Activating oxygen deficient TiO2 in the visible region by Bi2MoO6 for CO2 photoreduction to methanol†
Risov
Das
ab,
Kousik
Das
 ab,
Sathyapal R.
Churipard
ab,
Sathyapal R.
Churipard
 ab and
Sebastian C.
Peter
ab and
Sebastian C.
Peter
 *ab
*ab
aNew Chemistry Unit, Jawaharlal Nehru Centre for Advanced Scientific Research, Jakkur, Bangalore-560064, India. E-mail: sebastiancp@jncasr.ac.in; sebastiancp@gmail.com
bSchool of Advanced Materials, Jawaharlal Nehru Centre for Advanced Scientific Research, Jakkur, Bangalore-560064, India
First published on 5th April 2022
Abstract
Fast photogenerated charge recombination and inappropriate bandgap for visible light driven charge generation hinders the performance of TiO2. In this study, TiO2 was activated for visible light driven CO2 reduction in the presence of Bi2MoO6 as an electron donor. Furthermore, the introduction of oxygen vacancies resulted in enhanced CO2 adsorption and conversion. The best catalyst gives 27.1 μmol g−1 h−1 methanol formation. DRIFTS was used to explain the methanol formation mechanism on oxygen deficient TiO2.
Rapid escalations in the demand for energy globally have set researchers on a quest to design and develop new energy production and storage technologies that can meet this demand sustainably. One such route is the use of abundantly available solar energy to convert anthropogenic CO2 to energy. As an added benefit, this can also mitigate the greenhouse effects caused by the rise in CO2 levels in the atmosphere.1 Transition metal oxide-based semiconductors are considered to be promising photocatalysts due to their suitable band energy and excellent stability against photodegradation. For example, TiO2,2 WO3,3 Bi2MoO6,4 BiVO45etc. have been extensively used for the photocatalytic hydrogen evolution reaction (HER) and CO2 reduction reaction (CO2RR).6
Despite having promising photocatalytic activity, the practical application of TiO2 is constrained by its poor visible light activity and its tendency to undergo extensive electron–hole recombination, reducing the extent of photogenerated electrons (e−) available for photoconversion. Similarly, Bi2MoO6, a promising visible light driven photocatalyst shows low quantum efficiency owing to its poor charge separation and slow carrier mobility.7 Establishing a heterojunction between two semiconductors is an excellent strategy to significantly increase the photoconversion efficiency by not only modulating the light sensitivity towards the visible region but also promoting the charge carrier separation.8 In addition, surface engineering leading to the generation of oxygen vacancies (Ov) can also increase the lifetime of photogenerated e−s by creating trap states between the conduction and valence band.9 Here, Bi2MoO6 is used as an e− donor to activate TiO2 under visible light. In addition, Ov was created deliberately to offer optimum CO2 chemisorption opportunities at the TiO2 surface. With the help of this dual strategy, we were able to achieve a dramatic increment of the CO2RR performance by suppressing the drawbacks of the individual components. CH3OH was obtained as the major product, which is regarded as a high energy dense fuel.10
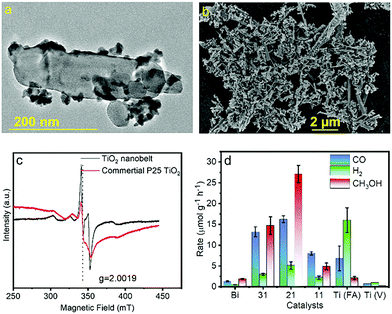
