 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enantiopure ferrocene-1,2-disulfoxides: synthesis and reactivity†‡
Min
Wen
,
William
Erb
 *,
Florence
Mongin
*,
Florence
Mongin
 ,
Marielle
Blot
and
Thierry
Roisnel
,
Marielle
Blot
and
Thierry
Roisnel

Univ Rennes, CNRS, ISCR (Institut des Sciences Chimiques de Rennes) – UMR 6226, F-35000 Rennes, France. E-mail: william.erb@univ-rennes1.fr
First published on 14th January 2022
Abstract
The rational use of directed deprotometallation, sulfur oxidation and sulfoxide/lithium exchange allowed the synthesis of enantiopure ferrocene-1,2-disulfoxide derivatives. Not only do they represent the first members of this original family, but some of them have shown promise as ligands in rhodium-catalysed conjugate addition.
While bis-sulfoxides were mentioned sporadically in a few studies over a century ago,1–3 their widespread development did not take place until the 1980s, especially with C2 symmetric derivatives,4 given their interest in asymmetric synthesis and catalysis.5–8
Among the various examples reported, (S,S)-S,S′-di(4-tolyl)benzene-1,2-disulfoxide (A), (R,R,P)-S,S′-di(4-tolyl)-1,1′-binaphthalene-2,2′-disulfoxide (not shown), (R,R)-S,S′-di(tert-butyl)benzene-1,2-disulfoxide (B) and (S,S)-S,S′-diferrocenylethane-1,2-disulfoxide (S,S-Ferbisox), respectively disclosed by the groups of Shibasaki,9 Dorta,10 Liao,11 Khiar and Fernández12 (Fig. 1), deserve to be mentioned in view of their interest as ligands in organometallic catalysis. However, ligands in which the phenylene bridge would be replaced by a 1,2-ferrocenylene have never been documented to date.
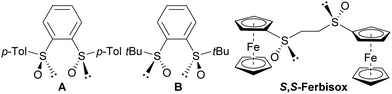 | ||
| Fig. 1 Aromatic bis-sulfoxides used as chiral ligands either in Pd-catalysed asymmetric allylic allylation (A) or in Rh-catalysed conjugate additions (B and S,S-Ferbisox). | ||
Beginning in 1993, Kagan and co-workers reported key reactions to prepare enantiopure ferrocenesulfoxides and convert them by diastereoselective deprotolithiation to planar chiral ferrocenes.13,14 In this approach, 4-tolylsulfinyl15–17 and tert-butylsulfinyl18 have been particularly studied, not only as directing groups, but also because they can be easily transformed, by reduction to sulfides,15,19,20 by oxidation to sulfones,21 by sulfoxide/lithium exchange15 followed by trapping,16,17 or simply by removal.18
Taking into account their unique three-dimensional structure and the possibility of finely adjusting their steric and electronic properties, ferrocenic ligands are privileged structures in asymmetric catalysis.22,23 However, despite the reported advantages of bis-sulfoxide ligands in asymmetric catalysis, examples with a ferrocene core are scarce.12,24 Since ferrocene-1,2-disulfoxides have never been described before, we report here the first study devoted to their synthesis and functionalization. Furthermore, we validate their potential as new ligands for asymmetric catalysis.
Liao and co-workers synthesized B from its monosulfoxide by deprotolithiation followed by interception with (R)-S-tert-butyl-tert-butanethiosulfinate.11 In our hands, reacting (R)-S-tert-butylferrocenesulfoxide (R-FcSOtBu) with tert-butyllithium25 in tetrahydrofuran (THF) before similar trapping with Ellman's reagent26 afforded the ferrocene-1,2-disulfoxide 1 in a similar yield (Scheme 1, top). In parallel, when the Andersen method (reaction of the organolithium with (−)-menthyl (S)-4-toluenesulfinate)27 was applied to the lithiated derivative of (S)-S-tert-butylferrocenesulfoxide (S-FcSOtBu), (S,S,RP)-S-tert-butyl-S′-(4-tolyl)ferrocene-1,2-disulfoxide (2) was obtained in 88% yield28 (Scheme 1, middle).
