII complex [Cr(dpc)2]+ (dpc = 3,6-di-tert-butyl-1,8-di(pyridine-2-yl)-carbazolato) shifted the emission to lower energy (λem = 1067 nm, 77 K). However, this went along with a poor quantum yield (Φ < 0.00089%).23
We investigate the effect of cyclometalation on the spin–flip emission of CrIII reporting the structure and photophysical properties of Cr(ppy)3 as a 3d3 structural analog of the 5d6 complex IrIII(ppy)3.8,9 Cr(ppy)3 was first prepared in 1967 by treating CrCl3x3THF with 2-(ortho-lithiophenyl)pyridine24 and later (1991) by treating CrPh3(thf)325 with 2-phenylpyridine.26 Despite its existence for decades, neither the stereochemistry, nor emission properties of Cr(ppy)3 were reported.24,26
Single crystals of the facial isomer fac-Cr(ppy)3 suitable for X-ray diffraction (XRD) analysis were obtained after heating CrPh3(thf)3 with 2-phenylpyridine in THF and recrystallisation from CH2Cl2/toluene (Fig. 1). fac-Cr(ppy)3 crystallised in the tetragonal space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 21c as fac-Ir(ppy)3 obtained from sublimation,27 while Ir(ppy)3 crystallised from solution as twinned crystals in the trigonal space group P
21c as fac-Ir(ppy)3 obtained from sublimation,27 while Ir(ppy)3 crystallised from solution as twinned crystals in the trigonal space group P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , resulting in superimposed Ir-coordinated C/N positions.28
, resulting in superimposed Ir-coordinated C/N positions.28
In the present XRD analysis, the C/N assignment and the facial configuration were confirmed by inspection of the thermal ellipsoids of the atoms of the alternative assignment and the resulting R factors. The Cr–C distances are shorter than the Cr–N distances (dav(Cr–C) = 2.059(2) Å, dav(Cr–N) = 2.145(2) Å; ESI,† Table S1 and Fig. S1). The Cr–N distances in [Cr(bpy)3][PF6]329 (bpy = 2,2′-bipyridine; d(Cr–N) = 2.042(5) Å) are significantly smaller, substantiating a trans influence of the carbon donor in fac-Cr(ppy)3. Quantum chemical calculations yield d(Cr–C) = 2.063 Å and d(Cr–N) = 2.146 Å in very good agreement with the XRD data (ESI,† Table S1 and Fig. S1). The geometry of the [CrC3N3] coordination sphere in fac-Cr(ppy)3 is close to octahedral, similar to the [CrN6] coordination of [Cr(bpy)3]3+ (ESI,† Table S2). π⋯π and CH⋯π contacts are observed between Δ and Λ isomers in the solid state of fac-Cr(ppy)3 (Fig. 1), while no such contacts appear in the salt [Cr(bpy)3][PF6]3.29 Analytical data of fac-Cr(ppy)3 are compiled in the ESI† (Fig. S3 and S4).
While polypyridine CrIII complexes are reduced either at the ligand to give radical anions or at the metal center to give CrII,17,20,22,30,31fac-Cr(ppy)3 is not reduced up to potentials of −2.2 V vs. ferrocene (ESI,† Fig. S3), likely due to the electron-rich ppy− ligand. An irreversible oxidation is observed at a peak potential Ep = +0.24 V vs. ferrocene, which is assigned to an oxidation of the Cr-aryl moiety with a covalent Cr–C bond.
