Open Access Article
Open Access ArticleRhodium-catalyzed regioselective addition of thioacids to terminal allenes: enantioselective access to branched allylic thioesters†
A.
Ziyaei Halimehjani
*ab and
B.
Breit
 *b
*b
aFaculty of Chemistry, Kharazmi University, P. O. Box 15719-14911, 49 Mofateh Street, Tehran, Iran. E-mail: ziyaei@khu.ac.ir
bInstitut für Organische Chemie, Albert-Ludwigs-Universität Freiburg, Albert Strasse 21, 79104 Freiburg im Breisgau, Germany. E-mail: bernhard.breit@chemie.uni-freiburg.de
First published on 13th January 2022
Abstract
Rhodium-catalyzed regio- and enantioselective hydrothiolation of terminal allenes with thioacids is reported for the atom-economic synthesis of chiral branched allylic thioesters. By using a rhodium(I) catalyst system, diversities of terminal allenes and thioacids afforded the corresponding branched thioesters in excellent regioselectivity, high yield, and good enantioselectivity. This method was also explored for Fmoc-protected aminothioacids for diastereoselective synthesis of the corresponding thioesters.
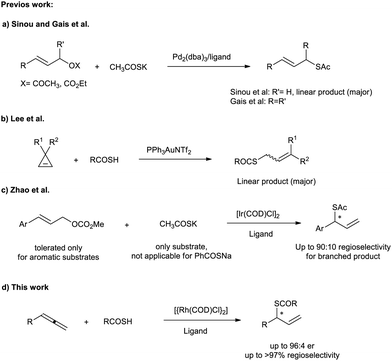
Rhodium-catalyzed regio- and stereoselective coupling of allenes with pronucleophiles is a powerful method for atom-economic synthesis of branched allylic products.1 In this context, recently, we reported an enantioselective synthesis of allylic thioethers and sulfones via intermolecular hydrothiolation reaction of terminal allenes with thiols.2 Due to the difficulties associated to the direct synthesis of chiral thiols from simple starting materials and also the cleavage of C–S bonds in thioethers to furnish the corresponding thiols, thioesters are introduced as efficient precursors for the synthesis of free thiols.3 Chiral thioesters and related thiols are widely present in nature4 and biological systems,5 and found extensive applications as valuable intermediates in organic synthesis.6 The catalytic synthesis of chiral thioesters, thiols and generally the thiolation chemistry has been an active area of research in synthetic organic chemistry and is a challenging task and remains largely unexplored. Although considerable progress in the synthesis of thioesters and thiols via C–S bond formation has been made in recent years,7 there are limited strategies for the synthesis of allylic thioesters, especially chiral branched allylic thioesters. Sinou et al. reported palladium(0)-catalyzed allylic substitution of various allylic acetates and carbonates with potassium thioacetate to furnish the corresponding linear allylic thioacetates as major product (Scheme 1a).8 An asymmetric version of this reaction for symmetrical allylic carbonates was developed by Gais et al. employing the Trost ligand, affording branched allylic esters in good yield and enantioselectivities.9 In 2012, Lee et al. has shown that the addition of thioacids to 3,3-disubstituted cyclopropenes catalyzed by PPh3AuNTf2 afforded the corresponding linear allylic thioesters as the major regioisomer (Scheme 1b).10 According to our knowledge, the only report for the synthesis of chiral branched allylic thioesters has been published by Zhao et al. via a direct iridium-catalyzed asymmetric allylation of KSAc with active allylic carbonates in the presence of KOAc (Scheme 1c).11 This method suffers from some drawbacks such as low regioselectivity and yield for unactivated allylic carbonates and a lack of diversity in thioacid salts. Based on our previous experience on the Rh-catalyzed regioselective and stereoselective allylation reaction using N,O,S,C-nucleophiles/pronucleophiles,12 herein, we report the first enantioselective intermolecular hydrothiolation reaction of allenes with thioacids using a rhodium catalyst system to provide chiral branched allylic thioesters (Scheme 1d).