TiO2 and Bi2MoO6 and composites were synthesised by solvothermal processes. Pre-synthesized TiO2 was added to an autoclave along with Bi(NO3)3 and Na2MoO4 to make a suitable interface between TiO2 and Bi2MoO6 for facile e− transfer within the heterojunction. The formation of the heterojunction is depicted via TEM and SEM images in Fig. 1a and b, which demonstrate the anchoring of Bi2MoO6 nano-seeds on TiO2 nano ribbons. The HRTEM image in Fig. S1 (ESI†) depicts the (101) plane of TiO2 and (131) plane of Bi2MoO6, which are in intimate contact with each other to form the heterostructure in the composite catalyst. The nano-seed like morphology of pristine Bi2MoO6 and the ribbon like morphology of pure TiO2 were confirmed from EDX colour mapping which showed that the seed like morphology contains Bi, Mo and O (Fig. S2a–d, ESI†) and the ribbon like morphology mainly consists of Ti and O (Fig. S3a–d, ESI†). The ratio of Bi2MoO6 and TiO2 was varied during the synthesis process to optimize the composite composition for best CO2 photoreduction activity. The weight ratios of Bi2MoO6 and TiO2 were varied from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, to 2
1, to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and termed as Bi3@Ti1, Bi2@Ti1 and Bi1@Ti1. Henceforth this nomenclature will be used throughout the manuscript. The PXRD patterns of the TiO2, Bi2MoO6, and Bi2MoO6/TiO2 heterostructures are shown in Fig. S4a (ESI†). Six distinctive peaks observed for TiO2 at 2θ = 25.28° (101), 37.80° (004), 48.05° (200), 53.89° (105), 55.06° (211) and 62.69° (204) corroborate with anatase TiO2 (I41/amd).11 In the case of Bi2MoO6, the diffraction peaks at 28.25°, 32.59°, 33.07°, 46.72°, 47.07°, 55.46°, 55.53° and 56.16° could be perfectly indexed to the (131), (002), (060), (202), (260), (331), (133) and (191) planes of orthorhombic Bi2MoO6 (Pca21).12 For the Bi2MoO6/TiO2 heterostructures, all of the peaks can be assigned to either TiO2 or Bi2MoO6. The absence of extra peaks proves the formation of a pure heterojunction. The (101) plane of TiO2 with very less intensity is observed in the XRD patterns of the composite, which is due to the lower diffraction ability of TiO2 compared to Bi2MoO6. With an increase in the amount of TiO2, the peak intensity also increases (Fig. S4a, ESI†). Instead of using commercially available TiO2 (P25), here TiO2 was treated with a base for increasing Ov in it. The presence of more Ov in the TiO2 nano ribbons compared to P25 was understood from EPR spectroscopy (Fig. 1c). Ov induces the formation of Ti3+ along with Ti4+ (Ti4+O2 → Ti3+Ti4+O(2−X) + X/2O2).
1 and termed as Bi3@Ti1, Bi2@Ti1 and Bi1@Ti1. Henceforth this nomenclature will be used throughout the manuscript. The PXRD patterns of the TiO2, Bi2MoO6, and Bi2MoO6/TiO2 heterostructures are shown in Fig. S4a (ESI†). Six distinctive peaks observed for TiO2 at 2θ = 25.28° (101), 37.80° (004), 48.05° (200), 53.89° (105), 55.06° (211) and 62.69° (204) corroborate with anatase TiO2 (I41/amd).11 In the case of Bi2MoO6, the diffraction peaks at 28.25°, 32.59°, 33.07°, 46.72°, 47.07°, 55.46°, 55.53° and 56.16° could be perfectly indexed to the (131), (002), (060), (202), (260), (331), (133) and (191) planes of orthorhombic Bi2MoO6 (Pca21).12 For the Bi2MoO6/TiO2 heterostructures, all of the peaks can be assigned to either TiO2 or Bi2MoO6. The absence of extra peaks proves the formation of a pure heterojunction. The (101) plane of TiO2 with very less intensity is observed in the XRD patterns of the composite, which is due to the lower diffraction ability of TiO2 compared to Bi2MoO6. With an increase in the amount of TiO2, the peak intensity also increases (Fig. S4a, ESI†). Instead of using commercially available TiO2 (P25), here TiO2 was treated with a base for increasing Ov in it. The presence of more Ov in the TiO2 nano ribbons compared to P25 was understood from EPR spectroscopy (Fig. 1c). Ov induces the formation of Ti3+ along with Ti4+ (Ti4+O2 → Ti3+Ti4+O(2−X) + X/2O2).