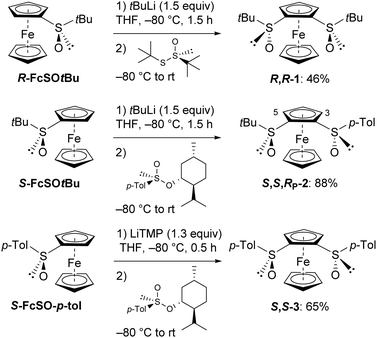 | ||
| Scheme 1 Access to (R,R)-S,S′-di-tert-butylferrocene-1,2-disulfoxide (1), (S,S,RP)-S-tert-butyl-S′-(4-tolyl)ferrocene-1,2-disulfoxide (2) and (S,S)-S,S′-di(4-tolyl)ferrocene-1,2-disulfoxide (3).30 | ||
As we also needed a ligand bearing two 4-tolylsulfoxides, we next turned to (S,S)-S,S′-di(4-tolyl)ferrocene-1,2-disulfoxide (3). It was prepared from (S)-S-(4-tolyl)ferrocenesulfoxide (R-FcSO-p-Tol), this time using a lithium amide to avoid the well-known sulfoxide/lithium exchange with tert-butyllithium. While such sulfoxides are usually deprotonated using lithium diisopropylamide,13,14 we employed the stronger lithium 2,2,6,6-tetramethylpiperidide (LiTMP)29 after checking its ability to deprotonate R-FcSO-p-Tol (80% yield after deuteriolysis). Thus, using this base, the bis-sulfoxide 3 (ferrocene analogue of A) was isolated in 65% yield (Scheme 1, bottom).
Although these reactions work well, we reasoned that a diastereoselective oxidation of sulfide could represent a complementary approach towards ferrocene-1,2-disulfoxides. In 1995, Shibasaki and co-workers showed that the oxidation of (S)-S-(4-tolyl)-2-(4-tolylthio)benzenesulfoxide using 3-chloroperbenzoic acid (m-CPBA) only allowed a moderate diastereoselectivity (61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39) in favour of the benzene-1,2-disulfoxide of C2 symmetry.9 In the ferrocene series, the Ugi group showed as early as 1985, from (R,SP)-2-(tert-butylthio)- and (R,SP)-2-(4-tolylthio)-α-(dimethylamino)ethylferrocenes, that the reaction result depended on the oxidizing agent, the (RC,SS,SP)-sulfoxides being mainly formed with m-CPBA and the (RC,RS,SP)- with sodium periodate.18,31 However, in 1998, the Hua group reported that the treatment of (R)-2-(tert-butylthio)ferrocenemethanol with m-CPBA gave the stereoisomer for which the oxygen of the sulfinyl group is directed to the unsubstituted site of ferrocene.15,21
39) in favour of the benzene-1,2-disulfoxide of C2 symmetry.9 In the ferrocene series, the Ugi group showed as early as 1985, from (R,SP)-2-(tert-butylthio)- and (R,SP)-2-(4-tolylthio)-α-(dimethylamino)ethylferrocenes, that the reaction result depended on the oxidizing agent, the (RC,SS,SP)-sulfoxides being mainly formed with m-CPBA and the (RC,RS,SP)- with sodium periodate.18,31 However, in 1998, the Hua group reported that the treatment of (R)-2-(tert-butylthio)ferrocenemethanol with m-CPBA gave the stereoisomer for which the oxygen of the sulfinyl group is directed to the unsubstituted site of ferrocene.15,21
Accordingly, we selected m-CPBA to attempt the stereoselective oxidation of the two enantiomers of S-tert-butyl-2-(phenylthio)ferrocenesulfoxide 4 (see ESI‡). In both cases, by carrying out the reactions at 0 °C, only one stereoisomer was obtained in high yield. In addition, the 1H and 13C NMR spectra of the bis-sulfoxide S,S,RP-5 being similar to those of S,S,RP-2, the stereochemistry of the compounds 5 could be unambiguously assigned (Scheme 2).
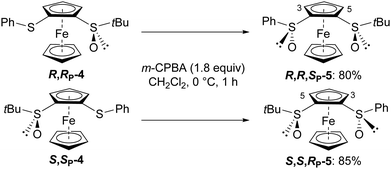 | ||
| Scheme 2 Stereoselective oxidation of (R,RP)- and (S,SP)-S-tert-butyl-2-(phenylthio)ferrocenesulfoxides (4) to (R,R,SP)- and (S,S,RP)-S-tert-butyl-S′-phenylferrocene-1,2-disulfoxides (5), respectively.30 | ||
The regioselectivity of deprotolithiation on ferrocene-1,2-disulfoxides is highly predictable since the bulky sulfinyl group (tBu or Ar) remains in the exo position.14 In our case, the oxygen oriented to the 3-position (see Schemes 1 and 2) should direct its deprotolithiation. Logically, when S,S,RP-2 and R,R,SP-5 were successively treated with LiTMP and chlorotrimethylsilane in THF at −80 °C, the 3-silylated derivatives 6a and 7a were obtained, although in low yields (Scheme 3). These could result from an unfavourable steric hindrance between the two sulfinyl groups, since the starting materials were recycled in the two reactions carried out at low temperature.