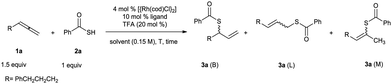
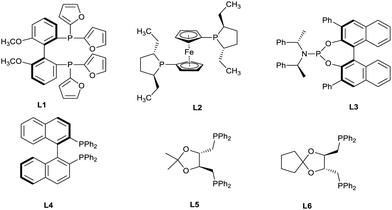
We began by investigating the reaction of hexa-4,5-dien-1-ylbenzene (1a) with thiobenzoic acid (2a). No reaction occurred under catalyst-free conditions (Table 1, entry 1). Further experiments were designed to find a suitable ligand for regio- and enantioselective synthesis of branched thioester 3a using 4 mol% of [Rh(COD)Cl]2, 10 mol% of a ligand, and 20 mol% of TFA as additive in 1,2-dichloroethane (DCE). After ligand screening, we were delighted to identify that (+)-Binap and (+)-DIOP were the most promising ligands, providing 3a in high yield, good regioselectivity and moderate enantioselectivity (Table 1, entries 2–6). Solvent screening at room temperature revealed that (+)-DIOP afforded excellent regioselectivity and high ee (85%) in acetonitrile (Table 1, entry 8). By using 8 mol% of ligand, the ee was improved to 87% with similar regioselectivity and yield (Table 1, entry 9). No improvement was observed using other DIOP ligands such as (R,R)-DTBM-DIOP (DTBM = (3,5-di-tert-butyl-4-methoxy) and (R,R)-Cp-DIOP (Cp = cyclopentyl) (Table 1, entry 10). Varying other parameters in the model reaction such as the amount of TFA, the type of additive, rhodium source, concentration of the reaction, and substrates equivalents didn’t improve the reaction significantly (Table 1, entries 11–16). It is notable that premixing of the ligand with [Rh(COD)Cl]2 in acetonitrile for 10 minutes is crucial to get the optimal yield and enantioselectivity (Table 1, entry 17).
| Entry | Ligand | Solvent | T (°C)/t (h) | Yielda [%] |
3a (B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M)b M)b |
eec [%] |
|---|---|---|---|---|---|---|
a Isolated yield of branched thioester.
b Determined by crude NMR.
c Determined by HPLC analysis on a chiral stationary phase.
d 8 mol% of ligand was used.
e 30 mol% of TFA was used.
f 10 mol% of TFA was used.
g [{Rh(cod)OAc}2] was used as rhodium source.
h Solvent (0.1 M) was used.
i Solvent 0.2 M was used.
j Allene/thioacid ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5.
k Starting materials and catalyst were added at the same time. 1.5.
k Starting materials and catalyst were added at the same time.
|
||||||
| 1 | — | DCE | rt/24 | Trace | — | — |
| 2 | L1 | DCE | rt/16 | 20 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
— |
| 3 | L2 | DCE | rt/16 | 5 | — | — |
| 4 | L3 | DCE | rt/16 | 10 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
— |
| 5 | L4 | DCE | 40/3 | 82 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
21 |
| 6 | L5 | DCE | 40/3 | 80 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
−69 |
| 7 | L4 | CH3CN | rt/16 | 80 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
10 |
| 8 | L5 | CH3CN | rt/16 | 85 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
−85 |
| 9 | L5 | CH 3 CN | rt/16 | 85 |
97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
−87 |
| 10d | L6 | CH3CN | rt/16 | 45 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
85 |
| 11de | L5 | CH3CN | rt/16 | 63 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
−83 |
| 12df | L5 | CH3CN | rt/16 | 55 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
−87 |
| 13dg | L5 | CH3CN | rt/16 | 10 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
— |
| 14dh | L5 | CH3CN | rt/16 | 62 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
−85 |
| 15di | L5 | CH3CN | rt/16 | 70 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
−85 |
| 16dj | L5 | CH3CN | rt/16 | 62 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
−82 |
| 17dk | L5 | CH3CN | rt/16 | 52 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
−86 |
|
||||||
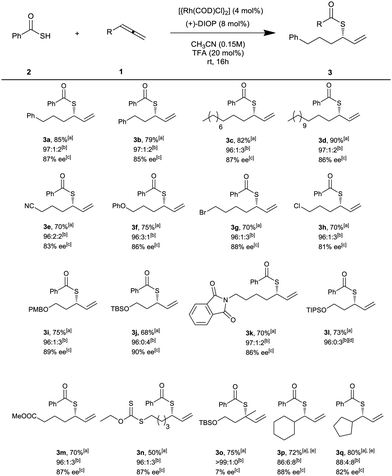
With the optimized reaction conditions in hand, we investigated the scope of allenes in the regio- and enantioselective synthesis of branched allylic thioesters (Scheme 2). A variety of aliphatic allenes were used successfully in this protocol to give the corresponding thioesters in high yields, excellent regioselectivity (>96%) and high ee. Beside simple linear alkyl-substituted allenes (3a–d), allenes with various functional groups such as nitrile (3e), ether (3f), protected alcohols (3j, 3l, 3o) (TBS-, TIPS-, and PMB-protected), phthalimide (3k), ester (3m), halide (3g–h), and xanthate (3n) were tolerated well in this protocol. For 1,1′-disubstituted allene, the corresponding thioester (3o) was obtained in high yield and excellent regioselectivity, but with poor enantioselectivity. Cyclohexylallene and cyclopentylallene afforded the corresponding thioesters (3p, 3q) in high yield and enantioselectivity, albeit with lower regioselectivity. It is notable that internal allenes are not suitable substrates for this reaction. In addition, unfortunately, aryl-substituted allenes were not compatible in this coupling reaction. The absolute configurations of products were determined as S by chemical correlation with known compounds (see ESI†).
