Supramolecular cancer photoimmunotherapy based on precise peptide self-assembly design
Yamei
Liu
a,
Lu
Zhang
b,
Rui
Chang
a and
Xuehai
Yan
 *acd
*acd
aState Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, 100190 Beijing, China
bState Key Laboratory of Polymer Physics & Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, 130022 Changchun, China
cSchool of Chemical Engineering, University of Chinese Academy of Sciences, 100049 Beijing, China
dCenter for Mesoscience, Institute of Process Engineering, Chinese Academy of Sciences, 100190 Beijing, China. E-mail: yanxh@ipe.ac.cn
First published on 11th January 2022
Abstract
Combinational photoimmunotherapy (PIT) is considered to be an ideal strategy for the treatment of highly recurrent and metastatic cancer, because it can ablate the primary tumor and provide in situ an autologous tumor vaccine to induce the host immune response, ultimately achieving the goal of controlling tumor growth and distal metastasis. Significant efforts have been devoted to enhancing the immune response caused by phototherapy-eliminated tumors. Recently, supramolecular PIT nanoagents based on precise peptide self-assembly design have been employed to improve the efficacy of photoimmunotherapy by utilizing the stability, targeting capability and flexibility of drugs, increasing tumor immunogenicity and realizing the synergistic amplification of immune effects through multiple pathways and collaborative strategy. This review summarizes peptide-based supramolecular PIT nanoagents for phototherapy-synergized cancer immunotherapy and its progress in enhancing the effect of photoimmunotherapy, especially focusing on the design of peptide-based PIT nanoagents, the progress of bioactive peptides combined photoimmunotherapy, and the synergistic immune-response mechanism.
1. Introduction
With the development of tumor immunology and promising clinical antitumor effects, immunotherapy has gradually become an important antitumor strategy. Unlike traditional surgery, chemotherapy, and radiotherapy that directly act on tumor tissues, tumor immunotherapy achieves the goal of killing and even eradicating tumors by stimulating and enhancing the host's immune response. Importantly, the strong immune memory generated by a systemic antitumor immune response stimulated by cancer immunotherapy can prevent tumor metastasis and provide long-term protection against tumor recurrence.1 Although immunotherapy can activate the systemic antitumor immune response to solve the problem of metastasis and recurrence, it is relatively incapable in eliminating primary solid tumors. This is mainly because exclusive immunotherapy does not directly ablate cancer cells, but only relies on activating the immune system, while weak immunogenicity and an immunosuppressive tumor microenvironment hinder this process.2 In actual clinical treatment, the removal of primary large-size tumors is preferred through conventional means such as chemotherapy, radiotherapy and surgery so as to inhibit its excessive proliferation, and then mediate a durable immune response by immunotherapy to recover the immune system and prevent tumor recurrence and metastasis, because the conventional tumor therapies cause significant impairment of the immune system.3Phototherapy including photodynamic therapy (PDT) and photothermal therapy (PTT), as an advanced local and precise treatment modality, can selectively kill cancer cells that take up photosensitizers capable of generating cytotoxic reactive oxygen species (ROS) and/or heat upon light irradiation, thereby removing primary solid tumors without damaging the surrounding normal tissues.4 Phototherapy with the assistance of appropriate light-absorbing agents and light input have been found to efficiently induce immunogenic cell death (ICD) of tumors with the release of tumor-associated antigens (TAAs) and damage-associated molecular patterns (DAMPs), which may activate an immune response against the antigens of dead tumor cells.5 Phototherapy usually aims at directly killing the tumor cells to eradicate the tumor tissue, and the immune response mediated by this process can achieve inhibition of tumor recurrence and metastasis by reducing the immune regulation inhibition or stimulating the host immune response. Therefore, a combination of phototherapy and immunotherapy, called photoimmunotherapy (PIT), is an ideal strategy to eliminate the primary tumor, meanwhile triggering the systemic immune response to clean up residual tumor cells and inhibit distant metastasis. The major advantage of photoimmunotherapy is that it can be applied to the tumor treatment repeatedly over a long period, unlike radiotherapy, which cannot be administered multiple times at the same site. Based on these therapeutic advantages, photoimmunotherapy has obtained encouraging results both in preclinical and clinical research of a number of cancers.6,7 Especially, the first EGFR targeted NIR-PIT drug (ASP-1929, Akalux™, Rakten Medical Inc.) and a diode laser system (BioBlade™, Rakten Medical Inc.) were conditionally approved and registered for clinical use by the Pharmaceuticals and Medical Devices Agency in Japan in September 2020,8,9 which demonstrates that photoimmunotherapy is very promising in clinical cancer treatment.
