Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Acetylene storage performance of [Ni(4,4′-bipyridine)2(NCS)2]n, a switching square lattice coordination network†
Shi-Qiang
Wang
 a,
Shaza
Darwish
a,
Xiao-Qing
Meng
b,
Ze
Chang
b,
Xian-He
Bu
b and
Michael J.
Zaworotko
a,
Shaza
Darwish
a,
Xiao-Qing
Meng
b,
Ze
Chang
b,
Xian-He
Bu
b and
Michael J.
Zaworotko
 *a
*a
aBernal Institute, Department of Chemical Sciences, University of Limerick, Limerick V94 T9PX, Republic of Ireland. E-mail: Michael.Zaworotko@ul.ie
bSchool of Materials Science and Engineering, Nankai University, Tianjin 300350, China
First published on 30th December 2021
Abstract
We report that the previously reported square lattice coordination network [Ni(4,4′-bipyridine)2(NCS)2]n, sql-1-Ni-NCS, undergoes acetylene induced switching between closed (nonporous) and open (porous) phases. The resulting stepped sorption isotherms exhibit temperature controlled steps, consistent high uptake and benchmark working capacity (185 cm−3 g−1 or 189 cm−3, 1–3.2 bar, 288 K) for acetylene storage.
Acetylene (C2H2) is an important chemical feedstock and is also used as a fuel for oxyacetylene torches.1 However, its highly flammable and explosive nature renders its handling to be more challenging than most gases.2,3 Current C2H2 storage technology involves desensitization by dissolving C2H2 in acetone pre-dispersed in a porous monolith that completely fills a gas cylinder.2,3 The solubility of C2H2 in acetone reaches 470.4 cm−3 g−1 at 15 bar and 288 K.4 Nevertheless, charging C2H2 into acetone dramatically expands the volume of acetone and results in relatively low volumetric uptake (192.3 cm−3).4 In addition, discharging C2H2 releases acetone vapour,3 precluding its use in production of fine chemicals and electronic materials. Solid sorbents (e.g. porous coordination networks, PCNs5,6) offer potential to address these handicaps and broaden the utility of C2H2 in this “age of gas”.7
Switching coordination networks (CNs) can be classified as third generation CNs as they can undergo guest-induced structural transformation(s) between “closed” nonporous and “open” porous phases.8 They represent a small but growing subset of flexible metal–organic frameworks (FMOFs) or soft porous crystals (SPCs).9–11 Their potential utility is related to their sorption isotherms. Whereas rigid CNs typically exhibit Langmuir (type I) isotherms, switching CNs feature stepped or type F-IV sorption isotherms,8,12 making them distinctive from most metal–organic materials (MOMs13,14) by enhancing working capacity for gas storage (Fig. 1).8,12,15
With respect to C2H2 storage, a key performance parameter is the working capacity at practically relevant gas delivery (Pde) and storage (Pst) pressures. We recently proposed that a pressure range of 1–15 bar might be used to define working capacity as this range is compatible with existing C2H2–acetone technology.4 Another important but largely understudied parameter is sorption kinetics, which must be sufficiently fast for gas loading and unloading. To date, more than 100 CNs have been investigated with respect to C2H2 sorption and most are rigid CNs with high sorption uptake below 1 bar.16–27 Whereas high uptake can make a sorbent suitable for C2H2 sequestration, the type I isotherms typical of rigid sorbents are unlikely to offer a working capacity that is close or equal to their uptake as would be ideal for C2H2 storage/delivery.4 Conversely, switching CNs with a type F-IV isotherm can exhibit a working capacity that equals saturation uptake (Fig. 1). Furthermore, an appropriate hysteresis gap would enable C2H2 to be stored at lower pressure (i.e. between Pgd and Pga) than its charging pressure (i.e. Pga and above). A feature of switching CNs is that Pga and Pgd can be calculated by applying the Clausius–Clapeyron equation,4,8 thereby providing an opportunity to calculate C2H2 working capacity at higher pressures and avoiding experimental explosion risks. To our knowledge, only two switching CNs, the 3D pillar-layered CN MOF-508 and the 2D square lattice (sql) CN sql-1-Cu-BF4 (ELM-11) have been studied for their C2H2 storage properties.4,19,28 In this contribution, we report that the sql CN [Ni(bpy)2(NCS)2], sql-1-Ni-NCS (1 = bpy = 4,4′-bipyridine) exhibits C2H2 induced switching and evaluate its C2H2 storage performance by means of variable temperature C2H2 sorption studies and in situ synchrotron PXRD studies.
