 Open Access Article
Open Access ArticleSchiff base capped gold nanoparticles for transition metal cation sensing in organic media†
Miroslava
Čonková‡
ab,
Verónica
Montes-García‡
 c,
Marcin
Konopka
ab,
Artur
Ciesielski
c,
Marcin
Konopka
ab,
Artur
Ciesielski
 *bc,
Paolo
Samori
*bc,
Paolo
Samori
 *c and
Artur R.
Stefankiewicz
*c and
Artur R.
Stefankiewicz
 *ab
*ab
aFaculty of Chemistry, Adam Mickiewicz University, Uniwersytetu Poznańskiego 8, 61-614 Poznań, Poland. E-mail: ars@amu.edu.pl
bCenter for Advanced Technology, Adam Mickiewicz University, Uniwersytetu Poznańskiego 10, 61-614 Poznań, Poland
cUniversité de Strasbourg, CNRS, ISIS, 8 allée Gaspard Monge, 67000 Strasbourg, France
First published on 13th April 2022
Abstract
We report a fast and ultrasensitive colorimetric method for the detection of transition metal ions (Fe3+, Cu2+, Ni2+) in a mixture of toluene–acetonitrile using Schiff base functionalized gold nanoparticles. We achieved limits of detection for the three metal ions at least two orders of magnitude lower than the EU recommended limits. Finally, our methodology was assessed for the determination of nickel in the organic waste of a relevant industrial reaction.
The fine chemical, petroleum, and pharmaceutical industries have been identified as major producers of chemical waste because the vast majority of technological processes are still carried out in organic solvents with the use of metal-based catalysts.1 Organic medium hitherto remains essential not only for chemical reactions to proceed but also for the extraction and purification steps, necessary to achieve sufficient product purity. Despite the continuous development and improvement of synthetic methodologies, waste including that containing transition metal ions derived from decomposed catalysts is also often a source of contamination of the final product, including in active pharmaceutical ingredients (APIs).2 Although some of the transition metal ions employed in catalytically relevant processes (e.g., Cu2+, Ni2+, and Fe3+) bear several important biological roles,3–5 their excessive content in the human body can lead to serious health problems.6 EU recommended limits in APIs are 20 ppm7 and 300 ppm7 for Ni2+ and Cu2+, respectively. No specific limit has yet been established so far for Fe3+, although overexposure to this metal can lead to iron poisoning,8 including heart diseases9 and cancer.10 Analytical methods commonly exploited for trace metal ion determination, despite the high capital cost, are ion-coupled-plasma spectroscopy (ICP-MS)11,12 and atomic absorption spectrometry (AAS).12 However, the direct determination of trace elements in non-aqueous mixtures by these techniques remains problematic due to time-consuming and highly invasive sample pre-treatment (e.g., mineralization, high temperature/pressure) often leading to variation in metal content.12 Within this context, the development of simple and effective methods to detect transition metal ions in organic media is highly sought after.
Low-dimensional nanostructures possess the highest surface-to-volume ratios and unique optoelectronic properties, which are highly susceptible to their interaction with the environment. The latter can be tuned via the chemical functionalization of their surface with receptors of the analyte of choice, enabling the development of chemical sensors with electrical or optical readouts featuring key performance indicators beyond the state-of-the-art.13 Among low-dimensional nanostructures, noble metal nanoparticles (NPs) represent versatile platforms for the fabrication of (bio)-chemical sensors due to their high chemical stability, surface-to-volume ratio, and distinctive optoelectronic properties. The extraordinary plasmonic phenomenon has given rise to a rapidly developing field of optical nanosensors,14 where the exposure to target analytes can induce a localized surface plasmon resonance (LSPR) shift of the metallic NPs (in solution or deposited into a solid platform) and may be accompanied by a visual colour change. In particular, colorimetric sensing, where a specific analyte can trigger a significant visual colour change, is very attractive due to its simplicity, cost-effectiveness, and unprecedented selectivity among the traditional detection methodologies.15
On the other hand, Schiff bases derived from 2-hydroxybenzaldehydes and appropriate amine or hydrazide create a very effective chelating system for binding to metal cations.16,17 They have also been employed for colorimetric ion sensing, reaching in some cases limits of detection in the micromolar range.18–21 However, their synergetic combination with metal NPs for colorimetric cation sensing has been barely exploited. For instance, gold nanoparticles (AuNPs) functionalized with Schiff bases can effectively detect Cu2+, Al3+, or Fe3+, exhibiting sensitivities in the micromolar range.22–24 Yet most examples combining AuNPs and Schiff bases were investigated in aqueous solutions, despite the high stability of AuNPs capped by Schiff base ligands in organic media.25,26 Examples of sensors combining AuNPs with Schiff base ligands capable of efficiently sensing selected metal ions in organic solvents remain very few.24,27
To fill this gap, we report here a new type of colorimetric sensor based on Schiff-base decorated AuNPs for the detection of industrially relevant transition metal cations (Cu2+, Ni2+, and Fe3+) in organic solvents. The employed chelating system (L1) was designed to act both as a stabilizing agent of AuNPs and as a supramolecular receptor for the analyte of interest.