Intelligent nanosystems provide a simple and precise method for achieving a safer and more effective therapeutic outcome through combined photoimmunotherapy.10,11 Peptides are ideal building blocks for constructing a variety of nanostructures due to their inherent advantages such as biocompatibility, biodegradability, structural programmability and assembly flexibility.12,13 Moreover, peptides on their own afford bioactivity such as an immune-modulating capability as they consist of protein segments or recognition motifs. Some peptide sequences not only have a potential affinity for tumor cells resulting in enhanced tumor accumulation of drugs, but also contain abundant enzyme cleavage sites that can be used to further induce the formation of stimulus-response nanostructures.14,15 Noteworthily, some peptides are immune-related drugs or immunomodulators that can activate or promote the host to produce relevant immune responses.16,17 Therefore, the introduction of peptides could endow PIT agents with the potential to further activate anticancer immunity through a combination with conventional immune therapies. All in all, compared with molecular PIT agents, precisely designed supramolecular peptide-based PIT agents not only improve the tumor targeting ability of light-absorbing agents, but also enhance immunogenicity and regulate the subsequent immune response, ultimately enhancing the effect of antitumor immunotherapy. More importantly, supramolecular cancer photoimmunotherapy based on peptide-based PIT agents offers the opportunity to integrate multiple treatment modalities into a single platform, which is consistent with the therapeutic principles in clinical therapy and provides an important tool for the treatment of cancer prone to recurrence and metastasis.
In this review, we focus on recent advances in supramolecular PIT agents based on precise peptide self-assembly design for combinational cancer photoimmunotherapy (Scheme 1). We introduce the role of peptides and put forward general design principles in the design of PIT agents in terms of the functions of peptides: (1) self-assembled peptides: as a carrier for photosensitizers and drug delivery; (2) functional peptides: constructing multifunctional PIT agents with active targeting or stimulus responsiveness; (3) immunorelated peptides: as a synergistic immunomodulator for amplifying the immune response. Subsequently, we summarize the application of peptide-based PIT agents in PDT-synergized immunotherapy, PTT-synergized immunotherapy, and chemotherapy-combined PIT, and highlight the unique effect of peptides in regulating photosensitizer self-assembly, enhancing drug accumulation and penetration, and boosting PIT effects. Finally, we discuss the challenges and future perspectives of peptide-based PIT agents in the tumor therapy field. Based on this review, we aim to elucidate the key role of peptides in the design of photoimmunotherapy drugs, which may facilitate the clinical antitumor application of peptide-based nanomedicines in the future.
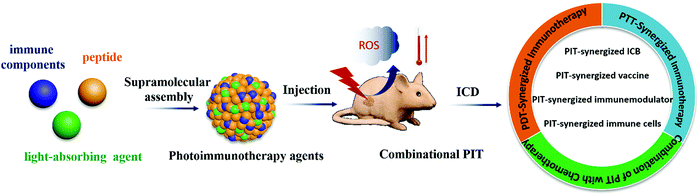 | ||
| Scheme 1 Schematic illustration of peptide-based supramolecular nanoagents for combinational cancer photoimmunotherapy (PIT). | ||
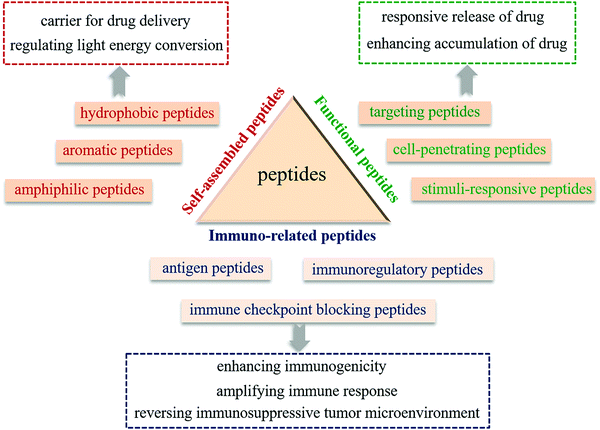
2. The roles and functions of peptides for the design of PIT agents
Peptide-modulated self-assembly of photosensitizers, as a versatile strategy, provides a means to overcome the physicochemical and pharmacokinetic challenges of hydrophobic photosensitizers.18 The peptide segment can not only modulate the self-assembly performance of hydrophobic photosensitizers to stabilize the assemblies in water, but also regulate light energy conversion of photosensitizers, which is essential for phototherapy. On the other hand, as they are commonly derived from the functional domains of native proteins, peptides possess inherent biological functions, including tumor targeting, tumor cell penetration, and stimuli-responsiveness, which are closely associated with tumor enrichment of therapeutic agents.17 Simultaneously, peptides as immunotherapeutic agents including antigen, adjuvant, immune checkpoint inhibitor or immunomodulator can effectively activate the innate and adaptive immune system, which leads to an enhanced efficacy for cancer immunotherapy.16 In recent years, our group developed a versatile peptide-modulated