sql-1-Ni-NCS was hydrothermally synthesized in 1999 by Zhang et al.29,30 we have developed an alternate route by heating the 1D chain coordination polymer {[Ni(bpy)(NCS)2(H2O)2]·bpy} obtained by water slurry.31 While the crystal structure (Fig. S1, ESI†) and spectroscopic properties of sql-1-Ni-NCS are known for two decades,29,30 its sorption properties were unstudied until we reported its CO2 sorption properties at low (≤1 bar, 195 K) and high (≤38 bar, 273–298 K) temperatures/pressures.31 Interestingly, it is the “softest” switching CN with respect to Pgavs. its Fe and Co analogues.31,32 This prompted us to study its C2H2 sorption properties since C2H2 generally offers stronger sorbent–sorbate interactions than CO2.4,8
The 195 K C2H2 sorption isotherm of sql-1-Ni-NCS reveals that the Pga is 2.9 kPa (Fig. 2), below the Pga for CO2 (4.0 kPa).31 The C2H2 uptake plateaus at 185 cm−3 g−1, which suggests 4 C2H2 molecules per formula unit (sql-1-Ni-NCS·4C2H2). This value is 34% higher than the CO2 saturation uptake (138 cm−3 g−1) of sql-1-Ni-NCS·3CO2.31 A second step appeared at ca. 60 kPa but does not plateau before 120 kPa. At temperatures above 205 K, the second step was not observed while the initial plateau retained the same saturation uptake. The BET surface area and total pore volume of sql-1-Ni-NCS were calculated to be 697.3 m2 g−1 and 0.41 cm3 g−1, respectively. Pga/Pgd values were observed to be 2.9/1.3, 4.1/1.8, 6.8/3.0, 10.2/4.4, 14.9/6.3 and 21.3/9.0 kPa at 195, 199, 205, 210, 215 and 220 K, respectively (Fig. 2 and Fig. S2, ESI†). These temperature and Pga/Pgd values were fitted to the Clausius–Clapeyron equation (Fig. 3a and Fig. S3, ESI†), which was used to calculate formation (ΔfH) and dissociation (ΔdH) enthalpies (absolute values) of ca. 28.5 and 27.7 kJ mol−1, respectively. These ΔH values are comparable to those (28.4/28.2 kJ mol−1) calculated for CO2 induced phase transition.31
 | ||
| Fig. 3 (a) Linear fit of gate sorption pressure (LnPgate) and temperature (1000/T) using the Clausius–Clapeyron equation; (b) calculated Pga, Pgd and the hysteresis gap (Pga − Pgd) for sql-1-Ni-NCS. | ||
P ga/Pgd can be calculated at various temperatures once ΔH has been determined.4,8 We were therefore able to calculate switching pressure vs temperature from 195 to 298 K for sql-1-Ni-NCS (Fig. 3b and Table S1, ESI†). These plots reveal that the C2H2 switching pressure and the hysteresis gap between Pga and Pgd increase at elevated temperature in a manner similar to that of its CO2 switching pressures.31 For example, Pga/Pgd were calculated to be 4.4/1.7, 8.5/3.2, and 12.7/4.7 bar at 273, 288, and 298 K, respectively. The corresponding hysteresis gaps were found to be 2.7, 5.3 and 8.0 bar at 273, 288 and 298 K, respectively. These data suggest that C2H2 can be stored by sql-1-Ni-NCS at lower pressure (e.g., 3.2 bar, 288 K) than the charging pressure (e.g., ≥8.5 bar, 288 K).