The presence of more Ti3+ compared to Ti4+ in the TiO2 nanoribbons was depicted from X-ray photoelectron spectroscopy (XPS) in Fig. S5 (ESI†). The deconvoluted spectra of P25 also indicated the presence of Ti3+ although in much lower quantity compared to the TiO2 nanoribbons. The 3d1 e− in Ti3+ generates an EPR signal.13 Hence, the more intense EPR signal of the TiO2 nano ribbons compared to P25 clearly indicates the presence of more Ti3+ or more Ov in the TiO2 nano ribbons. These Ov act as hole scavengers (Ti3+ + h+ → Ti4+) and oxygen deficient TiO2 lowers the CO2 adsorption energy on the TiO2 surface, which in turn facilitates the CO2 reduction process (Fig. S4b, ESI†).14 CO2 Temperature Programmed Desorption (TPD) in Fig. S6 (ESI†) revealed that the TiO2 with vacancies has better CO2 uptake capacity (1.85 μmol g−1) compared to pure TiO2 (0.53 μmol g−1) (Table S1, ESI†). The CO2 photoreduction performance of all the catalysts was tested under solar simulated 450 W Xe light illumination in a sealed quartz tube. Methanol was obtained as the major product and CO as a minor product. The CO2 reduction performance (rate of product formation) of all the catalysts is summarized in Fig. 1d and Table S2 (ESI†). Among all the composite catalysts, Bi2@Ti1 exhibited the best catalytic activity. It achieved a methanol formation rate of 27.1 μmol g−1 h−1 (Fig. S7, ESI†), which is better than most of the TiO2 based catalysts reported for methanol (Table S3, ESI†). Interestingly, pristine TiO2 produced more CO compared to methanol under UV-Visible light illumination. Upon switching the illuminated light from the UV-visible to visible region the activity reduced drastically as e−s and holes cannot be generated by visible light (>400 nm) illumination on TiO2 due to its large band gap. On the other hand, pristine Bi2MoO6 also could not perform satisfactorily due to fast charge recombination. In the composite material photogenerated e−s of Bi2MoO6 were transferred to the neighbouring TiO2 nano ribbons and activated TiO2 for photocatalysis under visible light. The optimum supply of e−s from Bi2MoO6 and the presence of Ov in TiO2 for CO2 adsorption and activation rendered Bi2@Ti1 the best catalyst for methanol formation. The presence of more Bi2MoO6 facilitates more e− for the kinetically demanding methanol formation process (6e−) in comparison to the production of CO (2e−).15 Therefore, better methanol formation rate was observed for Bi3@Ti1 compared to Bi1@Ti1 and pristine TiO2. Furthermore, the role of each component of the CO2 photoreduction reaction (CO2RR) was confirmed from a series of control experiments. Finally, the CO2RR was carried out with 13CO2 and the obtained methanol and CO were examined by GCMS, which clearly shows the formation of 13CO (Fig. S8a and c, ESI†) and 13CH3OH (Fig. S8b and d, ESI†) confirming that the generated products are solely coming from the CO2RR. Cyclability of the spent catalyst showed an unaltered product formation rate for 6 consecutive cycles meaning that the catalyst is stable for repeated performance (Fig. S9, ESI†).
Photocatalytic CO2RR in the absence of CO2, light, catalyst and 0.1M NaOH solution showed neither gaseous nor any liquid product formation, indicating the crucial role played by these components. After 6 cycles the catalysts were examined via XRD (Fig. S10, ESI†), TEM (Fig. S11a, ESI†) and SEM (Fig. S11b, ESI†) analyses to understand the structural and morphological integrity. Post catalysis samples displayed similar XRD patterns, and morphology compared to the pristine catalyst. All the catalysts has produced some amount of H2. However, upon composite formation with Bi2MoO6, the HER was suppressed dramatically compared to pristine TiO2. Therefore, it can be inferred that Bi2@Ti1 not only performed better towards CO2 reduction but also suppressed the e− wasting competitive HER, which is considered as one of the biggest challenges in photocatalytic CO2RR. Improved activity of composites and the role of Bi2MoO6 as an e− donor can be understood by the analysis of the band structure and charge transfer process. As depicted in Fig. 2a, the absorbance spectra revealed that TiO2 can only harvest the UV part of the solar spectrum. Therefore, TiO2 could not perform under visible light illumination. On the other hand, Bi2MoO6 has an absorption edge in the visible region. Therefore, the composite materials have UV as well as visible light absorption capability. The agreement between the absorption onset of Bi2@Ti1 and overall apparent quantum yield (AQY%) was understood upon different wavelength chopped light illumination (Fig. 2b). The CO2 reduction process terminated at more than 475 nm light irradiation and the AQY% trends followed the Bi2@Ti1 absorption pattern meaning that the photocatalysis proceeded via excited e−s of Bi2MoO6. Therefore, the highest AQY of 0.89% was obtained by 400 ± 10 nm light irradiation where Bi2MoO6 shows maximum absorption.
