Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Wavelength dependent photoextrusion and tandem photo-extrusion reactions of ninhydrin bis-acetals for the synthesis of 8-ring lactones, benzocyclobutenes and orthoanhydrides†
Wei
Sun
a,
Surajit
Kayal
 b,
William A. T.
Raimbach
a,
Xue-Zhong
Sun
b,
Mark E.
Light
a,
Magnus W. D.
Hanson-Heine
b,
William A. T.
Raimbach
a,
Xue-Zhong
Sun
b,
Mark E.
Light
a,
Magnus W. D.
Hanson-Heine
 b,
Michael W.
George
b,
Michael W.
George
 bc and
David C.
Harrowven
bc and
David C.
Harrowven
 *a
*a
aChemistry, University of Southampton, Highfield, Southampton, SO17 1BJ, UK. E-mail: dch2@soton.ac.uk
bSchool of Chemistry, University of Nottingham, Nottingham NG7 2RD, UK
cDepartment of Chemical and Environmental Engineering, University of Nottingham Ningbo China, 199 Taikang East Road, Ningbo 315100, China
First published on 23rd December 2021
Abstract
Ninhydrin bis-acetals give access to 8-ring lactones, benzocyclo-butenes and spirocyclic orthoanhydrides through photoextrusion and tandem photoextrusion reactions. Syntheses of fimbricalyxlactone B, isoshihunine and numerous biologically-relevant heterocycles show the value of the methods, while TA-spectroscopy and TD-DFT studies provide mechanistic insights on their wavelength dependence.
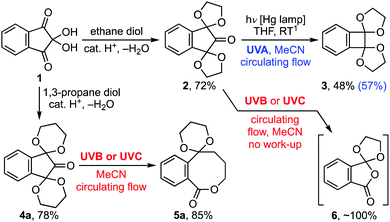
The photodecarbonylation of ninhydrin bis-acetal 2 to benzocyclobutene 3 was developed by Butenschön et al. to provide low cost access to benzocyclobutenedione (Scheme 1).1 Indeed, it remains the most convenient method for its synthesis in spite of the modest yield attained for the photoextrusion of carbon monoxide from 2. Consequently, when a need for benzocyclobutenedione arose, we decided to develop the reaction as a flow photochemical process in order to facilitate its optimisation with respect to wavelength, residence time, solvent and acetal protecting group.2,3 In the event, we uncovered conditions leading to a modest yield improvement for benzocyclobutene 3 and discovered a wavelength dependence for the reaction giving high-yielding access to 8-ring lactone 5a and orthoanhydride 6. Herein we describe our development of those methods, together with demonstrations of their usefulness in heterocyclic and natural products synthesis, and a detailed mechanistic study by transient absorption (TA) spectroscopy and TD-DFT analysis.
 | ||
| Scheme 1 The discovery of wavelength and acetal dependence on the outcome of photoextrusion reactions involving ninhydrin bis-acetals. | ||
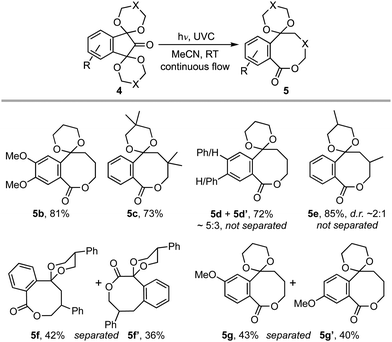
Our investigation began with an examination of the wavelength dependence on the photoextrusion of carbon monoxide from bis-acetal 2. While all wavelengths studied led to the evolution of gas, which was vented from the system using a circulating flow technique (see ESI†), only UVA irradiation gave benzocyclobutene 3 as a significant product.3,4 An optimum yield of 57% was realised when a 0.05 M solution of 2 in acetonitrile was irradiated with 30 × 1.5 W UVA LEDs for residence time of 3 h. By contrast, irradiation of bis-acetal 2 with UVB or UVC