Ligand L1 was designed to combine a moiety that is capable of chemisorbing on gold surfaces and a versatile coordination pocket that can efficiently bind metal cations. Thus, an α-lipoic acid moiety was chosen as the anchoring site, through the formation of an Au–S linkage,28–31 while the mono(salicylaldehyde)-iminoacetylhydrazone ligand providing the N,O,O binding pocket16,32–34 was employed for coordinating metal cations, as confirmed in the control experiment with ligand L2 (see ESI†). Ligand L1 was synthesized from α-lipoic acid via a three-step protocol in a high 75% overall yield without column chromatography (see Scheme S1, ESI†). The final ligand L1 was obtained as a mixture of two geometrical isomers (in Z:E = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio calculated from integrals of H-9cis, H-9trans and H-11cis, H-11trans). Detailed synthetic procedures and characterization can be found in the ESI,† Fig. S1–S8.
1 ratio calculated from integrals of H-9cis, H-9trans and H-11cis, H-11trans). Detailed synthetic procedures and characterization can be found in the ESI,† Fig. S1–S8.
The synthesis of monodispersed AuNPs in toluene was performed by following a previously reported protocol, where oleylamine was used as a surfactant.35 The resulting oleylamine capped gold nanoparticles (OL@AuNPs) featured a plasmon band at 526 nm, ascribed to the dipole resonance of individual AuNPs (Fig. 1b). The interactions between the gold nanoparticle surface and the amine group of oleylamine are weaker than dative Au–S bonds, and hence this favours the ligand exchange reaction between oleylamine and ligand L1. The ligand exchange reaction should be achieved by adding approximately 890 L1 molecules per nanoparticle (calculated from the size of the metal core, for details see ESI†). To maximize the number of L1 molecules on the AuNPs surface, and hence have the maximum number of receptors, we performed the ligand exchange reaction with different concentrations of ligand L1 (i.e., from 7.5 to 60 μM) and we studied the stability of the AuNPs in time (see ESI,† Fig. S12). The highest concentration of ligand L1 that lead to a stable system during at least 96 hours was 15 μM; higher concentrations lead to a fast and irreversible aggregation of the system (see ESI,† Fig. S12C and D).