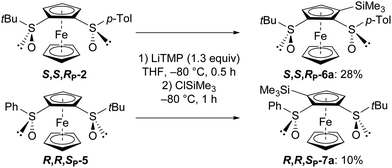 | ||
| Scheme 3 Access to (S,S,RP)-S-tert-butyl-S′-(4-tolyl)-3-(trimethylsilyl)ferrocene-1,2-disulfoxide (6a) and (R,R,SP)-S-tert-butyl-S′-phenyl-3-(trimethylsilyl)ferrocene-1,2-disulfoxide (7a).30 | ||
However, by performing the deprotometallation of S,S,RP-5 at −50 °C, subsequent electrophilic trapping using respectively N,N-dimethylmethyleneiminium iodide, hexachloroethane or N-fluorobenzenesulfonimide (NFSI) led to the amine 7b, the chloride 7c and the fluoride 7d with better yields (Scheme 4). As expected, starting from R,R,SP-5 afforded the enantiomer of 7d in similar yield (60%; see ESI‡).
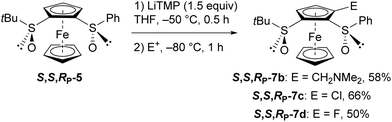 | ||
| Scheme 4 Access to the 3-substituted (S,S,RP)-S-tert-butyl-S′-phenylferrocene-1,2-disulfoxides 7b-d. (E+ = electrophile).30 | ||
In order to obtain other original ferrocene sulfoxides, we next studied deprotolithiation-trapping sequences from the sulfide S,SP-4, easily obtained from S-FcSOtBu (see ESI‡). Indeed, the phenylthio being a weak ortho-directing group,32 but removable after oxidation, it can play here the role of protecting group of both positions 2 and 3. However, because the oxygen of the sulfinyl group is not directed towards a free position, deprotolithiation of S,SP-4 is less likely than that of FcSOtBu. To overcome this issue, Tokitoh's group showed in 2012 that an S-phenylsulfinyl group can direct a first deprotonation-trapping sequence, and be converted into R-phenylsulfinyl, able to direct a second functionalization.33 Although long, this strategy allows the functionalization of the two positions adjacent to the sulfoxide. However, to our knowledge, the direct functionalization of the unfavourable position of a ferrocenesulfoxide has never been reported.
Pleasingly, when S,SP-4 was reacted with n-butyllithium in THF at rt before the addition of ferrocenecarboxaldehyde or chlorotrimethylsilane, the derivatives 8a and 8b were obtained with satisfactory yields (Scheme 5, top). tert-Butylsulfoxide is also a potent group for inducing 2,1′-dideprotolithiation in the presence of an excess of n-butyllithium in THF at rt.13 In our hands, the bis-functionalized derivatives 9a-c were also obtained from S,SP-4 by increasing the amount of base to 2.5 equiv. and using respectively chlorotrimethylsilane, chlorodiphenylphosphine or dichlorodimethylphosphine as electrophile (Scheme 5, bottom left).
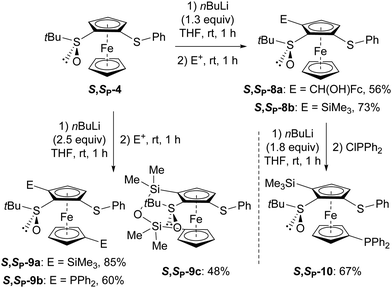 | ||
| Scheme 5 Access to the 5-substituted and 5,1′-disubstituted (S,SP)-S-tert-butyl-2-(phenylthio)ferrocenesulfoxides 8a-b, 9a-c and 10 (E+ = electrophile).30 | ||
However, when the substituent introduced next to the sulfoxide is a poor directing group such as the trimethylsilyl group of compound S,SP-8b, only monodeprotolithiation at the free cyclopentadienyl ring occurred, even in the presence of an excess of base; this was nicely exemplified with the synthesis of the 1′-phosphino derivative 10 (Scheme 5, bottom right).