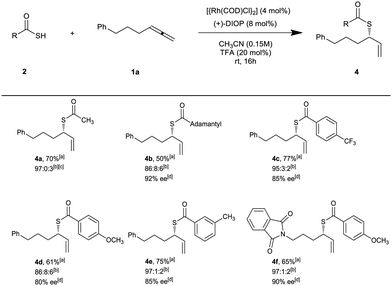
Next, the generality of this protocol was examined using aliphatic and aromatic thioacids as coupling partner (Scheme 3). Thioacids were prepared in one or two steps from the corresponding carboxylic acid or acyl halides (see ESI†).13 Both aromatic and aliphatic thioacids are suitable substrates for this reaction, affording the corresponding thioesters 4a–f in high yields, regioselectivity and enantioselectivity. Adamantane-1-carbothioic S-acid as a bulky thioacid afforded the corresponding thioester 4b in 92% ee (Scheme 3).
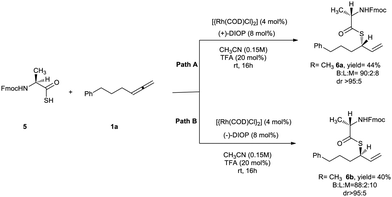
Due to the importance of Fmoc-protected thioester peptide precursors in chemical ligation,14 finally, the scope of this reaction was further explored by the use of Fmoc-protected-aminothioacid 5 as coupling partner in the reaction with allene 1a under optimized reaction conditions (Scheme 4). By using L-alanine-based thioacid the corresponding branched thioester 6a was obtained in excellent diastereoselectivity with moderate yield (Scheme 4, path A). It is notable that by using Fmoc-D-alanine thioacid and (−)-DIOP, the opposite enantiomer of 6a was obtained. It means that the catalyst has complete control on the diastereoselectivity of the reaction. In addition, similar diastereoselectivity was observed using Fmoc-L-alanine thioacid and (−)-DIOP (Scheme 4, path B).
In conclusion, we have developed the first method for the regio- and enantioselective intermolecular hydrothiolation of terminal allenes with thioacids for the preparation of chiral branched thioesters in good to excellent yields. The method is broadly applicable for a wide range of terminal allenes with various functional groups and aromatic and aliphatic thioacids. In addition, this method was explored for Fmoc-protected aminothioacids for diastereoselective synthesis of the corresponding thioesters under complete control of catalyst. This method provides an efficient access to chiral thioesters, which are suitable substrates for the synthesis of chiral thiols after deprotection, and are efficient intermediates in further organic synthesis.
We are grateful to the Alexander von Humboldt foundation for supporting this work. In addition, we are thankful to the research council of Kharazmi University for supporting this work.
Conflicts of interest
There are no conflicts to declare.Notes and references
-
(a) B. Breit, Acc. Chem. Res., 2016, 49, 1524–1536 CrossRef PubMed
; (b) D. Berthold, A. G. A. Geissler, S. Giofré and B. Breit, Angew. Chem., Int. Ed., 2019, 58, 9994–9997 CrossRef CAS PubMed
.