The optical properties of fac-Cr(ppy)3 were probed in solution and in the solid state (ESI,† Fig. S5–S10). The absorption band at 440 nm (ε = 3000 M−1 cm−1 in CH2Cl2, Fig. 2a) and around 450 nm in a KBr pellet (ESI,† Fig. S7) is assigned to transitions with ILCT/MLCT character (calcd. at 414 nm, ƒ = 0.0016 (E); 409 nm, ƒ = 0.0041 (A)) according to time-dependent DFT calculations and CT number analyses (Fig. 2a; ESI,† Fig. S2).32,33 The energetically next higher absorption band possesses a larger MLCT character (calcd. at 395 nm, ƒ = 0.0294 (E); 387 nm, ƒ = 0.0016 (A)) while the largely metal centered 4T2 states are calculated at 350/348 nm (ƒ = 0.0108/0.0025 (A/E)). The MLCT bands are responsible for the yellow-orange colour of the complex (Fig. 2b, inset). Excited states with MLCT/ILCT character are compatible with the oxidation of fac-Cr(ppy)3 at Ep = 0.24 V and the reduction below −2.2 V vs. ferrocene (ESI,† Fig. S3), similar to the redox processes assigned for fac-Ir(ppy)3 at 0.31 and −2.7 V in DMF.34
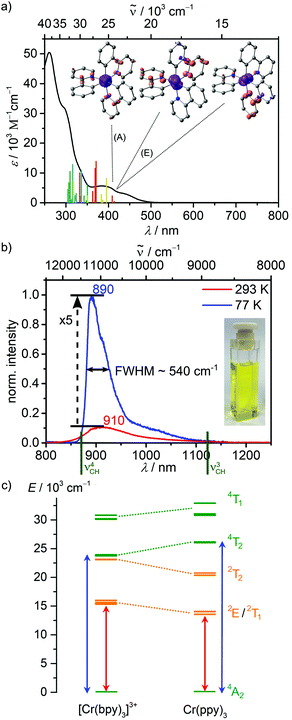 | ||
| Fig. 2 (a) UV/Vis absorption spectrum of fac-Cr(ppy)3 (black) in CH2Cl2 at 293 K including TD-DFT calculated transitions. The code indicates the character of the transition according to CT number analysis (green: MC, red: ILCT yellow: MLCT, orange: LMCT, blue: LLCT) and difference densities of the three lowest energy transitions (E and A, isosurface at 0.005 a.u., purple = electron depletion, orange = electron gain). (b) Emission spectra of fac-Cr(ppy)3 in 2-MeTHF at 293 K (red) and 77 K (blue) (λexc = 420 nm) with the energies of CH overtones19ν3 and ν4 indicated in green and a photograph of fac-Cr(ppy)3 in 2-MeTHF (inset). (c) Quartet (green) and doublet (orange) energy levels of [Cr(bpy)3]3+ and fac-Cr(ppy)3 from CASSCF(7,12)-NEVPT2 calculations showing the increased ligand field splitting (blue arrows) and decreased spin–flip state energy (red arrows) for fac-Cr(ppy)3 (labels according to octahedral symmetry, although the actual symmetry is lower). | ||
Excitation of fac-Cr(ppy)3 dissolved in deaerated 2-MeTHF or CH2Cl2 at 293 K results in sharp emission bands peaking at 910 nm with FWHM293K ≈ 1185/1068 cm−1, respectively (Fig. 2b; ESI,† Fig. S5). The band shifts to 890 nm and sharpens to FWHM77K ≈ 540 cm−1 at 77 K in frozen 2-MeTHF, revealing some fine structure (890, 910 (sh), 1020 (sh) nm). The integrated emission intensity increases ca. fivefold (Fig. 2b). The excitation spectra at 298 and 77 K in 2-MeTHF solution follow the absorption spectrum (ESI,† Fig. S6). This confirms that the emission arises from initially excited ILCT/MLCT states (350–500 nm) of fac-Cr(ppy)3, which efficiently evolve to the emissive state in 2-MeTHF (ESI,† Fig. S6a). This also holds for excitation and emission properties in CH2Cl2 at 293 K (ESI,† Fig. S5 and S6b) and in the solid state (ESI,† Fig. S7). The luminescence lifetimes at 293 and 77 K in 2-MeTHF amount to 9.5 and 48 μs, respectively and to 9.3 μs in CH2Cl2 at 293 K (ESI,† Fig. S8 and S9). In the powder the decay is biexponential with 10 μs (76%) and 3 μs (24%) (ESI,† Fig. S10), possibly due to energy migration in the solid via the π⋯π and CH⋯π contacts (Fig. 1) as has been observed e.g. for Ir(ppy)3.35,36
Nanosecond laser flash photolysis experiments of fac-Cr(ppy)3 in Ar-saturated CH2Cl2 yielded a transient absorption (TA) spectrum with excited state absorptions (ESA) throughout the visible region, superimposed to the ground state bleach below 500 nm (ESI,† Fig. S11). The TA spectrum of fac-Cr(ppy)3 differs from reported TA spectra of pyridine and acetylacetonato CrIII complexes displaying only a single ESA around 520–540 nm.22,37–40 The apparent ESA bands at 485 nm and 640 nm decay monoexponentially with τ = 10.2 μs and 9.9 μs, respectively (ESI,† Fig. S11 and S12), in agreement with the luminescence decay.