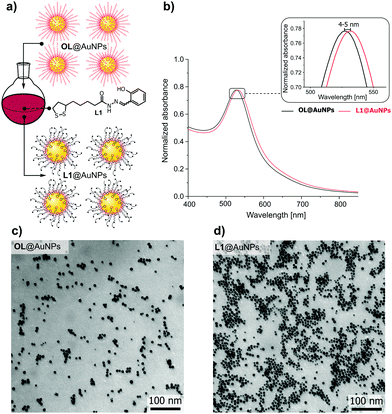 | ||
| Fig. 1 (a) Schematic representation of the ligand exchange reaction; (b) UV-Vis comparison of OL@AuNPs and L1@AuNPs; (c) TEM image of OL@AuNPs (d) TEM image of L1@AuNPs. | ||
The size and the morphology studies of both OL@AuNPs and L1@AuNPs were performed by transmission electron microscopy (TEM) and dynamic light scattering measurements (DLS). TEM images of OL@AuNPs revealed monodispersed spherical nanoparticles with uniform shape and core size 11.5 ± 1.2 nm (Fig. 1c), which is in good agreement with DLS measurements, revealing an average hydrodynamic diameter of 14.3 ± 4.4 nm (for details see ESI,† Fig. S13 and S14). In the case of L1@AuNPs, TEM images revealed that the AuNP size remains constant (core size: 10.8 ± 1.1 nm, Fig. 1d) as ligand L1 is not expected to modify the NP size. This is in good agreement with DLS measurements, which showed an average hydrodynamic diameter of L1@AuNPs of 15.45 ± 4.26 nm (see ESI,† Fig. S13).
To evaluate the sensing performance, our system was tested for the selected transition metal ions (Cu2+, Ni2+, and Fe3+). To rule out nonspecific acetonitrile-induced aggregation we have verified the stability of L1@AuNPs in the presence of acetonitrile up to 23% (see ESI,† Fig. S15). To demonstrate the selective interactions between ligand L1 and metal cations, colloidal dispersions of OL@AuNPs were exposed to selected metal cations and no aggregation was observed (see Fig. S16a, S17a, and S18a in ESI†). These experiments also exclude any non-specific interaction with AuNPs. To eliminate the counter ion influence, all experiments were performed by using NO32− salts. After 10 minutes of analyte addition (for a detailed description see ESI†), a visible colour change from red to purple was observed indicating the aggregation of L1@AuNPs (See Fig. 2). The response time observed for our system is better or comparable with other known colorimetric sensors (see ESI,† Table S4).36–40 The aggregation of L1@AuNPs is triggered by the coordination reaction between the N-acylhydrazone moiety and the metal cation, giving rise to octahedral complexes (Fig. 2). Solely based on naked-eye observation, the colour change from red to purple can be observed in the 5–7.5 μM concentration range (Fig. S19 in ESI†). To be easily comparable to the EU recommended limits, we converted the concentration in which colour change was noted into ppb. More specifically, for Cu2+ cations, the visible colour change took place after the addition of 5 μM, which corresponds to 366 ppb. For Fe3+ cations it was 7.5 μM (458 ppb) and 5 μM of Ni2+ cations, which corresponds to 204 ppb (see ESI,† Fig. S19). As compared to the allowed limits of transition metal ions in APIs and an excipient,7 our sensor exhibited a sensitivity 2 and 3 orders higher.
The sensing performance of the L1@AuNPs was quantitatively assessed via UV-Vis spectroscopy. The UV-vis absorbance spectrum of L1@AuNPs upon each metal ion addition at different concentrations showed a red shift of the LSPR band (Fig. 3a, b, and c, for Cu2+, Fe3+, and Ni2+ ions, respectively). The calibration curves were obtained by plotting known concentrations of Cu2+, Ni2+ or Fe3 ions (1–10 μM) against the LSPR maximum (Fig. 3d and Table S1 in ESI†) and the linear response can be fitted to the formula: LSPRmax = a × [metal ion] + b. For calculating the limit of detection (LoD) of each metal ion, the 3SB/m41 equation was used, where SB is the standard deviation of a blank sample and m is the slope of each calibration curve, also known as sensitivity (S). The colorimetric sensor in toluene–acetonitrile solutions showed extremely low LoDs, in the range 1.4–11.2 nM (see ESI,† Table S1) for the assessed metal ions.