light gave rise to a complex mixture of products on work-up that varied from experiment to experiment and contained no benzocyclobutene 3, vide infra. Curiously, when the same reaction was performed on the homologous ninhydrin bis-acetal 4a, 8-ring lactone 5a was given in high yield (Scheme 1). Various analogues of 5 carrying substituents on the acetals and arene also gave the reaction in high yield (Table 1). In general, bis-1,3-dioxanes with a symmetrical arene and/or acetal substitution pattern gave a single product in 80–90% yield, e.g.5a–c. Substrates lacking those symmetry elements also gave high yields but led to isomeric mixtures, e.g. lactones 5d–g. Thought this limited the yield attained for individual products to ∼40%, in the context of medium ring synthesis such an outcome remains competitive.5
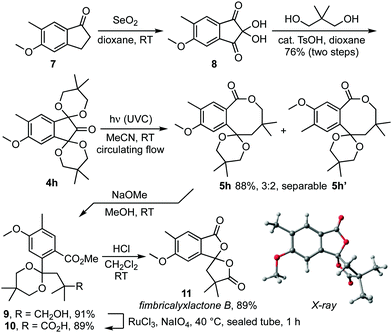
To demonstrate the utility of the method, we next applied it in a total synthesis of fimbricalyxlactone B 11, a natural product from the roots of Strophioblachia fimbricalyx Boerl. (Euphorbiaceae) (Scheme 2).6 Thus, bis-acetal 4h was formed from the corresponding 1-indanone 7 in 76% yield through sequential SeO2 oxidation and acetalisation with 2,2-dimethylpropandiol.7 Exposure of an acetonitrile solution of 4h to UVC light under circulating flow next gave a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of lactones 5h and 5h′ that was readily separated by column chromatography. Methanolysis of the major isomer 5h then gave ester 9 which was advanced to fimbricalyxlactone B 11 by ruthenium tetroxide oxidation to carboxylic acid 10 and acetal hydrolysis with HCl.8 Importantly, the spectral and physical data recorded on our sample matched those reported for the natural product. Its identity was also confirmed by X-ray crystallographic analysis (Scheme 2, CCDC 1908866).†6,9
2 mixture of lactones 5h and 5h′ that was readily separated by column chromatography. Methanolysis of the major isomer 5h then gave ester 9 which was advanced to fimbricalyxlactone B 11 by ruthenium tetroxide oxidation to carboxylic acid 10 and acetal hydrolysis with HCl.8 Importantly, the spectral and physical data recorded on our sample matched those reported for the natural product. Its identity was also confirmed by X-ray crystallographic analysis (Scheme 2, CCDC 1908866).†6,9
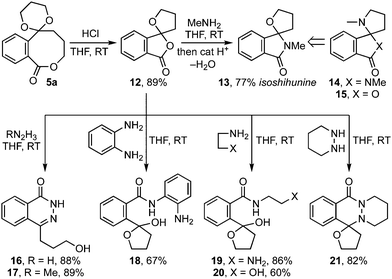
The potential of these 8-ring lactones to serve as intermediates in heterocyclic synthesis was next demonstrated with the conversion of 5a to spirolactone 12 on acetal hydrolysis (Scheme 3).9 In turn, its treatment with methylamine gave isoshihunine 13 in 77% yield, from which the alkaloids shihunidin 14 and shihunine 15 can each be derived.10,11 Furthermore, reaction with hydrazines gave high yielding access to phthalazones (e.g.16, 17, 21), which are important pharmacophores present in the chemotherapeutic agent olaparib (Lynparza®),12 and azelastine (Astelin, Optivar, Allergodil), a frontline drug treatment for mild rhinitis.13 By contrast, o-phenylenediamine, ethanolamine and ethylenediamine gave lactols 18 – 20 respectively.