The related sql CN sql-1-Cu-BF4 was reported to exhibit a type F-IVm isotherm with three complete C2H2 sorption steps at 195 K.4,8 In contrast, sql-1-Ni-NCS exhibited a type F-IVs isotherm with a single C2H2 sorption step. Although the C2H2 uptake of sql-1-Cu-BF4 (245 cm−3 g−1 at the 3rd plateau) is higher than that of sql-1-Ni-NCS (185 cm−3 g−1 at the 1st plateau), its working capacity is lower between 1–15 bar under ambient temperatures. This is because only the uptake between the second and the third step of sql-1-Cu-BF4 can be utilised in this pressure range. The working capacity (163 cm−3 g−1) of sql-1-Cu-BF4 is therefore 66.7% of the sorption uptake.4 In contrast, the uptake of sql-1-Ni-NCS can be fully exploited (185 cm−3 g−1) under the same conditions so its working capacity is 13.5% above that of sql-1-Cu-BF4. With respect to other parameters, the switching pressures (Pga/Pgd) and hysteresis gaps are comparable (Fig. S4 and S5, ESI†): Pgd values for sql-1-Ni-NCS are slightly (0.01–0.34 bar) lower than Pgd3 of sql-1-Cu-BF4 in the range 195–298 K; Pga values are 0.04–0.40 bar lower between 195–278 K and 0.08–1.31 bar higher between 283–298 K than those (Pga3) of sql-1-Cu-BF4. For example, at 288 K, the Pga/Pgd values are 8.1/3.5 and 8.5/3.2 bar for sql-1-Cu-BF4 and sql-1-Ni-NCS, respectively.
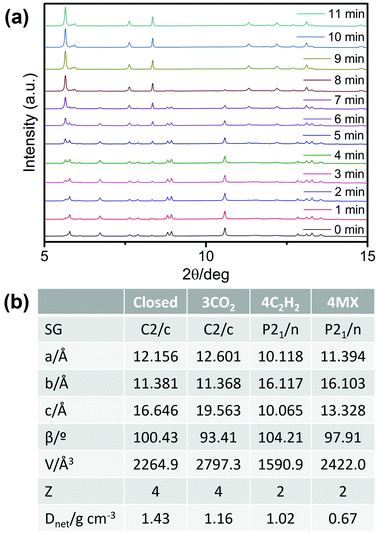
To gain insight into the nature of the phase transformation induced by C2H2, in situ synchrotron PXRD experiments were conducted. As shown in Fig. 4a, the phase transformation was complete within 8 min under 0.5 bar C2H2 at 195 K. Such sorption kinetics are adequate for practical utility and comparable with the CO2 sorption kinetics.31 From a structural perspective, synchrotron PXRD refinement revealed that the unit-cell parameters of sql-1-Ni-NCS·4C2H2 differ from those of sql-1-Ni-NCS·3CO2 (Fig. 4b, Fig. S6 and S7, ESI†). For instance, sql-1-Ni-NCS·3CO2 retained the same space group, C2/c, as the closed phase of sql-1-Ni-NCS, while sql-1-Ni-NCS·4C2H2 adopted space group P21/n as did the m-xylene loaded phase (sql-1-Co-NCS·4MX) previously reported by us.33 The Z value is 4 in the closed phase and 2 in the C2H2-loaded phase. The normalized unit-cell volume changes from 2264.9 Å3 in the closed phase to 3181.8 (i.e., 1590.9 × 2) Å3 in the C2H2-loaded phase, correspond to a 40.5% increase in unit cell volume. Attempts to solve the crystal structure of sql-1-Ni-NCS·4C2H2 were unsuccessful, but MX molecules reside in both interlayer spaces and square cavities in sql-1-Co-NCS·4MX (Fig. S8, ESI†).
Volumetric working capacity for gas storage is also a key performance parameter since container volume is necessarily limited. The network density (excluding C2H2) of sql-1-Ni-NCS·4C2H2 was calculated to be 1.02 g cm−1 (Fig. 4b), and therefore the volumetric working capacity of C2H2 is ca. 189 cm−3. This value is higher than sql-1-Cu-BF4 (174 cm−3) and MOF-508 (106 cm−3).4,28 When compared to the industrial adsorbent acetone, which has a volumetric working capacity (170 cm−3) between 1–15 bar at 288 K,4sql-1-Ni-NCS outperforms it by 11.2% at a safer working pressure range (1–3.2 bar, 288 K).