photosensitizer self-assembly strategy and constructed a series of peptide-based PIT agents for tumor supramolecular theranostics.19–22 We focus on building block design, intermolecular interactions as well as the relationships between such interactions and the formed supramolecular nanostructures, and controllable regulation of the light energy conversion mode, and these provide insights into the relationship between the supramolecular nanostructures and the therapeutic efficiency, such as supramolecular photothermal effects and supramolecular immunotherapy effect.16,23 We summarize the roles that peptides play in the design of PIT agents and their functions, with inclusion of our own recent progress (Fig. 1).2.1 Self-assembled peptides
Photoimmunotherapy usually relies on the use of highly hydrophobic photosensitizers. However, limited solubility and the non-targeting property of photosensitizers result in low bioavailability and large side effects and thus hinder their application. Hence, functional nanocarriers that can efficiently load and deliver photosensitizers to the tumor site have been developed to reduce the system toxicity and improve therapeutic efficiency. Among them, peptide-based delivery systems have been attracting more attention owing to their intrinsic biocompatibility and biodegradability, structural stability, and tunable functionality.24 Diverse peptide building blocks, including hydrophobic peptides, amphiphilic peptides, and aromatic peptides have been designed to construct functional drug delivery systems for the loading of hydrophobic photosensitizers. In some case, photosensitizers not only exist as loaded drugs, but also participate in peptide self-assembly through non-covalent interactions, forming a self-delivery system. Therefore, instead of physical encapsulation of photosensitizers, a peptide-modulated photosensitizer self-assembly strategy can enhance the stability of nanostructures through multiple noncovalent interactions and reduce toxicity by reducing unnecessary ingredients, and the resulting structures and functions can be flexibly tuned by altering the kinetics and thermodynamics of self-assembly.4,25 Incorporation of a photosensitizer and other immune components into assembled peptide nanostructures may allow for prolonged circulation of PIT agents and may facilitate the accumulation of therapeutics at tumor sites.In addition to supramolecular self-assembly, we recently developed a covalently-triggered self-assembly strategy.26–28 The integration of a covalent reaction and the self-assembly of peptides synthesized in situ enable the simultaneous encapsulation of photosensitizers and enhancement of the assemblies’ stability. These covalent reactions can be obtained through the inherent reaction activity of amino acids, e.g. Schiff's base reaction,29 disulfide exchange,30 tyrosine crosslinking,31 enzymatic reaction32 and so on. Furthermore, physicochemical properties and the functions of PIT agents can be flexibly regulated by changing the covalent reaction conditions such as the reaction time, temperature, concentration and proportion of reactant etc.26,33
Apart from serving as a delivery carrier, peptide-based assemblies themselves have the function of immune stimulation, which can induce the local inflammatory microenvironment to recruit and activate immune cells.34 For example, we constructed an injectable self-assembled peptide hydrogel capable of activating the T cell response without the assistance of antigens, immune regulatory factors, and adjuvants, and efficiently suppressed tumor growth.35 Meanwhile, the nano/micro structures formed increase the density of immune components (such as antigens) on the surface of the delivery platform and the affinity with targeting receptors through multivalent effects, thus mediating a highly efficient and durable immune response after photoimmunotherapy.36
2.2 Functional peptides