The mechanistic course of the photoextrusion reaction was next studied by transient absorption (TA) spectroscopy following excitation of bis-acetal 4a in CH3CN at 266 nm (Scheme 4 and Fig. S1, ESI†). Analysis of the ps-TA spectra (Fig. S1a, ESI†) allowed us to extract the time constants associated with spectral evolution using global kinetic analysis.14 The data could be fitted using two exponential decays with time constants τ1 = 1.8 ± 0.4 ps and τ2 = 1.8 ± 0.2 ns (Fig. S1b and c, ESI†). The observed transient spectrum at 1 ps time delay showed an absorption band at 585 nm which was assigned to the nπ* (S1) excited state 1[4a] following comparison of the extracted spectrum with that computed for the S1 state (Fig. S7, ESI†). Decay of the S1 state was associated with the formation of a new transient species with an absorption band at 454 nm. This was attributed to biradical 1[22] based on comparison with results reported for the photoexcitation of 1,1,3-triphenyl-3-hydroxy-2-indanone.15 Additionally, the result aligns with a recent TA study of the C–C bond cleavage of cyclopentanone in cyclohexane following 255 nm photoexcitation, which Kao et al. found occurred in ≤1 ps.16 Likewise the τ2 component [1.8 ± 0.2 ns] for the rate of formation of 3[22] from 1[22] is consistent with previously reported ISC timescales for acyl–alkyl diradicals.17
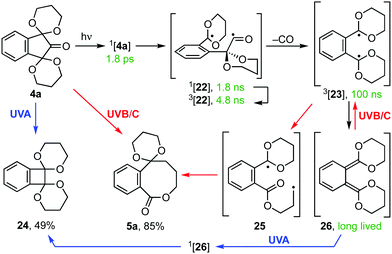 | ||
| Scheme 4 Proposed mechanistic course of the photoextrusion reactions of bis-acetal 4a [UVA (broad), 355–390 nm; UVB (narrow), 310 nm; UVC (narrow), 254 nm] | ||
Spectral evolution by ns-TA spectroscopy was also monitored (Fig. S1d, ESI†). Three exponentials are required to describe these data, with time constants τ1 = 4.8 ± 0.4 ns, τ2 = 100 ± 20 ns, and τ3 = long-lived (Fig. S1e and f, ESI†).17 The τ1 component was associated with a decay of the 460 nm band for 3[22] with simultaneous formation of a new transient at 550 nm, attributed to the decarbonylation product 3[23]. A similar transient spectral feature was reported for the decarbonylation of diphenylmethylacyl radical (Ph2MeCCO˙) in isooctane, which had a rate constant k = 1.5 × 108 s−1.15,18 Decay of the 550 nm transient species 3[23], with τ2 = 100 ± 20 ns, was associated with the formation of a long-lived transient at 440 nm assigned to quinodimethane 26. This is in line with the rate constant measured by Fujiwara et al. for the formation of o-xylylene from 2-indanone following photoexcitation at 266 nm in cyclohexane (k = 1.4 ± 0.1 × 107 s−1).19 Thus, the wavelength dependence of the reaction centres on the fate of long-lived intermediate 26 (Scheme 4). Irradiation of 26 with UVA leads to a singlet excited state 1[26] that can relax to benzocyclobutene 24. By contrast, its irradiation with UVB or UVC light gives rise to higher singlet excited states of 26 from which ISC to 3[23] is allowed. Collapse of 3[23] to biradical 25 then provides access to 8-ring lactone 5a.
Applying this mechanistic understanding to ninhydrin bis-acetal 2 (Scheme 5) led us to conclude that the course of that reaction was also dictated by the nature of the excitation of quinodimethane intermediate 28. As before, its irradiation with UVA gives a singlet excited state 1[28] from which benzocyclobutene 3 is derived. Similarly, irradiation with UVB or UVC light leads to biradical 3[29], which in turn collapses to biradical 30. However, in this case, ring closure to ε-lactone 27 is outpaced by the extrusion of ethene to biradical 31, a precursor of orthoanhydride 6. Indeed, the near quantitative formation of orthoanhydride 6 on photolysis of bis-acetal 2 with UVB or UVC light was confirmed by analysis of a concentrated product mixture before aqueous work-up (see ESI† for characterisation data).