Compared to [Cr(bpy)3]3+ (λem = 729 nm, HClaq),41 the spin–flip emission band of fac-Cr(ppy)3 is bathochromically shifted by ≈ 2730 cm−1. CASSCF(7,12)-NEVPT2 calculations on geometry-optimised [Cr(bpy)3]3+ and fac-Cr(ppy)3 complexes correctly predict the bathochromic shift of the spin–flip luminescence (1773 cm−1; Fig. 2c; ESI,† Table S5). With respect to the carbazolato pyridine CrIII complex [Cr(dpc)2]+23 the luminescence band of fac-Cr(ppy)3 appears at higher energy by ≈ 1860 cm−1 (77 K). Obviously, the combination of σ/π-donating carbanionic donors and π-accepting pyridines along all axes x, y and z in fac-Cr(ppy)3, achieves a metal–ligand bond covalence in-between that of [Cr(bpy)3]3+41 and [Cr(dpc)2]+.23
Contrary to [Cr(dpc)2]+ emitting only at 77 K,23fac-Cr(ppy)3 emits at 293 K in fluid 2-MeTHF or CH2Cl2 (Φ = 0.03% in CH2Cl2; Φ = 0.02% as powder). Although these quantum yields are still below the record values of molecular rubies of up to Φ = 20% in solution,17–22 this marks a great advancement for open-shell complexes made from abundant transition metals emitting in the NIR region above 800 nm in fluid solution.
As the quantum yields increase at lower temperature, a thermally activated decay pathway is likely operative.41 For the tris(bidentate) CrIII complex [Cr(bpy)3]3+ (Φ = 0.25 ± 0.02%, τ = 69 ± 2 μs in 1 M HCl at 296–297 K) an activation barrier for thermal non-radiative deactivation of 43 ± 1 kJ mol−1 has been determined.41 A thermally activated trigonal twist has been suggested as possible non-radiative pathway for tris(bidentate) quasi-cage CrIII complexes.42 Compared to the rather flexible bpy ligand in [Cr(bpy)3]3+, the more rigid 1,10-phenanthroline ligand phen endows a higher Φ and τ in [Cr(phen)3]3+ (Φ = 1.2 ± 0.1%, τ = 304 ± 4 μs in 1 M HCl at 296–297 K).41 Therefore, trigonal twisting and intraligand torsional modes might be operating in the excited fac-Cr(ppy)3 complex as well, leading to thermally activated surface crossing. This implies that the relaxed excited doublet state is distorted relative to the ground state (see below).