In summary, we herein report a switching transformation in a 2D sql CN, sql-1-Ni-NCS, induced through exposure to C2H2. The C2H2 switching pressure, Pga/Pgd, was controlled by temperature with retention of the saturation uptake. The type F-IV isotherm exhibited by sql-1-Ni-NCS enabled working capacity to be 100% of uptake capacity and the relatively high density resulted in benchmark volumetric working capacity at practically relevant conditions. When combined with other features such as fast sorption kinetics, hydrophobicity, and ease of scale-up,31sql-1-Ni-NCS is a promising candidate for enhancing the working capacity of gas storage and highlights the general potential that layered CNs offer for high working capacity. This is perhaps counterintuitive since such CNs are nonporous in their closed phases. Further studies to explore the storage potential of sql-1-Ni-NCS and related switching adsorbent layered materials (SALMAs) for other gases and vapours are in progress.
M. J. Z. gratefully acknowledges the support of the Irish Research Council (IRCLA/2019/167) and Science Foundation Ireland (16/IA/4624). Z. C. and X.-H. B. acknowledge the National Science Foundation of China (NSFC) (21531005) and the Programme of Introducing Talents of Discipline to Universities (B18030). We thank Dr Claire Murray and Dr Chiu C. Tang at the Diamond Light Source, UK, for providing access to the synchrotron X-ray diffraction beamline i11 (EE20500). S.-Q. W. would also like to thank his colleagues, Mr Daniel O’Hearn, Dr Andrey Bezrukov and Dr Soumya Mukherjee, for their assistance at Diamond Light Source.
Conflicts of interest
There are no conflicts to declare.Notes and references
- H. J. Arpe, Acetylene. Industrial Organic Chemistry, Wiley, 5th edn, 2010, pp 91–105 Search PubMed.
- R. E. Gannon, R. M. Manyik, C. Dietz, H. Sargent, R. Thribolet and R. Schaffer, Acetylene, Kirk-Othmer Encyclopedia of Chemical Technology, Wiley, 2003 Search PubMed.
- P. Pässler, W. Hefner, K. Buckl, H. Meinass, A. Meiswinkel, H.-J. Wernicke, G. Ebersberg, R. Müller, J. Bässler, H. Behringer and D. Mayer, Acetylene, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, 2011 Search PubMed.
- S.-Q. Wang, X.-Q. Meng, M. Vandichel, S. Darwish, Z. Chang, X.-H. Bu and M. J. Zaworotko, ACS Appl. Mater. Interfaces, 2021, 13, 23877–23883 CrossRef CAS PubMed.
- C. Janiak and J. K. Vieth, New J. Chem., 2010, 34, 2366–2388 RSC.
- D. J. O'Hearn, A. Bajpai and M. J. Zaworotko, Small, 2021, 2006351 CrossRef PubMed.
- S. Kitagawa, Angew. Chem., Int. Ed., 2015, 54, 10686–10687 CrossRef CAS PubMed.
- S.-Q. Wang, S. Mukherjee and M. J. Zaworotko, Faraday Discuss., 2021, 231, 9–50 RSC.
- A. Schneemann, V. Bon, I. Schwedler, I. Senkovska, S. Kaskel and R. A. Fischer, Chem. Soc. Rev., 2014, 43, 6062–6096 RSC.
- Z. Chang, D.-H. Yang, J. Xu, T.-L. Hu and X.-H. Bu, Adv. Mater., 2015, 27, 5432–5441 CrossRef CAS PubMed.
- S. Horike, S. Shimomura and S. Kitagawa, Nat. Chem., 2009, 1, 695–704 CrossRef CAS PubMed.
- Q. Y. Yang, P. Lama, S. Sen, M. Lusi, K. J. Chen, W. Y. Gao, M. Shivanna, T. Pham, N. Hosono, S. Kusaka, J. J. Perry IV, S. Ma, B. Space, L. J. Barbour, S. Kitagawa and M. J. Zaworotko, Angew. Chem., Int. Ed., 2018, 57, 5684–5689 CrossRef CAS PubMed.
- J. J. Perry IV, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400–1417 RSC.
- T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2012, 113, 734–777 CrossRef PubMed.
- J. A. Mason, J. Oktawiec, M. K. Taylor, M. R. Hudson, J. Rodriguez, J. E. Bachman, M. I. Gonzalez, A. Cervellino, A. Guagliardi, C. M. Brown, P. L. Llewellyn, N. Masciocchi and J. R. Long, Nature, 2015, 527, 357–361 CrossRef CAS PubMed.