Peptides are endowed with strong biological diversity and play a variety of physiological functions in the human body. Therefore, self-assembly of functional peptides and photosensitizers hold great potential for the design of PIT agents. The covalent conjugation of photosensitizers to functional peptides is a frequently-used strategy to construct PIT agents, allowing for establishment of self-delivering and self-formulating systems.37 The control over the peptide sequence allows tuning the overall conjugate hydrophobicity and chargeability, both of which influence the stackings of photosensitizers and further adjust the light energy conversion pathway of PIT agents.38 Moreover, the introduction of functional peptide sequences with active targeting, cell penetration, lesion environmental response or tumor killing may benefit the PIT effect.For instance, the integration of tumor targeting peptides (such as RGD) that specifically recognize acceptors on the surface of tumor cells enables the PIT agents to be tumor targeting, thus enhancing the enrichment of PIT agents on the tumors.39 Cationic antimicrobial peptides, due to their inherent ability to bind to cell membranes, can form pores or destroy membranes and thereby deliver drugs or induce apoptosis of cancer cells.40 The tumor apoptosis induced by perforated peptides is beneficial to the leakage of deep tumor antigens for enhanced PIT. Introducing stimuli-responsive peptide sequences (such as caspase-specific DEVD peptide) as a linker of conjugates is one of the important strategies to trigger controlled drug release behavior.41 For example, the photosensitizer is usually conjugated with targeted peptide or other therapeutic agents including chemotherapeutic drugs, small immunodrugs, through the responsive peptide linker.42 Noteworthily, the self-assembly strategy of photosensitizer-peptide conjugates can on the one hand realize the combination of multiple functions of peptide sequences, and on the other hand integrate a variety of treatment methods through a robust nanoplatform, ultimately amplifying the PIT effect.
2.3 Immuno-related peptides
The immune function of peptides runs through the process of immune surveillance, defense, regulation and therapy. Peptide-based therapies have shown particular efficacy in regulating the immune response and improving the immunosuppressive tumor microenvironment. Peptides with minimal antigenic epitopes to bind with targeting receptors, as immunogens that cause effector cell immune responses in the body, are expected to become a new type of vaccine for the treatment of tumors.43 In peptide-based vaccines, peptides can not only act as antigens, but also adjuvants to enhance the immune response to antigens.44 In addition, peptides and peptide derivatives have strong biological activity and diversity, and play an irreplaceable regulatory role on the immune system.45 On the one hand, peptides can activate immune cell activity by promoting immune cell phagocytosis, proliferation, and differentiation, etc.46 On the other hand, as an immune checkpoint blocking agent, peptides can reshape the immunosuppressive tumor microenvironment by targeting immune suppressive receptors.47Although peptides play an irreplaceable role in immunotherapy, individual peptides still face various problems in immunotherapy. First, peptides, especially water-soluble peptides, are quickly eliminated from the body by enzymatic degradation, resulting in an unsustainable immune response in vivo. Next, the short amino acid sequence leads to a low affinity of the peptide to the receptor, resulting in low immunogenicity. In recent work, we proposed the supramolecular immunotherapy effects of peptide assemblies and clarified the mechanism by which supramolecular self-assembly of peptides affects the immunotherapy performance.16 The integration of immune-related peptides into PIT agents has complementary advantages, which not only avoids the rapid degradation of immune-active peptides in vivo, but also improves the intelligence and immune synergy of the system via a supramolecular immunotherapy effect. In addition, PIT agents constructed by supramolecular self-assembly between photosensitizers and immunopeptides may realize the synergistic effect of photoimmunotherapy and conventional immune therapies against cancer. During phototherapy, hyperthermia or ROS generated locally in the tumor mediates ICD and eliminates the primary tumor, meanwhile initiating an antitumor immune response.5 With the degradation of PIT agents, immune-related peptides play a sustainable and synergistic immunomodulatory role through a variety of immunomodulatory pathways, and finally realize the cascade amplification of immunotherapy, which is expected to conquer metastasis and recurrence in the treatment of advanced cancer.