- A. B. Pritzius and B. Breit, Angew. Chem., Int. Ed., 2015, 54, 3121–3125 CrossRef CAS PubMed
.
-
(a) T. Xu, T. Cao, M. Yang, R. Xu, X. Nie and S. Liao, Org. Lett., 2020, 22, 3692–3696 CrossRef CAS PubMed
; (b) J. Luo, M. Rauch, L. Avram, Y. Ben-David and D. Milstein, J. Am. Chem. Soc., 2020, 142, 21628–21633 CrossRef CAS PubMed
; (c) Q. Song, P. Zhang, S. Liang, X. Chen, P. Li and W. Li, Org. Lett., 2020, 22, 7859–7863 CrossRef CAS PubMed
.
-
(a) K. Chandru, A. Gilbert, C. Butch, M. Aono and H. J. Cleaves, Sci. Rep., 2016, 6, 29883 CrossRef CAS PubMed
; (b) A. L. Lehninger, D. L. Nelson and M. M. Cox, Principles of Biochemistry, Worth Publishing, New York, 3rd edn, 2000 Search PubMed
; (c) F. Pietrocola, L. Galluzzi, J. M. Bravo-SanPedro, F. Madeo and G. Kroemer, Cell Metab., 2015, 21, 805–821 CrossRef CAS PubMed
; (d) Y. E. Du, W. S. Byun, S. B. Lee, S. Hwang, Y. H. Shin, B. Shin, Y. J. Jang, S. Hong, J. Shin, S. K. Lee and D. C. Oh, Org. Lett., 2020, 22, 5337–5341 CrossRef CAS PubMed
; (e) K. Taori, V. J. Paul and H. Luesch, J. Am. Chem. Soc., 2008, 130, 1806–1807 CrossRef CAS PubMed
.
-
(a) J. Staunton and K. J. Weissman, Nat. Prod. Rep., 2001, 18, 380–416 RSC
; (b) K. A. Scott and J. T. Njardarson, Top. Curr. Chem., 2019, 376, 1–34 Search PubMed
; (c) M. Feng, B. Tang, S. Liang and X. Jiang, Curr. Top. Med. Chem., 2016, 16, 1200–1216 CrossRef CAS PubMed
.
-
(a) A. P. Pulis, A. Varela, C. Citti, P. Songara, D. Leonori and V. K. Aggarwal, Angew. Chem., Int. Ed., 2017, 56, 10835–10839 CrossRef CAS PubMed
; (b) S. Ujiwara and N. Kambe, Top. Curr. Chem., 2012, 251, 87–140 CrossRef
; (c) J. M. Villalobos, J. Srogl and L. S. Liebeskind, J. Am. Chem. Soc., 2007, 129, 15734–15735 CrossRef CAS PubMed
; (d) J. Clayden and P. MacLellan, Beilstein J. Org. Chem., 2011, 7, 582–595 CrossRef CAS PubMed
; (e) D. Crich and I. Sharma, Angew. Chem., Int. Ed., 2009, 48, 2355–2358 CrossRef CAS PubMed
; (f) D. Crich and K. Sasaki, Org. Lett., 2009, 11, 3514–3517 CrossRef CAS PubMed
; (g) H. Yang, H. Li, R. Wittenberg, M. Egi, W. Huang and L. S. Liebeskind, J. Am. Chem. Soc., 2007, 129, 1132–1140 CrossRef CAS PubMed
; (h) D. A. Alonso, S. Kitagaki, N. Utsumi and C. F. Barbas III, Angew. Chem., Int. Ed., 2008, 47, 4588–4591 CrossRef CAS PubMed
; (i) H. Cao, X. Liu, F. Bie, Y. Shi, Y. Han, P. Yan, M. Szostak and C. Liu, J. Org. Chem., 2021, 86, 10829–10837 CrossRef CAS PubMed
; (j) H. Ochiai, Y. Uetake, T. Niwa and T. Hosoya, Angew. Chem., Int. Ed., 2017, 56, 2482–2486 CrossRef CAS PubMed
.