- R. Matsuda, R. Kitaura, S. Kitagawa, Y. Kubota, R. V. Belosludov, T. C. Kobayashi, H. Sakamoto, T. Chiba, M. Takata, Y. Kawazoe and Y. Mita, Nature, 2005, 436, 238–241 CrossRef CAS PubMed.
- D. G. Samsonenko, H. Kim, Y. Sun, G.-H. Kim, H.-S. Lee and K. Kim, Chem. – Asian J., 2007, 2, 484–488 CrossRef CAS PubMed.
- D. Tanaka, M. Higuchi, S. Horike, R. Matsuda, Y. Kinoshita, N. Yanai and S. Kitagawa, Chem. – Asian J., 2008, 3, 1343–1349 CrossRef CAS PubMed.
- S. Xiang, W. Zhou, J. M. Gallegos, Y. Liu and B. Chen, J. Am. Chem. Soc., 2009, 131, 12415–12419 CrossRef CAS PubMed.
- J.-P. Zhang and X.-M. Chen, J. Am. Chem. Soc., 2009, 131, 5516–5521 CrossRef CAS PubMed.
- S. Xiang, W. Zhou, Z. Zhang, M. A. Green, Y. Liu and B. Chen, Angew. Chem., Int. Ed., 2010, 49, 4615–4618 CrossRef CAS PubMed.
- Z. Zhang, S. Xiang and B. Chen, CrystEngComm, 2011, 13, 5983–5992 RSC.
- J. Pang, F. Jiang, M. Wu, C. Liu, K. Su, W. Lu, D. Yuan and M. Hong, Nat. Commun., 2015, 6, 7575 CrossRef PubMed.
- S. Gao, C. G. Morris, Z. Lu, Y. Yan, H. G. W. Godfrey, C. Murray, C. C. Tang, K. M. Thomas, S. Yang and M. Schroder, Chem. Mater., 2016, 28, 2331–2340 CrossRef CAS.
- F. Moreau, I. da Silva, N. H. Al Smail, T. L. Easun, M. Savage, H. G. W. Godfrey, S. F. Parker, P. Manuel, S. Yang and M. Schröder, Nat. Commun., 2017, 8, 14085 CrossRef CAS PubMed.
- Y. He, F. Chen, B. Li, G. Qian, W. Zhou and B. Chen, Coord. Chem. Rev., 2018, 373, 167–198 CrossRef CAS.
- Y.-P. Li, Y. Wang, Y.-Y. Xue, H.-P. Li, Q.-G. Zhai, S.-N. Li, Y.-C. Jiang, M.-C. Hu and X. Bu, Angew. Chem., Int. Ed., 2019, 58, 13590–13595 CrossRef CAS PubMed.
- M. Bonneau, C. Lavenn, K. Sugimoto, A. Legrand, T. Ogawa, F.-X. Coudert, R. Réau, K.-i. Otake and S. Kitagawa, Res. Square, 2020 DOI:10.21203/rs.3.rs-102861/v1.
- Z. Yugen, J. Li, W. Deng, N. Masayoshi and I. Tsuneo, Chem. Lett., 1999, 195–196 Search PubMed.
- Y. Zhang, L. Jianmin, M. Nishiura and T. Imamoto, J. Mol. Struct., 2000, 519, 219–224 CrossRef CAS.
- S.-Q. Wang, S. Darwish, D. Sensharma and M. J. Zaworotko, Mater. Adv., 2022, 3 10.1039/D1MA00785H , accepted.
- S.-Q. Wang, Q.-Y. Yang, S. Mukherjee, D. O'Nolan, E. Patyk-Kazmierczak, K.-J. Chen, M. Shivanna, C. Murray, C. C. Tang and M. J. Zaworotko, Chem. Commun., 2018, 54, 7042–7045 RSC.
- S.-Q. Wang, S. Mukherjee, E. Patyk-Kaźmierczak, S. Darwish, A. Bajpai, Q.-Y. Yang and M. J. Zaworotko, Angew. Chem., Int. Ed., 2019, 58, 6630–6634 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, desorption isotherms, PXRD patterns, etc. See DOI: 10.1039/d1cc06638b |
| This journal is © The Royal Society of Chemistry 2022 |