While we have previously achieved 3-substituted ferrocene-1,2-disulfoxides from the 1,2-disulfoxides S,S,RP-2 and S,S,RP-5 (Schemes 3 and 4), the synthesis of 5-subtituted isomers would require another approach. Therefore, we performed the diastereoselective oxidation of the sulfide S,SP-8b to the required sulfoxide S,S,RP-11 (Scheme 6). We further used the newly formed sulfoxide as a directing group towards the 3,5-disubstituted ferrocene-1,2-disulfoxides 12a and 12b, successively using LiTMP at −80 °C for 0.5 h, and hexachloroethane or NFSI as an electrophile (Scheme 6).
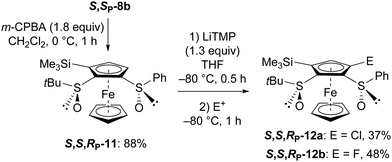 | ||
| Scheme 6 Access to the 5-substituted and 3,5-disubstituted S-tert-butyl-S′-(phenyl)ferrocene-1,2-disulfoxides 12a and 12b (E+ = electrophile).30 | ||
Interestingly, phenylsulfinyl is a traceless directing group that can be replaced by sulfoxide/lithium exchange.15 This was first exemplified in this series by treating 11 and 12a with tert-butyllithium in THF at −90 °C, leading to the 2,5-disubstituted 13a and the 2,4-disubstituted 13b, respectively. By starting from 7d, a reaction time of 10 min34 was found to be sufficient to isolate, after methanolysis, the 3-substituted 13c in high yield, a result which could be due to the good ability of fluorine to promote the introduction of lithium on a neighbouring site. It is also possible to intercept the lithiated intermediate with an electrophile such as methyl chloroformiate, as observed with the synthesis of the ester 13d (Scheme 7).
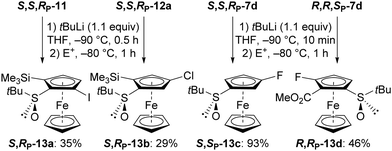 | ||
| Scheme 7 Access to the 2,5-disubstituted, 2,4-disubstituted, 3-substituted and 2,3-disubstituted S-tert-butylferrocenesulfoxides 13a-d (E+ = electrophile: I2 for 13a; ClSiMe3 for 13b but no trapping observed, a result that might be due to steric hindrance).30 | ||
We finally attempted the deprotonation of the monosubstituted cycle of S,SP-9a with n-butyllithium in THF at r.t. before trapping with chlorotrimethylsilane. However, instead of the expected product, we isolated (SP)-1-tert-butyl-2-(phenylthio)-5,1′-bis(trimethylsilyl)ferrocene (14). Although S-tert-butylbenzenesulfoxides can undergo sulfinyl replacement using hindered alkyllithiums, especially in the presence of an adjacent electron-withdrawing group,35,36 this is the first time this reaction has been reported in the ferrocene series (Scheme 8).
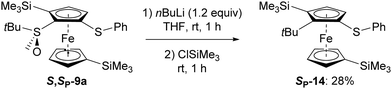 | ||
| Scheme 8 Unexpected replacement of a tert-butylsulfinyl group by a tert-butyl in the presence of n-butyllithium.30 | ||
Some of the newly synthesized ferrocene-1,2-disulfoxides (R,R-1, S,S,RP-2 and S,S-3) were ultimately evaluated as ligands in the rhodium-catalysed 1,4-addition of arylboronic acids to electron-deficient olefins.37–42 Inspired by the studies of Liao,11 Khiar and Fernández,12 the reaction between phenylboronic acid and 2-cyclohexenone was tested in the presence of potassium hydroxide and catalytic amounts of [Rh(C2H4)2Cl]2 and ferrocene-1,2-disulfoxide. Using 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane-water as a solvent at rt or 40 °C did not allow any reaction to take place. However, when a 10
1 dichloromethane-water as a solvent at rt or 40 °C did not allow any reaction to take place. However, when a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 toluene-water mixture was used, the expected product was formed quantitatively with S,S,RP-2 and S,S-3. The best enantioselectivity was observed in the case of S,S,RP-2, probably as the result of a steric hindrance intermediate between R,R-1 and S,S-3 (Scheme 9).
