Iridium-catalyzed asymmetric double allylic alkylation of azlactone: efficient access to chiral α-amino acid derivatives†
Abstract
An unprecedented Ir-catalyzed enantioselective double allylic alkylation of less bulky cyclic imine glycinate (azlactone) was rationally designed and developed, providing various bisallylated chiral amino acid derivatives. Control experiments revealed that this transformation proceeds in a sequential manner featuring quasi-dynamic kinetic resolution of the initially-formed monoallylation intermediates.
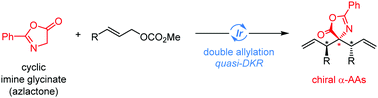
- This article is part of the themed collection: Chemical Communications HOT Articles 2022


 Please wait while we load your content...
Please wait while we load your content...