 | ||
| Fig. 3 (a) UV-Vis spectrum showing the LSPR band shift upon addition of Cu2+, (b) Fe3+ and (c) Ni2+; (d) linear change of LSPR maximum plotted as a function of metal ion concentration. | ||
As can be seen in Fig. 3d, the sensor showed a similar sensitivity in the case of Cu2+ and Fe3+ cations (S = 0.88 and 0.71 μM−1, respectively). However, the sensitivity towards the most catalytically valuable and the most toxic of all studied cations, i.e. Ni2+, was much higher in both linear regions (S = 1.7 and 5.84 μM−1). This high sensitivity towards Ni2+ can be further utilized in industrially valuable Ni-catalyzed reactions, such as the synthesis of Pictilisib42 or PDE472.43 Usually, Ni2+, Cu2+ and Fe3+ cations are not found as a mixture in industrial processes and hence we highlight that our colorimetric sensor can be used to detect all of them individually. When compared to other known colorimetric sensors based on Schiff bases, L1@AuNPs displayed 2–6 orders of magnitude lower LoD than structurally similar Schiff base capped AuNP sensors, which were described for Cu2+22,27 and Al3+23 detection.
To demonstrate that our sensor can be used for the quantitative evaluation of metal content in organic waste produced during catalytic processes used in the pharmaceutical industry, we decided to reproduce the synthetic protocol reported by Novartis Pharma AG, that utilizes nickel catalyzed Kumada coupling for the synthesis of PDE472, an inhibitor of phosphodiesterase type 4D and a recognized drug target for the treatment of asthma.43 The reaction was performed at a laboratory scale and the organic waste was analyzed by UV-vis spectroscopy with our sensor and by inductively coupled plasma mass spectrometry (ICP-MS). Right after the purification procedure, the toluene waste (50 μL) was added to the L1@AuNP dispersion (for more details see ESI†). The UV-vis spectrum was recorded after 10 minutes and showed a red shift of the LSPR band (4 nm, see ESI,† Fig. S22), similar to the red shift observed for low concentrations of Ni2+ ions (≤ 4 μM, see ESI,† Fig. S17d). On the basis of this UV-Vis experiment and the calibration curve obtained upon the addition of a known concentration of Ni2+ ions (Fig. S17d, ESI†), the Ni2+ content in the real sample was evaluated as 3.5 μM. The exact nickel content was determined by ICP-MS. The obtained value of 3.49 μM is in full agreement with our UV-vis results. It is worth highlighting that for ICP-MS measurements the organic waste had to be evaporated, dried and mineralized prior to measurement, which in total took several hours. On the other hand, for our sensing experiment, the organic waste was directly examined by UV-vis spectroscopy without any pre-treatment, which reduced the total analysis time to a few minutes.
We have devised a highly sensitive chemical sensor of divalent and trivalent metal ions based on Schiff base capped AuNPs via colorimetric detection. These low-dimensional sensitive elements were characterized by UV-vis spectroscopy, DLS, and TEM. The colorimetric response relies on the complexation of the metal ions with the Schiff base ligand, forming ML2-type complexes with first-row transition metal ions (Ni2+, Cu2+, Fe3+) in toluene–acetonitrile solution, which triggers the AuNP aggregation process. The metal coordination occurred in less than 10 minutes, which makes this sensor suitable for on-the-spot qualitative sensing. The sensitivity performance was excellent for all studied ions, and the colorimetric response visible solely by the naked eye (no instrumentation necessary) was as low as 204 ppb for Ni2+ ions, which is two orders of magnitude lower than the EU recommended limits. The estimated LoD for all studied metal ions was in the nanomolar range and the highest sensitivity was observed for the toxic and catalytically valuable Ni2+ cations.
Significantly, our chemical sensor outperforms other known Schiff base capped AuNP colorimetric sensors, with a 3–6 orders of magnitude lower LoD. Moreover, we demonstrated the applicability of our sensor for the evaluation of the Ni2+ content in organic waste generated during the synthesis of PDE472, a recognized drug target for the treatment of asthma. The modular strategy applied can tune the sensor on-demand and it can be refined to become suitable for the selective detection of transition metal ions for pharmaceutical and technological applications in organic solvents.