1 toluene-water mixture was used, the expected product was formed quantitatively with S,S,RP-2 and S,S-3. The best enantioselectivity was observed in the case of S,S,RP-2, probably as the result of a steric hindrance intermediate between R,R-1 and S,S-3 (Scheme 9).
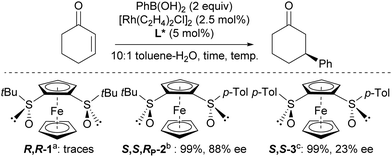 | ||
| Scheme 9 Rhodium-catalysed 1,4-addition of phenylboronic acid to 2-cyclohexenone. (a) 40 °C, 24 h then 60 °C, 24 h; (b) r.t., 2 h; (c) r.t. 0.5 h. | ||
In conclusion, the first ferrocene-1,2-disulfoxides were synthesized by two complementary routes and, taking advantage of the chiral sulfur atom, they were regioselectively functionalized towards more complex structures. We have found that it is possible to deprotonate the unfavourable position of a S-tert-butylferrocenesulfoxide and to use a phenylthio as a traceless directing group to reach ferrocene derivatives hardly accessible by following the routes already described. Finally, we reported the first promising results concerning the use of ferrocene-1,2-sulfoxide ligands in asymmetric catalysis.
Investigation, M. W., W. E., M. B., T. R.; writing – review and editing, F. M., W. E., M. W. and T. R. This work was supported by the ANR (Ferrodance project), the Université de Rennes 1, Rennes Métropole, the Fonds Européen de Développement Régional (FEDER; D8 VENTURE Bruker AXS diffractometer) and Thermofisher (generous gift of TMPH).
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. M. Crafts, Justus Liebigs Ann. Chem., 1862, 124, 110 Search PubMed.
- E. V. Bell and G. M. Bennett, J. Chem. Soc., 1927, 1798 RSC.
- E. V. Bell and G. M. Bennett, J. Chem. Soc., 1928, 3189 RSC.
- For a review, see: B. Delouvrié, L. Fensterbank, F. Nájera and M. Malacria, Eur. J. Org. Chem., 2002, 3507 CrossRef.
- B. M. Trost and M. Rao, Angew. Chem., Int. Ed., 2015, 54, 5026 CrossRef CAS PubMed.
- G. Sipos, E. E. Drinkel and R. Dorta, Chem. Soc. Rev., 2015, 44, 3834 RSC.
- S. Otocka, M. Kwiatkowska, L. Madalińska and P. Kiełbasiński, Chem. Rev., 2017, 117, 4147 CrossRef CAS PubMed.
- T. Jia, M. Wang and J. Liao, Top. Curr. Chem., 2019, 377, 1 CrossRef CAS PubMed.
- R. Tokunoh, M. Sodeoka, K.-I. Aoe and M. Shibasaki, Tetrahedron Lett., 1995, 36, 8035 CrossRef CAS.
- R. Mariz, X. Luan, M. Gatti, A. Linden and R. Dorta, J. Am. Chem. Soc., 2008, 130, 2172 CrossRef CAS PubMed.
- J. Chen, J.-M. Chen, F. Lang, X.-Y. Zhang, L.-F. Cun, J. Zhu, J.-G. Deng and J. Liao, J. Am. Chem. Soc., 2010, 132, 4552 CrossRef CAS PubMed.
- N. Khiar, A. Salvador, V. Valdivia, A. Chelouan, A. Alcudia, E. Álvarez and I. Fernández, J. Org. Chem., 2013, 78, 6510 CrossRef CAS PubMed.
- F. Rebière, O. Riant, L. Ricard and H. B. Kagan, Angew. Chem., 1993, 105, 644 CrossRef; F. Rebiere, O. Riant, L. Ricard and H. B. Kagan, Angew. Chem., Int. Ed. Engl., 1993, 32, 568 CrossRef.
- B. Ferber and H. B. Kagan, Adv. Synth. Catal., 2007, 349, 493 CrossRef CAS , and references therein.
- D. H. Hua, N. M. Lagneau, Y. Chen, P. M. Robben, G. Clapham and P. D. Robinson, J. Org. Chem., 1996, 61, 4508 CrossRef CAS PubMed.
- O. Riant, G. Argouarch, D. Guillaneux, O. Samuel and H. B. Kagan, J. Org. Chem., 1998, 63, 3511 CrossRef CAS.