Further insight of e− transfer was understood by band gap calculation through a Tauc plot (Fig. S12, ESI†) and band alignment calculation by Mott–Schottky measurements. It showed that TiO2 and Bi2MoO6 have bandgaps of 3.19 eV and 2.92 eV, respectively. From the Mott–Schottky measurements it can be said that both the semiconductors are n-type and hence their conduction band maxima (CBM) stay near to the Fermi level. Therefore, the CBM position was obtained by adding −0.1 V with the flat band potential (Vfb) obtained from Mott–Schottky plots (Fig S13a and b, ESI†). As depicted in Fig. 2c the CBM positions of Bi2MoO6 and TiO2 are situated at −0.71 V and −0.58 V. Therefore, the excited e−s of Bi2MoO6 can easily be transferred to TiO2 upon visible light irradiation, which can further be used for the CO2RR. Fig. 2c clearly shows that TiO2 has enough potential for CO2 to methanol or CO production by utilizing excited e−s of Bi2MoO6 because −0.36 V and −0.54 V is the CO2 to methanol and CO formation potential, respectively. Therefore, the leftover photogenerated holes in the valence band of Bi2MoO6 participated in the water oxidation reaction. The evolved O2 was quantified by gas chromatography (GC) which showed that the amount of evolved O2 is approximately matching with the stoichiometric amount (Fig. S14, ESI†). The valence band positions were determined using band gap value and CBM position. Time dependent photoluminescence study further elucidated the e− transfer pathway. Fig. 2d and Table S4 (ESI†) show that the pristine Bi2MoO6 had lower lifetime for the excited electrons (τavg = 2.2 ns) compared to Bi2@Ti1 (τavg = 2.7 ns). On the other hand, as shown in Fig. S15 (ESI†) static photo luminescence (PL) study showed that the composites have weaker PL intensities compared to pristine Bi2MoO6. This manifests that the excited e−s of pristine Bi2MoO6 quickly recombine with photogenerated holes, whereas in the composites the e− goes to neighbouring TiO2 instead of recombining. Therefore, the composites showed less PL intensity and more excited charge lifetime.16 Photoelectrochemical measurements including interfacial charge transfer resistance and light induce current generation capacity were understood from electrochemical impedance spectroscopy (Fig. S16a, ESI†) and transient photocurrent measurements (Fig. S16b, ESI†). Interestingly, Bi2@Ti1 has the least and pristine Bi2MoO6 has the highest charge transfer resistance. An interesting photocurrent generation feature was seen for TiO2 and the composites. TiO2 showed rapid photocurrent decay compared to the composites upon light off (Fig. S17, ESI†). Moreover, Bi2@Ti1 exhibited the highest photocurrent. Pristine TiO2 has delivered no extra current under visible light illumination. The featured photocurrent for TiO2 in Fig. S16b (ESI†) is due to UV-visible light irradiation. Pristine Bi2MoO6 has much less photocurrent production ability and high charge transfer resistance. Therefore, its performance as a photocatalyst is lacking. However, upon composite formation these drawbacks were overcome and the CO2RR performance increased.