The activity in Poznań was funded by the National Science Centre of Poland grant: SONATA BIS 2018/30/E/ST5/00032 (A.R.S.) and co-funded by The National Centre for Research and Development grant: POWR.03.02.00-00-I032/16 (M.C.). The activity in Strasbourg was financially supported by European Commission through the ERC project SUPRA2DMAT (GA-833707), the AMI project funded by the ERA-NET EuroNanoMed III program, the European Union and the Agence Nationale de la Recherche (ANR) GA-ANR-17-ENM3-0001-01, the Labex project CSC (ANR-10LABX-0026 CSC) within the Investissement d’Avenir program ANR-10-IDEX-0002-02, the International Center for Frontier Research in Chemistry (icFRC) and the Institut Universitaire de France (IUF). We thank Prof. Danuta Barałkiewicz and Dr Adam Sajnóg for ICP-MS measurements.
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. Abou-Shehada, J. H. Clark, G. Paggiola and J. Sherwood, Chem. Eng. Process., 2016, 99, 88–96 CrossRef CAS.
- European Medicine Agency, Guideline on the Specification Limits for Residues of Metal Catalysts, London, 2007 Search PubMed.
- R. Crichton, Inorganic Biochemistry of Iron Metabolism: From Molecular Mechanisms to Clinical Consequences, John Wiley & Sons, Ltd, 2001 Search PubMed.
- A. Sass-Kortsak, Adv. Clin. Chem., 1966, 8, 1–67 Search PubMed.
- F. Sunderman, Ann. Clin. Lab. Sci., 1977, 7, 377–398 CAS.
- R. R. Crichton, in Metal Chelation in Medicine, ed. R. J. W. Robert R Crichton, Robert C Hider, 2016, ch. 1, pp. 1–23 Search PubMed.
- European Medicine Agency, ICH guideline Q3D (R1) on elemental impurities, 2019.
- C. G. Fraga, Mol. Aspects Med., 2005, 26, 235–244 CrossRef CAS PubMed.
- M. L. Rasmussen, A. R. Folsom, D. J. Catellier, M. Y. Tsai, U. Garg and J. H. Eckfeldt, Atherosclerosis, 2001, 154, 739–746 CrossRef CAS PubMed.
- L. E. Beckman, G. F. Van Landeghem, C. Sikstrom, A. Wahlin, B. Markevarn, G. Hallmans, P. Lenner, L. Athlin, R. Stenling and L. Beckman, Carcinogenesis, 1999, 20, 1231–1233 CrossRef CAS PubMed.
- N. Lewen, S. Mathew, M. Schenkenberger and T. Raglione, J. Pharm. Biomed. Anal., 2004, 35, 739–752 CrossRef CAS PubMed.
- E. Bulska and A. Ruszczynska, Phys. Sci. Rev., 2017, 2, 1–14 CrossRef.
- R. Furlan de Oliveira, V. Montes-Garcia, A. Ciesielski and P. Samori, Mater. Horiz., 2021, 8, 2685–2708 RSC.
- C. Pezzato, S. Maiti, J. L. Chen, A. Cazzolaro, C. Gobbo and L. J. Prins, Chem. Commun., 2015, 51, 9922–9931 RSC.
- V. Montes-Garcia, M. A. Squillaci, M. Diez-Castellnou, Q. K. Ong, F. Stellacci and P. Samori, Chem. Soc. Rev., 2021, 50, 1269–1304 RSC.
- G. Markiewicz, A. Walczak, F. Perlitius, M. Piasecka, J. Harrowfield and A. R. Stefankiewicz, Dalton Trans., 2018, 47, 14254–14262 RSC.
- A. R. Stefankiewicz, J. Harrowfield, A. M. Madalan and J. M. Lehn, CrystEngComm, 2013, 15, 9128–9134 RSC.
- B. Kaur, N. Kaur and S. Kumar, Coord. Chem. Rev., 2018, 358, 13–69 CrossRef CAS.
- V. K. Gupta, A. K. Singh, M. R. Ganjali, P. Norouzi, F. Faridbod and N. Mergu, Sens. Actuators, B, 2013, 182, 642–651 CrossRef CAS.
- H. Sun, Y. Jiang, J. Nie, J.-H. Wei, B. X. Miao, Y. Zhao, L.-F. Zhang and Z.-H. Ni, Mater. Chem. Front., 2021, 5, 347–354 RSC.