- B. Ferber, S. Top, R. Welter and G. Jaouen, Chem. – Eur. J., 2006, 12, 2081 CrossRef CAS PubMed.
- N. D'Antona, D. Lambusta, R. Morrone, G. Nicolosi and F. Secundo, Tetrahedron: Asymmetry, 2004, 15, 3835 CrossRef.
- J. Priego, O. García Mancheño, S. Cabrera, R. Gómez Arrayás, T. Llamas and J. C. Carretero, Chem. Commun., 2002, 2512 RSC.
- M. Steurer, K. Tiedl, Y. Wang and W. Weissensteiner, Chem. Commun., 2005, 4929 RSC.
- N. M. Lagneau, Y. Chen, P. M. Robben, H.-S. Sin, K. Takasu, J.-S. Chen, P. D. Robinson and D. H. Hua, Tetrahedron, 1998, 54, 7301 CrossRef CAS.
- R. Gómez-Arrayás, J. Adrio and J. C. Carretero, Angew. Chem., Int. Ed., 2006, 45, 7674 CrossRef PubMed.
- N. A. Butt, D. Liu and W. Zhang, Synlett, 2014, 615 CAS.
- (R,R)-S,S′-di-tert-butylferrocene-1,1′-disulfoxide is known: M. Raghunath, W. Gao and X. Zhang, Tetrahedron: Asymmetry, 2005, 16, 3676 CrossRef CAS.
- R. Gómez Arrayás, I. Alonso, O. Familiar and J. C. Carretero, Organometallics, 2004, 23, 1991 CrossRef.
- D. A. Cogan, G. Liu, K. Kim, B. J. Backes and J. A. Ellman, J. Am. Chem. Soc., 1998, 120, 8011 CrossRef CAS , and references therein.
- K. K. Andersen, W. Gaffield, N. E. Papanikolaou, J. W. Foley and R. I. Perkins, J. Am. Chem. Soc., 1964, 86, 5637 CrossRef , and references therein.
- For the similar reaction in the benzene series, see: F. Han, G. Chen, X. Zhang and J. Liao, Eur. J. Org. Chem., 2011, 2928 CrossRef CAS.
- R. R. Fraser, T. S. Mansour and S. Savard, J. Org. Chem., 1985, 50, 3232 CrossRef CAS.
- The absolute configuration of planar chirality was named according to Schlögl’s nomenclature: K. Schlögl, Top. Stereochem., 1967, 1, 39 Search PubMed.
- R. Herrmann, G. Hübener and I. Ugi, Tetrahedron, 1985, 41, 941 CrossRef CAS.
- C. Pichon, B. Odell and J. M. Brown, Chem. Commun., 2004, 598 RSC.
- T. Sasamori, M. Sakagami, M. Niwa, H. Sakai, Y. Furukawa and N. Tokitoh, Chem. Commun., 2012, 48, 8562 RSC.
- R. J. Kloetzing and P. Knochel, Tetrahedron: Asymmetry, 2006, 17, 116 CrossRef CAS.
- J. Clayden, C. C. Stimson and M. Keenan, Chem. Commun., 2006, 1393 RSC.
- J. P. Flemming, M. B. Berry and J. M. Brown, Org. Biomol. Chem., 2008, 6, 1215 RSC.
- Y. Takaya, M. Ogasawara, T. Hayashi, M. Sakai and N. Miyaura, J. Am. Chem. Soc., 1998, 120, 5579 CrossRef CAS.
- T. Hayashi and K. Yamasaki, Chem. Rev., 2003, 103, 2829 CrossRef CAS PubMed.
- H. J. Edwards, J. D. Hargrave, S. D. Penrose and C. G. Frost, Chem. Soc. Rev., 2010, 39, 2093 RSC.
- P. Tian, H.-Q. Dong and G.-Q. Lin, ACS Catal., 2012, 2, 95 CrossRef CAS.
- M. M. Heravi, M. Dehghani and V. Zadsirjan, Tetrahedron: Asymmetry, 2016, 27, 513 CrossRef CAS.
- A. R. Burns, H. W. Lam and I. D. Roy, Org. React., 2017, 93, 1 CAS.
Footnotes |
| † Dedicated to Prof. Henri Kagan in recognition of his work on ferrocenes. |
| ‡ Electronic supplementary information (ESI) available. CCDC 2127411–2127413. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc07085a |
| This journal is © The Royal Society of Chemistry 2022 |