The methanol formation pathway was understood from the identification of intermediates formed during CO2 hydrogenation via operando DRIFTS (Fig. 3a). The peaks observed at 1372 cm−1 and 1583 cm−1 represent the bidentate and monodentate carbonates generated via adsorption of CO2 in the Ov–TiO2 surface.17 Most importantly, the intensity of the band at 1655 cm−1 corresponding to *COOH has increased with light irradiation time.18 The *COOH intermediate is regarded as the first and most common intermediate for CO2 hydrogenation.18
A tiny peak evolution at 2095 cm−1 corresponding to the *CO intermediate was also found, which upon desorption from the surface can produce gaseous CO.18 The most important intermediates of methanol formation are *OCH3 and *CHO. A monotonous evolution of the IR peaks at 1036 cm−1 and 1114 cm−1 implicitly indicates the presence of these 2 important intermediates.5 The *CO intermediate was rapidly converted to the *CHO intermediate; therefore, the intensity of the *CO intermediate remained negligible. On the other hand, the *OCH3 intermediate showed a prominent peak. Therefore, it can be inferred that the *OCH3 intermediate has chemisorbed on the Ov–TiO2 surface for longer time and got protonated on the O site for methanol formation.19 The absence of methane eliminates the probability of proton adsorption on the C centre of the *OCH3 intermediate (Fig. S18, ESI†). Based on the knowledge of these intermediates, the formate mechanism for methanol formation can be confirmed. A mechanistic scheme for methanol formation via a 6 proton coupled e− transfer process is shown in Fig. 3b. CO and H2O were obtained as the by-products through this mechanism. Upon *OCH3 protonation, the catalyst bed can be regenerated without any chemical corrosion. Moreover, as mentioned previously, the formed heterojunction allows TiO2 to utilize visible light energy more effectively via additional photo-generated e− transfer from Bi2MoO6 for the reaction, whereby the yield of methanol formation is greatly enhanced.
In summary, introducing Bi2MoO6 nanoparticles into Ov TiO2 allows e− transfer through the intimate interface. Thereby, the active sites of TiO2 were used for CO2 adsorption and photogenerated charges of Bi2MoO6 were used for the CO2RR. A series of photophysical and photoelectrochemical studies established the regions of best activity in the Bi2@Ti1 catalyst. Finally, the methanol formation mechanism was predicted based on the intermediate's information from operando DRIFTS. Further modification of the reaction conditions can lead to better increment of the performance and can be used for large scale application owing to the high durability of this composite.
Financial support from the Department of Science and Technology (DST) (DST/TMD/(EWO)/IC#5-2018/02) is gratefully acknowledged. SCP thanks DST for the SwarnaJayanti Fellowship (DST/SJF/CSA-02/2017-18). RD, KD and SRC thank CSIR and JNCASR for the research fellowships.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) W. Tu, Y. Zhou and Z. Zou, Adv. Mater., 2014, 26, 4607–4626 CrossRef CAS PubMed; (b) S. Roy, A. Cherevotan and S. C. Peter, ACS Energy Lett., 2018, 3, 1938–1966 CrossRef CAS; (c) S. C. Peter, ACS Energy Lett., 2018, 3, 1557–1561 CrossRef CAS; (d) W. Zhou, Q. Xie, J. Li, C. Zhengwei and F. Peng, Bull. Mater. Sci., 2019, 42, 12 CrossRef; (e) D.-H. Lan, Y.-X. Gong, N.-Y. Tan, S.-S. Wu, J. Shen, K.-C. Yao, B. Yi, C.-T. Au and S.-F. Yin, Carbon, 2018, 127, 245–254 CrossRef CAS.
- R. Das, S. Chakraborty and S. C. Peter, ACS Energy Lett., 2021, 6, 3270–3274 CrossRef CAS.
- L. J. Zhang, S. Li, B. K. Liu, D. J. Wang and T. F. Xie, ACS Catal., 2014, 4, 3724–3729 CrossRef CAS.
- R. Das, K. Das, B. Ray, C. P. Vinod and S. C. Peter, Energy Environ. Sci., 2022 10.1039/D1EE02976B.
- R. Das, S. Sarkar, R. Kumar, S. D. Ramarao, A. Cherevotan, M. Jasil, C. P. Vinod, A. K. Singh and S. C. Peter, ACS Catal., 2022, 12, 687–697 CrossRef CAS.