Non-radiative multiphonon relaxation, i.e. electronic to vibrational energy transfer to overtones of high-energy oscillators, such as C–H modes, might not be very important for fac-Cr(ppy)3 as the relevant overtones determined from a model ligand (Me2-bpy; ν3 = 8792 cm−1 (1137 nm) and ν4 = 11493 cm−1 (870 nm))19 possess only a small spectral overlap with the emission band of fac-Cr(ppy)3 at 910 nm (Fig. 2b). A further non-radiative pathway involving back-intersystem crossing to the metal centered quartet states appears unlikely as well, considering the strongfield ligand ppy−. TDDFT and CASSCF(7,12)-NEVPT2 calculations suggest an even larger ligand field splitting (energy difference between 4T2 and 4A2 states) in fac-Cr(ppy)3 than in [Cr(bpy)3]3+ (by 2285 cm−1; Fig. 2c). Finally, energy transfer between uncharged fac-Cr(ppy)3 complexes in solution and in the solid is a possible self-quenching pathway mediated by π⋯π and CH⋯π contacts (Fig. 1), while self-quenching of charged CrIII complexes requires high chloride concentrations to form ion clusters in solution.43,44
To obtain information on the potential distortion of the excited doublet state, step-scan FTIR spectra of fac-Cr(ppy)3 were collected (KBr pellet, λexc = 355 nm, 20 K). The spectra display ESA and bleaching bands (ESI,† Fig. S13a). The excited state FTIR spectrum consequently differs from the ground state spectrum (ESI,† Fig. S13b), confirming that the long-lived doublet state is distorted with respect to the ground state. At 20 K in KBr, this doublet state decays monoexponentially with τ = 9.8 μs, as derived from a global fit of decay traces at 1286, 1397, 1442, 1461 and 1475 cm−1 (ESI,† Fig. S14 and Table S3). DFT geometry optimisations and frequency analyses (ESI,† Fig. S15 and S16) substantiate that the lowest energy doublet state is indeed distorted relative to the quartet ground state: Cr–C and Cr–N distances change from 2.063 Å to 2.044, 2.053 and 2.055 Å and from 2.146 Å to 2.138, 2.147, 2.153 Å, respectively, while the X–Cr–Y angles remain rather constant (ESI,† Fig. S17 and Table S1). In agreement with the experimentally obtained data, the DFT calculated vibrational frequencies for the quartet ground and the excited doublet state show similar shifts in energy (ESI,† Fig. S15 and S16, calculated frequencies scaled by 0.975).
The NIR luminescence of fac-Cr(ppy)3 recorded in a KBr pellet between 290–5 K strongly increases below 100 K (ESI,† Fig. S18). While in solution the emission intensity increases ca. fivefold already at 77 K (Fig. 2b), the gain in intensity of the KBr pellet starts below ca. 80 K. This is compatible with a distorted excited state, which decays by thermal activation to the ground state. While the energy barriers appear higher in rigid matrices thanks to less distorted and hence more nested excited states the temperature dependence in frozen 2-MeTHF and KBr matrices is different (Fig. 2b, ESI,† Fig. S18).
Complexes cis-[Cr(bpy)2(Ar)2]+ reductively eliminate Ar–Ar under irradiation,45 while fac-Cr(ppy)3 is photostable in 2-MeTHF (λexc = 420 nm, ESI,† Fig. S19) thanks to the absence of cis-positioned Ar ligands. Similarly, trans-[Cr(cyclam)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C6H4R)2]+ appear to be photostable.46 In CH2Cl2, some photoreactivity is observed for fac-Cr(ppy)3 (ESI,† Fig. S20). Rather than photoreduction of CH2Cl2 itself (E = −2.6 V)47 by the excited complex (E(*Cr(ppy)3/[Cr(ppy)3]+) = −1.12 V), which is thermodynamically unfavourable, traces of HCl in the CH2Cl2 could lead to irreversible photooxidation of *Cr(ppy)3 (ESI,† Fig. S3) and H+ reduction. Photo- and redox stable cyclometalated CrIII complexes might by developed into useful photoreductants in the future.
C–C6H4R)2]+ appear to be photostable.46 In CH2Cl2, some photoreactivity is observed for fac-Cr(ppy)3 (ESI,† Fig. S20). Rather than photoreduction of CH2Cl2 itself (E = −2.6 V)47 by the excited complex (E(*Cr(ppy)3/[Cr(ppy)3]+) = −1.12 V), which is thermodynamically unfavourable, traces of HCl in the CH2Cl2 could lead to irreversible photooxidation of *Cr(ppy)3 (ESI,† Fig. S3) and H+ reduction. Photo- and redox stable cyclometalated CrIII complexes might by developed into useful photoreductants in the future.
fac-Cr(ppy)3 is the first CrIII spin–flip emitter with emission above 900 nm in solution at 293 K. The effect of carbanionic donors in place of pyridine donors is two-fold, namely the increase in energy of the metal centred quartet states, owing to the larger ligand field strength, and the decrease of the energy of the spin–flip states, owing to the stronger Cr–C bond covalence (nephelauxetic effect). These insights on the photophysics of the cyclometalated CrIII complex fac-Cr(ppy)3 are complementary to recent discoveries for first row transition metal CT emitters7,48–54 and demonstrate that fundamental ground-breaking studies can pave the way to fully exploiting Earth-abundant metals, e.g. in light harvesting, sensing, photocatalysis and optical devices.