- Z. Liao, Y. Liu, S.-F. Han, D. Wang, J.-Q. Zheng, X.-J. Zheng and L.-P. Jin, Sens. Actuators, B, 2017, 244, 914–921 CrossRef CAS.
- Y. Wang, X. Li, Y. Zhou and C. Liu, Int. J. Chem., 2012, 4, 90–95 CrossRef CAS.
- P. Huang, J. Li, X. Liu and F. Wu, Microchim. Acta, 2015, 183, 863–869 CrossRef.
- A. A. Jimoh, A. Helal, M. N. Shaikh, M. A. Aziz, Z. H. Yamani, A. Al-Ahmed and J. P. Kim, J. Nanomater., 2015, 2015, 1–7 CrossRef.
- W. Edwards, N. Marro, G. Turner and E. R. Kay, Chem. Sci., 2018, 9, 125–133 RSC.
- J. M. McMahon and S. R. Emory, Langmuir, 2007, 23, 1414–1418 CrossRef CAS PubMed.
- E. Oliveira, J. D. Nunes-Miranda and H. M. Santos, Inorg. Chim. Acta, 2012, 380, 22–30 CrossRef CAS.
- A. C. Savage and Z. Pikramenou, Chem. Commun., 2011, 47, 6431–6433 RSC.
- P. D. Beer, D. P. Cormode and J. J. Davis, Chem. Commun., 2004, 414–415, 10.1039/b313658b.
- Z. Krpetic, L. Guerrini, I. A. Larmour, J. Reglinski, K. Faulds and D. Graham, Small, 2012, 8, 707–714 CrossRef CAS PubMed.
- J. M. Abad, S. F. Mertens, M. Pita, V. M. Fernandez and D. J. Schiffrin, J. Am. Chem. Soc., 2005, 127, 5689–5694 CrossRef CAS PubMed.
- D.-H. Wang, Y. Zhang, R. Sun and D.-Z. Zhao, RSC Adv., 2016, 6, 4640–4646 RSC.
- Y. Gou, Y. Zhang, J. Qi, Z. Zhou, F. Yang and H. Liang, J. Inorg. Biochem., 2015, 144, 47–55 CrossRef CAS PubMed.
- J.-X. Yu, V. D. Kodibagkar, L. Liu, Z. Zhang, L. Liu, J. Magnusson and Y. Liu, Chem. Sci., 2013, 4, 2132 RSC.
- X. Huang, A. J. Shumski, X. Zhang and C. W. Li, J. Am. Chem. Soc., 2018, 140, 8918–8923 CrossRef CAS PubMed.
- K. Singh, V. Kumar, B. Kukkar, K. H. Kim and T. R. Sharma, Int. J. Environ. Sci. Technol., 2021 DOI:10.1007/s13762-021-03331-0.
- J. Das and P. Sarkar, Environ. Sci.: Water Res. Technol., 2016, 2, 693–704 RSC.
- T. Kiatkumjorn, P. Rattanarat, W. Siangproh, O. Chailapakul and N. Praphairaksit, Talanta, 2014, 128, 215–220 CrossRef CAS PubMed.
- T.-B. Wei, P. Zhang, B.-B. Shi, P. Chen, Q. Lin, J. Liu and Y.-M. Zhang, Dyes Pigm., 2013, 97, 297–302 CrossRef CAS.
- A. K. Yetisen, Y. Montelongo, M. M. Qasim, H. Butt, T. D. Wilkinson, M. J. Monteiro and S. H. Yun, Anal. Chem., 2015, 87, 5101–5108 CrossRef CAS PubMed.
- European Medicine Agency, ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology, 1995.
- Q. Tian, Z. Cheng, H. M. Yajima, S. J. Savage, K. L. Green, T. Humphries, M. E. Reynolds, S. Babu, F. Gosselin, D. Askin, I. Kurimoto, N. Hirata, M. Iwasaki, Y. Shimasaki and T. Miki, Org. Process Res. Dev., 2013, 17, 97–107 CrossRef CAS.
- P. W. Manley, M. Acemoglu, W. Marterer and W. Pachinger, Org. Process Res. Dev., 2003, 7, 436–445 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc00497f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |