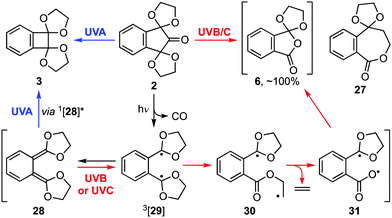 | ||
| Scheme 5 Proposed mechanism for the photoextrusion reactions of bis-acetal 2 [UVA (broad), 355–390 nm; UVB (narrow), 310 nm; UVC (narrow), 254 nm]. | ||
A TD-DFT analysis of quinodimethane 28 provided additional support for the mechanism (Fig. 1).20 In particular, it indicated that the energy provided by UVA irradiation (3.18 eV ≡ λmax 390 nm) mirrored that required for the π → π* transition S0 → S1, and that ISC between S1 of 1[28] and T1 of 3[29] was forbidden as both had ππ* character.21 Consequently, irradiation of 28 with UVA induces cyclisation to benzocyclobutene 3. By contrast, the greater energy provided by UVB and UVC irradiation facilitates ISC from 1[28] to 3[29] via higher excited states where both the orbital symmetry and energy requirements can each be fulfilled.21 As a result, irradiation of 28 with UVB or UVC gives orthoanhydride 6.
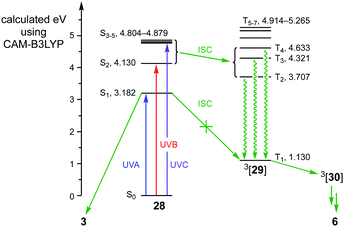 | ||
| Fig. 1 TD-DFT calculated energies for the singlet and triplet excited states of 28 and 29 respectively, and the mechanistic implications. | ||
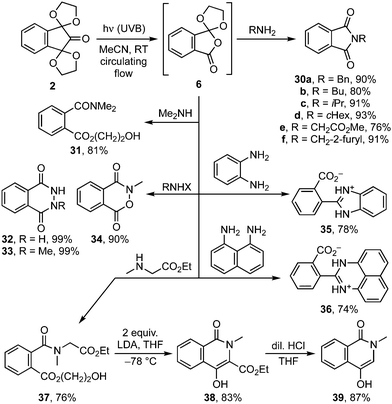
Finally, the use of orthoanhydride 6 as a phthalic anhydride surrogate (Scheme 6) was demonstrated with the phthalimide protection of various 1°-amines under mild conditions leading to 30a–f.22 Similarly, reactions with 2°-amines gave the expected amides, e.g.31 and 37, while bis-nucleophiles provided high yielding access to an array of biologically relevant nitrogen heterocycles 32–36, 38 and 39.
In conclusion, we have shown that the photodecarbonylation of ninhydrin bis-acetals is a wavelength dependent reaction that can be conducted on a gram scale to give benzocyclobutenes on UVA irradiation (60 W, ∼5 h), and orthoanhydrides or 8-ring lactones on UVB or UVC irradiation (36 W, ∼2 h). Notably, they formally constitute ring contraction, ring correlative, and ring expansion reactions, respectively. These versatile intermediates give access to a host of heterocycles, natural products, and pharmacophores. Mechanistic details have been delineated using computational and spectroscopic methods, including picosecond and nanosecond transient absorption spectroscopy. We are currently exploring further applications of the chemistry in target-oriented and natural products total synthesis.
Wei Sun and William Raimbach performed the synthetic chemistry under the supervision of David Harrowven. Wei Sun and Magnus Hanson-Heine performed the DFT analyses while Mark Light conducted the X-ray analysis. Transient absorption spectroscopic analyses were by Surajit Kayal and Xue-Zhong Sun under the supervision of Michael George.
We gratefully acknowledge financial support from EPSRC [EP/P013341/1, EP/L003325/1 and EP/K039466/1], the European Regional Development Fund [ERDF Interreg Va programme (Project 121)], and the University of Nottingham Green Chemicals Beacon.
Conflicts of interest
There are no conflicts of interest to declare.Notes and references
- D. Leinweber, M. Schnebel, R. Wartchow, H. G. Wey and H. Butenschoen, Eur. J. Org. Chem., 2002, 2385 CrossRef CAS; D. Leinweber and H. Butenschoen, Tetrahedron Lett., 1997, 38, 6385 CrossRef; D. Ng, Z. Yang and M. A. Garcia-Garibay, Tetrahedron Lett., 2002, 43, 7063 CrossRef.
- J. P. Knowles, L. D. Elliott and K. I. Booker-Milburn, Beilstein J. Org. Chem., 2012, 8, 2025 CrossRef CAS PubMed; K. Gilmore and P. H. Seeberger, Chem. Rec., 2014, 14, 410 CrossRef PubMed; C. Sambiagio and T. Noël, Trends Chem., 2020, 2, 92 CrossRef; M. B. Plutschack, B. Pieber, K. Gilmore and P. H. Seeberger, Chem. Rev., 2017, 117, 11796 CrossRef PubMed; D. Cambié, C. Bottecchia, N. J. W. Straathof, V. Hessel and T. Noël, Chem. Rev., 2016, 116, 10276 CrossRef PubMed; F. Politano and G. Oksdath-Mansilla, Org. Process Res. Dev., 2018, 22, 1045 CrossRef; T. H. Rehm, ChemPhotoChem, 2019, 3, 1 CrossRef; N. Hoffmann, Chem. Rev., 2008, 108, 1052 CrossRef PubMed; M. Oelgemoeller, Chem. Eng. Technol., 2012, 35, 1144 CrossRef.
- D. C. Harrowven, M. Mohamed, T. P. Gonçalves, R. J. Whitby, D. Bolien and H. F. Sneddon, Angew. Chem., Int. Ed., 2012, 51, 4405 CrossRef CAS PubMed; T. P. Gonçalves, M. Mohamed, R. J. Whitby, H. F. Sneddon and D. C. Harrowven, Angew. Chem., Int. Ed., 2015, 54, 4531 CrossRef PubMed; D. E. Collin, E. H. Jackman, N. Jouandon, W. Sun, D. C. Harrowven and B. Linclau, Synthesis, 2021, 1307 Search PubMed; M. A. Manning, W. Sun, M. E. Light and D. C. Harrowven, Chem. Commun., 2021, 57, 4556 RSC; W. Sun, W. A. T. Raimbach, L. D. Elliott, K. I. Booker-Milburn and D. C. Harrowven, Chem. Commun., 2022, 58, 383 RSC.
- B. D. Hook, W. Dohle, P. R. Hirst, M. Pickworth, M. B. Berry and K. I. Booker-Milburn, J. Org. Chem., 2005, 70, 7558 CrossRef CAS PubMed; L. D. Elliott, J. P. Knowles, P. J. Koovits, K. G. Maskill, M. J. Robertson-Ralph, G. Lejeune, L. J. Edwards, R. I. Robinson, I. R. Clemens, B. Cox, D. D. Pascoe, G. Koch, M. Eberle, M. B. Berry and K. I. Booker-Milburn, Chem. – Eur. J., 2014, 20, 15226 CrossRef PubMed.
- For our previous work on medium-ring synthesis see: W. Sun, D. C. Wilson, M. E. Light and D. C. Harrowven, Org. Lett., 2018, 20, 4346 CrossRef CAS PubMed; D. C. Harrowven, N. L’Helias, J. D. Moseley, N. J. Blumire and S. R. Flanagan, Chem. Commun., 2003, 2658 RSC.
- P. Seephonkai, S. G. Pyne, A. C. Willis and W. Lie, J. Nat. Prod., 2013, 76, 1358 CrossRef CAS PubMed.
- F. Johnson and N. Oberlender, J. Med. Chem., 1991, 34, 1662 CrossRef CAS PubMed; L. F. Fieser and W. C. Lothrop, J. Am. Chem. Soc., 1936, 58, 2050 CrossRef.
- H. Niwa, M. Nisiwaki, I. Tsukada, T. Ishigaki, S. Ito, K. Wakamatsu, T. Mori, M. Ishigaki and K. Yamada, J. Am. Chem. Soc., 1990, 112, 9001 CrossRef CAS; B. S. Lucas, L. M. Luther and S. D. Burke, Org. Lett., 2004, 6, 2965 CrossRef PubMed.