K. H. designed the project and wrote the paper, L. S. and R. N. performed synthesis, characterization and DFT, P. B. and G. N. S. step-scan FTIR spectroscopy and luminescence in KBr.
Support from the Deutsche Forschungsgemeinschaft [SPP 2102 “Light-controlled reactivity of metal complexes” GE 961/10-01; HE 2778/10-2 and INST 247/1018-1] is gratefully acknowledged. Parts of this research used the supercomputer Elwetritsch and advisory services offered by TU Kaiserslautern (https://elwe.rhrk.uni-kl.de). We thank Dr. Luca M. Carrella for XRD data collection and Prof. Dr. Christoph Kerzig and Maria-Sophie Bertrams for TA support and usage authorisation.
Conflicts of interest
There are no conflicts to declare.Notes and references
- C. Kreitner and K. Heinze, Dalton Trans., 2016, 45, 13631 RSC.
- T. C. Motley, L. Troian-Gautier, M. K. Brennaman and G. J. Meyer, Inorg. Chem., 2017, 56, 13579 CrossRef CAS PubMed.
- C. E. Housecroft and E. C. Constable, Coord. Chem. Rev., 2017, 350, 155 CrossRef CAS.
- A. R. B. M. Yusoff, A. J. Huckaba and M. K. Nazeeruddin, Top. Curr. Chem., 2017, 375, 39 CrossRef PubMed.
- J. Steube, L. Burkhardt, A. Päpcke, J. Moll, P. Zimmer, R. Schoch, C. Wölper, K. Heinze, S. Lochbrunner and M. Bauer, Chem. – Eur. J., 2019, 25, 11826 CrossRef CAS PubMed.
- Z. Tang, X.-Y. Chang, Q. Wan, J. Wang, C. Ma, K.-C. Law, Y. Liu and C.-M. Che, Organometallics, 2020, 39, 2791 CrossRef CAS.
- W. Leis, M. A. Argüello Cordero, S. Lochbrunner, H. Schubert and A. Berkefeld, J. Am. Chem. Soc., 2022, 144, 1169 CrossRef CAS PubMed.
- K. A. King, P. J. Spellane and R. J. Watts, J. Am. Chem. Soc., 1985, 107, 1431 CrossRef CAS.
- T. Hofbeck and H. Yersin, Inorg. Chem., 2010, 49, 9290 CrossRef CAS PubMed.
- M. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 1999, 75, 4 CrossRef CAS.
- C. Adachi, M. A. Baldo, S. R. Forrest and M. E. Thompson, Appl. Phys. Lett., 2000, 77, 904 CrossRef CAS.
- C. Förster and K. Heinze, Chem. Soc. Rev., 2020, 49, 1057 RSC.
- O. S. Wenger, J. Am. Chem. Soc., 2018, 140(42), 13522 CrossRef CAS PubMed.
- C. Bizzarri, E. Spuling, D. M. Knoll, D. Volz and S. Bräse, Coord. Chem. Rev., 2018, 373, 49 CrossRef CAS.
- B. M. Hockin, C. Li, N. Robertson and E. Zysman-Colman, Catal. Sci. Technol., 2019, 9, 889 RSC.
- O. S. Wenger, Chem. – Eur. J., 2019, 25, 6043 CrossRef CAS PubMed.
- S. Otto, M. Grabolle, C. Förster, C. Kreitner, U. Resch-Genger and K. Heinze, Angew. Chem., Int. Ed., 2015, 54, 11572 CrossRef CAS PubMed.
- S. Otto, C. Förster, C. Wang, U. Resch-Genger and K. Heinze, Chem. – Eur. J., 2018, 24, 12555 CrossRef CAS PubMed.