-
(a) J. Luo, M. Rauch, L. Avram, Y. Diskin-Posner, G. Shmul, Y. Ben-David and D. Milstein, Nat. Catal., 2020, 3, 887–892 CrossRef CAS
; (b) W. Yu, J. Han, D. Fang, M. Wang and J. Liao, Org. Lett., 2021, 23, 2482–2487 CrossRef PubMed
; (c) H. J. Ai, F. Zhao, H. Q. Geng and X. F. Wu, ACS Catal., 2021, 11, 3614–3619 CrossRef CAS
; (d) X. Qi, Z. P. Bao, X. T. Yao and X. F. Wu, Org. Lett., 2020, 22, 6671–6676 CrossRef CAS PubMed
; (e) H. J. Ai, W. Lu and X. F. Wu, Angew. Chem., Int. Ed., 2021, 60, 17178–17184 CrossRef CAS PubMed
; (f) M. Roggen and E. M. Carreira, Angew. Chem., Int. Ed., 2012, 51, 8652–8655 CrossRef PubMed
; (g) S. Zheng, N. Gao, W. Liu, D. Liu, X. Zhao and T. Cohen, Org. Lett., 2010, 12, 4454–4457 CrossRef CAS PubMed
; (h) S. Zheng, W. Huang, N. Gao, R. Cui, M. Zhang and X. Zhao, Chem. Commun., 2011, 47, 6969–6971 RSC
; (i) W. Huang, S. Zheng, J. Tang and X. Zhao, Org. Biomol. Chem., 2011, 9, 7897–7903 RSC
; (j) M. R. Monaco, S. Prevost and B. List, J. Am. Chem. Soc., 2014, 136, 16982–16985 CrossRef CAS PubMed
; (k) V. Hirschbeck, P. H. Gehrtz and I. Fleischer, Chem. – Eur. J., 2018, 24, 7092–7107 CrossRef CAS PubMed
.
- S. Divekar, M. Safi, M. Soufiaoui and D. Sinou, Tetrahedron, 1999, 55, 4369–4376 CrossRef CAS
.
- B. J. Lussem and H. J. Gais, J. Org. Chem., 2004, 69, 4041–4052 CrossRef PubMed
.
- R. J. Mudd, P. C. Young, J. A. Jordan-Hore, G. M. Rosair and A. L. Lee, J. Org. Chem., 2012, 77, 7633–7639 CrossRef CAS PubMed
.
- N. Gao and X. Zhao, Eur. J. Org. Chem., 2013, 2708–2714 CrossRef CAS
.
-
(a) J. P. Schmidt and B. Breit, Angew. Chem., Int. Ed., 2020, 59, 23485–23490 CrossRef CAS PubMed
; (b) A. M. Haydl, K. Xu and B. Breit, Angew. Chem., Int. Ed., 2015, 54, 7149–7153 CrossRef CAS PubMed
; (c) T. M. Beck and B. Breit, Angew. Chem., Int. Ed., 2017, 56, 1903–1907 CrossRef CAS PubMed
; (d) V. Khakyzadeh, Y. H. Wang and B. Breit, Chem. Commun., 2017, 53, 4966–4968 RSC
; (e) Y. H. Wang and B. Breit, Chem. Commun., 2019, 55, 7619–7622 RSC
.
- M. Toriyama, H. Kamijo, S. Motohashi, T. Takido and K. Itabashi, Phospho. Sulf. Silic., 2003, 178, 1661–1665 CrossRef CAS
.
-
(a) V. P. Terrier, H. Adihou, M. Arnould, A. F. Delmas and V. Aucagne, Chem. Sci., 2016, 7, 339–345 RSC
; (b) N. Naruse, K. Ohkawachi, T. Inokuma, A. Shigenaga and A. Otaka, Org. Lett., 2018, 20, 2449–2453 CrossRef CAS PubMed
; (c) J. B. Blanco-Canosa and P. E. Dawson, Angew. Chem., Int. Ed., 2008, 47, 6851–6855 CrossRef CAS PubMed
; (d) A. Kar, J. Mannuthodikayil, S. Singh, A. Biswas, P. Dubey, A. Das and K. Mandal, Angew. Chem., Int. Ed., 2020, 59, 14796–14801 CrossRef CAS PubMed
; (e) R. Okamoto, K. Morooka and Y. Kajihara, Angew. Chem., Int. Ed., 2012, 51, 191–196 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details, characterization data, and copies of 1H and 13C NMR spectra for all compounds. See DOI: 10.1039/d1cc06470c |
| This journal is © The Royal Society of Chemistry 2022 |