3. Application of peptide-based PIT agents in combinational cancer photoimmunotherapy
The clinical successes of near infrared photosensitizer-antibody conjugate drugs have promoted the progress of photoimmunotherapy for the treatment of cancer, especially of superficial tumors.48 Combining phototherapy with immunotherapy has been found to achieve synergistic effects, promoting cancer ablation and even preventing the recurrence and metastasis of cancers.5 Peptide-modulated photosensitizer self-assembly endows a unique phototherapy–immunotherapy synergistic mechanism to promote immune activation and amplify the immunotherapy effect. The following sections discuss the recent studies on the effects of peptide-based PIT agents on cancer treatment in immunotherapy combined with PDT/PTT and photoimmunotherapy combined with chemotherapy.3.1 PDT-synergized immunotherapy
PDT is an accurate targeted therapy modality based on local activation of photosensitizers by irradiating with appropriate laser doses, which cause the generation of ROS and induce the death of tumor cells.49 PDT has been used in the clinical treatment of cancer for many years and was initially intended to directly destruct tumors.50 Later studies found that PDT can induce ICD, which releases antigens and DAMPs. This process enhances the immunogenicity of the tumor microenvironment and initiates the downstream antitumor immune response, including stimulating dendritic cell (DC) maturation, antigen presentation, and subsequent activation of cytotoxic T lymphocytes, thus achieving the synergy of PDT with immunotherapy.5,51Cell apoptosis induced by conventional PDT has a lower immunogenicity than necrosis due to the slow release of DAMPs during apoptosis, which could affect the presentation of tumor-associated antigens and the initiation of antitumor immune responses.52 Specific damage to the plasma membrane is expected to induce rapid release of intracellular contents, cause cell necrosis, and initiate a stronger antitumor immune response than conventional PDT.53 Based on this, an enzyme-driven membrane-targeted nanoagent (PCPK) has been designed for enhanced antitumor photodynamic immunotherapy, in which the hydrophobic photosensitizer PpIX was conjugated to the tumor cell PM-targeted peptide (KKKKKKSKTKC-OMe) through hydrophilic PEG sequences and hydrophobic alkyl chains.54 PM-targeted K-Ras-derived peptides can form a farnesylated product that migrates from the cytoplasm to the PM through enzymatic conversion by the protein farnesyltransferase (PFTase).55 After uptake by tumor cells, PCPK is driven by intracellular PFTase to target and tightly anchor to the intracellular endoplasmic membrane. After 660 nm laser irradiation, the resulting cytotoxic ROS destroy the structure of the PM, thereby killing tumor cells. Subsequently, selective destruction of the PM would mediate an ICD process of tumor cells, leading to rapid release of intracellular contents and enhancing the immunogenicity of tumor cells.
In tumor tissue, there are some specific T cells, which can recognize the short peptide antigen presented by the major histocompatibility complex (MHC) on the surface of cancer cells, and then kill tumor cells.56 However, the expression of MHC molecules on the surface of some tumor cells is low or absent, which hinders the recognition and presentation of peptide antigens and it is therefore difficult to stimulate the body to produce a strong immune response. The introduction of tumor-associated/specific antigens may strengthen antitumor PDT-immunotherapy. The covalent conjugation strategy can integrate photosensitizers and antigen peptides into a single platform to enhance immunogenicity in the tumor microenvironment. Li et al. designed a photosensitizer-antigen peptide conjugation (PpIX–PEG8–KVPRNQDWL), in which a photosensitizer protoporphyrin IX (PpIX) is covalently conjugated to the melanoma specific antigen peptide (KVPRNQDWL) with a flexible PEG chain (PEG8) for synergistic melanoma therapy (Fig. 2A).57 The resulting photosensitizer–peptide conjugate can self-assemble into spherical nanoparticles (PPMA) in an aqueous solution, as an effective self-delivery system for PDT amplified immunotherapy against malignant melanoma. The chimeric antigenic peptide not only enhances the aqueous solubility of PpIX, which is conducive to enhancing the generation of ROS under light irradiation, but also improves the stability of the obtained nanoparticles in water, which is beneficial to enhance the accumulation on tumor tissue via an enhanced permeation and retention (EPR) effect. The results confirm that PPMA can mediate apoptosis and/or necrosis of cancer cells through the effect of PDT under laser irradiation. In addition, the melanoma-specific antigen peptides in PPMA can achieve effective antigen cross-presentation and activate specific T cells for immunotherapy. Importantly, PDT has been shown to have an enhanced immunological effect on the antitumor immune response of PPMA, and CD8+ T cells can be effectively recruited in vivo by combining photodynamic immunotherapy.