- C. Wang, S. Otto, M. Dorn, E. Kreidt, J. Lebon, L. Sršan, P. Di Martino-Fumo, M. Gerhards, U. Resch-Genger, M. Seitz and K. Heinze, Angew. Chem., Int. Ed., 2018, 57, 1112 CrossRef CAS PubMed.
- S. Treiling, C. Wang, C. Förster, F. Reichenauer, J. Kalmbach, P. Boden, J. P. Harris, L. Carrella, E. Rentschler, U. Resch-Genger, C. Reber, M. Seitz, M. Gerhards and K. Heinze, Angew. Chem., Int. Ed., 2019, 58, 18075 CrossRef CAS PubMed.
- J.-R. Jiménez, B. Doistau, C. M. Cruz, C. Besnard, J. M. Cuerva, A. G. Campaña and C. Piguet, J. Am. Chem. Soc., 2019, 141, 13244 CrossRef PubMed.
- F. Reichenauer, C. Wang, C. Förster, P. Boden, N. Ugur, R. Báez-Cruz, J. Kalmbach, L. M. Carrella, E. Rentschler, C. Ramanan, G. Niedner-Schatteburg, M. Gerhards, M. Seitz, U. Resch-Genger and K. Heinze, J. Am. Chem. Soc., 2021, 143, 11843 CrossRef CAS PubMed.
- N. Sinha, J.-R. Jiménez, B. Pfund, A. Prescimone, C. Piguet and O. S. Wenger, Angew. Chem., Int. Ed., 2021, 60, 23722 CrossRef CAS PubMed.
- K. Madeja, E. Hüsing and N. Ahrens, Z. Chem., 1967, 7, 22 CrossRef CAS.
- W. Herwig and H. Zeiss, J. Am. Chem. Soc., 1959, 81, 4798 CrossRef CAS.
- H. Drevs, Z. Anorg. Allg. Chem., 1991, 605, 145 CrossRef CAS.
- R. J. F. Berger, H.-G. Stammler, B. Neumann and N. W. Mitzel, Eur. J. Inorg. Chem., 2010, 1613 CrossRef CAS.
- J. Breu, P. Stössel, S. Schrader, A. Starukhin, W. J. Finkenzeller and H. Yersin, Chem. Mater., 2005, 17, 1745 CrossRef CAS.
- K. V. Goodwin, W. T. Pennington and J. D. Petersen, Inorg. Chem., 1989, 28, 2016 CrossRef CAS.
- C. C. Scarborough, S. Sproules, T. Weyhermüller, S. DeBeer and K. Wieghardt, Inorg. Chem., 2011, 50, 12446 CrossRef CAS PubMed.
- P. M. Becker, C. Förster, L. M. Carrella, P. Boden, D. Hunger, J. van Slageren, M. Gerhards, E. Rentschler and K. Heinze, Chem. – Eur. J., 2020, 26, 7199 CrossRef CAS PubMed.
- F. Plasser, J. Chem. Phys., 2020, 152, 084108 CrossRef CAS PubMed.
- S. Mai, F. Plasser, J. Dorn, M. Fumanal, C. Daniel and L. González, Coord. Chem. Rev., 2018, 361, 74 CrossRef CAS.
- A. B. Tamayo, B. D. Alleyne, P. I. Djurovich, S. Lamansky, I. Tsyba, N. N. Ho, R. Bau and M. E. Thompson, J. Am. Chem. Soc., 2003, 125, 7377 CrossRef CAS PubMed.
- C. Tonnel, M. Stroet, B. Caron, A. J. Clulow, R. C. R. Nagiri, A. K. Malde, P. L. Burn, I. R. Gentle, A. E. Mark and B. J. Powell, Angew. Chem., Int. Ed., 2017, 56, 8402 CrossRef PubMed.
- S. Sanderson, G. Vamvounis, A. E. Mark, P. L. Burn, R. D. White and B. W. Philippa, J. Chem. Phys., 2021, 154, 164101 CrossRef CAS PubMed.