- Some discrepancies were noted in the 13C NMR data. These data were reported tentatively in the isolation paper as they had been attained from a spectrum recorded on a 9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of fimbricalyxlactones A and B.
1 mixture of fimbricalyxlactones A and B. - P. M. Hare, C. T. Middleton, K. I. Mertel, J. M. Herbert and B. Kohler, Chem. Phys., 2008, 347, 383 CrossRef CAS PubMed; H. Sun and H. Frei, J. Phys. Chem. B, 1997, 101, 205 CrossRef.
- V. G. Ore, M. D. Hordia and N. S. Arasimhan, Tetrahedron, 1990, 46, 2483 CrossRef CAS; G. A. Flynn, Chem. Commun., 1980, 862 RSC; E. Breuer and S. Zbaida, Tetrahedron, 1975, 31, 499 CrossRef.
- X.-W. Li, M. Huang, K. Lo, W. L. Chen, Y.-Y. He, Y. Xu, H. Zheng, H. Hu and J. Wang, Molecules, 2019, 24, 2673 CrossRef CAS PubMed; H. Chen, X. Li, Y. Xu, K. Lo, H. Zheng, H. Hu, J. Wang and Y. Lin, Molecules, 2018, 23, 1185 CrossRef PubMed; J. Bastida, M. Selles, C. Codina, F. Viladomat and J. L. Leon de la Luz, Planta Med., 1996, 62, 575 CrossRef PubMed; G. B. Bodem and E. Leete, J. Org. Chem., 1979, 44, 4696 CrossRef; E. Leete and G. B. Bodem, J. Am. Chem. Soc., 1976, 98, 6321 CrossRef; E. Breuer and S. Zbaida, Tetrahedron, 1975, 31, 499 CrossRef; Y. Inubushi, Y. Tsuda, T. Konita and S. Matsumoto, Chem. Pharm. Bull., 1964, 12, 749 CrossRef PubMed.
- P. S. Fong, D. S. Boss, T. A. Yap, A. Tutt, P. Wu, M. Mergui-Roelvink, P. Mortimer, H. Swaisland, A. Lau, M. J. O'Connor, A. Ashworth, J. Carmichael, S. B. Kaye, J. H. Schellens and J. S. de Bono, N. Engl. J. Med., 2009, 361, 123 CrossRef CAS PubMed.
- The results were analysed with Glotaran (http://glotaran.org).
- G. Kuzmanich, J. Xue, J. C. Netto-Ferreira, J. C. Scaiano, M. Platz and M. A. Garcia-Garibay, Chem. Sci., 2011, 2, 1497 RSC.
- M. H. Kao, R. K. Venkatraman, M. N. Ashfold and A. J. Orr-Ewing, Chem. Sci., 2020, 11, 1991 RSC.
- Y. P. Tsentalovich, O. B. Morozova, N. I. Avdievich, G. S. Ananchenko, A. V. Yurkovskaya, J. D. Ball and M. D. Forbes, J. Phys. Chem. A, 1997, 101, 8809 CrossRef CAS.
- C. Chatgilialoglu, D. Crich, M. Komatsu and I. Ryu, Chem. Rev., 1999, 99, 1991 CrossRef CAS PubMed.
- M. Fujiwara, K. Mishima, K. Tamai, Y. Tanimoto, K. Mizuno and Y. Ishii, J. Phys. Chem. A, 1997, 101, 4912 CrossRef CAS.
- A. Dreuw and M. Head-Gordon, Chem. Rev., 2005, 105, 4009 CrossRef CAS PubMed.
- M. A. El-Sayed, J. Chem. Phys., 1963, 38, 2834 CrossRef CAS.
- V. V. Sureshbabu, N. Narendra, in Amino Acids, Peptides and Proteins in Organic Chemistry: Protection Reactions, Medicinal Chemistry, Combinatorial Synthesis, ed.: A. B. Hughes, Wiley–VCH, Weinheim, 2011, vol. 4, pp. 1–97.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental accounts with spectral details and copies of NMR spectra are available as supplementary information. CCDC 1908866. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc06800h |
| This journal is © The Royal Society of Chemistry 2022 |