- J.-K. G. Wei Zhou, S. Shen, J. Pan, J. Tang, L. Chen, C.-T. Au and S.-F. Yin, Acta Phys. -Chim. Sin., 2020, 36, 1906048 Search PubMed.
- H. Yu, L. Jiang, H. Wang, B. Huang, X. Yuan, J. Huang, J. Zhang and G. Zeng, Small, 2019, 15, 1901008 CrossRef PubMed.
- (a) L. Etgar, P. Gao, Z. Xue, Q. Peng, A. K. Chandiran, B. Liu, M. K. Nazeeruddin and M. Grätzel, J. Am. Chem. Soc., 2012, 134, 17396–17399 CrossRef CAS PubMed; (b) R. Hao, G. Wang, H. Tang, L. Sun, C. Xu and D. Han, Appl. Catal., B, 2016, 187, 47–58 CrossRef CAS; (c) E. Vesali-Kermani, A. Habibi-Yangjeh, H. Diarmand-Khalilabad and S. Ghosh, J. Colloid Interface Sci., 2020, 563, 81–91 CrossRef CAS PubMed.
- (a) Y. Zhang, Z. Xu, G. Li, X. Huang, W. Hao and Y. Bi, Angew. Chem., Int. Ed., 2019, 58, 14229–14233 CrossRef CAS PubMed; (b) Y. Fang, Z. Liu, J. Han, Z. Jin, Y. Han, F. Wang, Y. Niu, Y. Wu and Y. Xu, Adv. Energy Mater., 2019, 9, 1803406 CrossRef.
- (a) A. Cherevotan, J. Raj, L. Dheer, S. Roy, S. Sarkar, R. Das, C. P. Vinod, S. Xu, P. Wells, U. V. Waghmare and S. C. Peter, ACS Energy Lett., 2021, 6, 509–516 CrossRef CAS; (b) D. Bagchi, J. Raj, A. K. Singh, A. Cherevotan, S. Roy, K. S. Manoj, C. P. Vinod and S. C. Peter, Adv. Mater., 2022, 2109426 CrossRef CAS PubMed.
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Nature, 2008, 453, 638–641 CrossRef CAS PubMed.
- Z. Jia, F. Lyu, L. C. Zhang, S. Zeng, S. X. Liang, Y. Y. Li and J. Lu, Sci. Rep., 2019, 9, 7636 CrossRef CAS PubMed.
- X. Pan, M.-Q. Yang, X. Fu, N. Zhang and Y.-J. Xu, Nanoscale, 2013, 5, 3601–3614 RSC.
- (a) W. Pipornpong, R. Wanbayor and V. Ruangpornvisuti, Appl. Surf. Sci., 2011, 257, 10322–10328 CrossRef CAS; (b) J.-B. Pan, B.-H. Wang, J.-B. Wang, H.-Z. Ding, W. Zhou, X. Liu, J.-R. Zhang, S. Shen, J.-K. Guo, L. Chen, C.-T. Au, L.-L. Jiang and S.-F. Yin, Angew. Chem., Int. Ed., 2021, 60, 1433–1440 CrossRef CAS PubMed.
- Y. Fang and X. Wang, Chem. Commun., 2018, 54, 5674–5687 RSC.
- Y. P. Xie, Y. Yang, G. Wang and G. Liu, J. Colloid Interface Sci., 2017, 503, 198–204 CrossRef CAS PubMed.
- T. H. Tan, B. Xie, Y. H. Ng, S. F. B. Abdullah, H. Y. M. Tang, N. Bedford, R. A. Taylor, K.-F. Aguey-Zinsou, R. Amal and J. Scott, Nat. Catal., 2020, 3, 1034–1043 CrossRef CAS.
- X. Li, Y. Sun, J. Xu, Y. Shao, J. Wu, X. Xu, Y. Pan, H. Ju, J. Zhu and Y. Xie, Nat. Energy, 2019, 4, 690–699 CrossRef CAS.
- Z. Sun, T. Ma, H. Tao, Q. Fan and B. Han, Chem, 2017, 3, 560–587 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc00490a |
| This journal is © The Royal Society of Chemistry 2022 |