- S. Otto, A. M. Nauth, E. Ermilov, N. Scholz, A. Friedrich, U. Resch-Genger, S. Lochbrunner, T. Opatz and K. Heinze, ChemPhotoChem, 2017, 1, 344 CrossRef CAS.
- F. A. Baptista, D. Krizsan, M. Stitch, I. V. Sazanovich, I. P. Clark, M. Towrie, C. Long, L. Martinez-Fernandez, R. Improta, N. A. P. Kane-Maguire, J. M. Kelly and S. J. Quinn, J. Am. Chem. Soc., 2021, 143, 14766 CrossRef CAS PubMed.
- N. Serpone and M. Z. Hoffman, J. Phys. Chem., 1987, 91, 1737 CrossRef CAS.
- E. A. Juban and J. K. McCusker, J. Am. Chem. Soc., 2005, 127, 6857 CrossRef CAS PubMed.
- A. M. McDaniel, H.-W. Tseng, N. H. Damrauer and M. P. Shores, Inorg. Chem., 2010, 49, 7981 CrossRef CAS PubMed.
- M. W. Perkovic, M. J. Heeg and J. F. Endicott, Inorg. Chem., 1991, 30, 3140 CrossRef CAS.
- R. Sriram, M. Z. Hoffman, M. A. Jamieson and N. Serpone, J. Am. Chem. Soc., 1980, 102, 1754 CrossRef CAS.
- M. A. Jamieson, N. Serpone and M. Z. Hoffman, Coord. Chem. Rev., 1981, 39, 121 CrossRef CAS.
- B. E. Olafsen, G. V. Crescenzo, L. P. Moisey, B. O. Patrick and K. M. Smith, Inorg. Chem., 2018, 57, 9611 CrossRef CAS PubMed.
- D. L. Grisenti, W. W. Thomas, C. R. Turlington, M. D. Newsom, C. J. Priedemann, D. G. VanDerveer and P. S. Wagenknecht, Inorg. Chem., 2008, 47, 11452 CrossRef CAS PubMed.
- A. Kotsinaris, G. Kyriacou and C. Lambrou, J. Appl. Electrochem., 1998, 28, 613 CrossRef CAS.
- M. Dorn, J. Kalmbach, P. Boden, A. Päpcke, S. Gómez, C. Förster, F. Kuczelinis, L. M. Carrella, L. Büldt, N. Bings, E. Rentschler, S. Lochbrunner, L. González, M. Gerhards, M. Seitz and K. Heinze, J. Am. Chem. Soc., 2020, 142, 7947 CrossRef CAS PubMed.
- L. A. Büldt, X. Guo, R. Vogel, A. Prescimone and O. S. Wenger, J. Am. Chem. Soc., 2017, 139, 985 CrossRef PubMed.
- P. Herr, C. Kerzig, C. B. Larsen, D. Häussinger and O. S. Wenger, Nat. Chem., 2021, 13, 956 CrossRef CAS PubMed.
- K. S. Kjær, N. Kaul, O. Prakash, P. Chábera, N. W. Rosemann, A. Honarfar, O. Gordivska, L. A. Fredin, K. E. Bergquist, L. Häggström, T. Ericsson, L. Lindh, A. Yartsev, S. Styring, P. Huang, J. Uhlig, J. Bendix, D. Strand, V. Sundström, P. Persson, R. Lomoth and K. Wärnmark, Science, 2019, 363, 249 CrossRef PubMed.
- A. K. Pal, C. F. Li, G. S. Hanan and E. Zysman-Colman, Angew. Chem., Int. Ed., 2018, 57, 8027 CrossRef CAS PubMed.
- M. Gernert, L. Balles-Wolf, F. Kerner, U. Müller, A. Schmiedel, M. Holzapfel, C. M. Marian, J. Pflaum, C. Lambert and A. Steffen, J. Am. Chem. Soc., 2020, 142, 8897 CrossRef CAS PubMed.
- B. Hupp, C. Schiller, C. Lenczyk, M. Stanoppi, K. Edkins, A. Lorbach and A. Steffen, Inorg. Chem., 2017, 56, 8996 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2124885. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d2cc00680d |
| This journal is © The Royal Society of Chemistry 2022 |