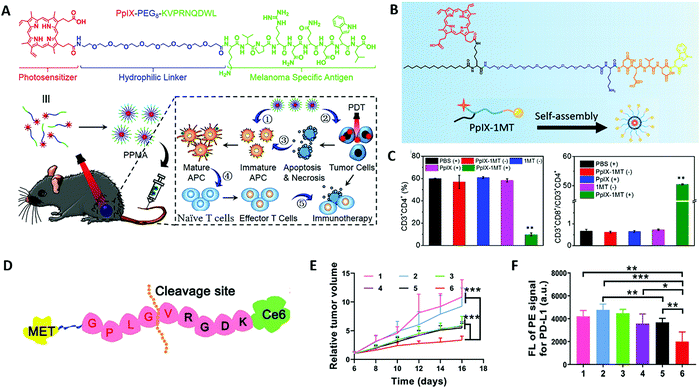 | ||
| Fig. 2 (A) The chemical structure and the proposed mechanism of PPMA for photodynamic therapy amplified immunotherapy against melanomas. Adapted with permission from ref. 57. Copyright 2021 John Wiley and Sons. (B) Structure of the chimeric peptide PpIX-1MT. (C) Ratio of CD4+ T cells to CD3+ lymphocytes and ratio of CD3+CD8+ T cells to CD3+CD4+ T cells after different treatments. Adapted with permission from ref. 41. Copyright 2021 American Chemical Society. (D) The design of self-delivery NPs for combination breast cancer immunotherapy. (E) Change in relative tumor volume during the treatment. (F) Expression of PD-L1 in tumor cells after various treatments were determined by flow cytometry. Adapted with permission from ref. 62. Copyright 2021 John Wiley and Sons. | ||
In the process of tumor occurrence and development, tumor cells are able to evade an immune response through different mechanisms and pathways (immune suppression and immune tolerance), thereby rapidly proliferating and metastasizing. For example, tumor cells can inhibit the killing capability of immune cells such as cytotoxic T lymphocytes and natural killer cells (NK) by secreting and expressing immunosuppressive factors such as programmed cell death 1 (PD-1) and its ligand PD-L1, cytotoxic T lymphocytes antigen 4 (CTLA-4) and indoleamine-2, 3-dioxygenase (IDO) to achieve immune evasion.58 Many research studies suggest that failure of tumor immunotherapy is caused by the immunosuppression that occurs not only in the tumor microenvironment but in tumor-draining lymph nodes.59,60 Once the immune checkpoint is activated, even if lymphocytes that recognize cancer cells are produced in large numbers in vivo, they cannot attack cancer cells. Therefore, the introduction of immune checkpoint blockade therapy through covalent conjugation of a peptide checkpoint inhibitor and a photosensitizer may achieve better therapeutic effects. The upregulated PD-1 or PD-L1 that is a crucial immunosuppressive molecule can inhibit the activity of T cells and lead to immune evasion of tumor cells. Blocking the interaction between PD-1 and PD-L1 on the surfaces of T cells and tumor cells enables T cells to reverse the activity and normalize the antitumor response. In an example reported recently, PpIX conjugated IDO inhibitor nanoparticles were designed against both primary and metastasis lung tumors.41 The porphyrin-peptide conjugation PpIX-1MT consists of three parts including palmitic acid grafted PpIX as the hydrophobic core of the nanoparticle, poly (ethylene glycol) (PEG) chain as the hydrophilic shell of the nanoparticles, and a caspase-3-sensitive DEVD sequence linked 1MT, which is an IDO inhibitor (Fig. 2B). During the treatment process, PpIX-1MT nanoparticles could induce tumor cell apoptosis by PDT. Then, the apoptotic tumor cells release caspase-3, which can cleave the DEVD sequence to release 1MT from the nanoparticles to efficiently activate CD8+ T cells via inhibiting the IDO pathway (Fig. 2C).
In general, the release of photosensitizers on demand in tumor cells is indispensable because the traditional photosensitizer monomer produces more reactive oxygen species than the aggregates owing to aggregation-caused quenching (ACQ).61 It is a common strategy to introduce the tumor microenvironment stimulus-responsive peptide linker between the photosensitizer and other immune components to ensure the stability of the obtained photoimmunoagent in the bloodstream, thus avoiding drug leakage and silencing the PDT effect to avoid phototoxicity. The Gao group subtly designed enzymatically cleavable self-delivery nanoparticles (MA-pepA-Ce6 NPs) by conjugating a photosensitizer (chlorin e6, Ce6) with programmed cell death ligand 1 (PD-L1) inhibitor (Metformin, MET) through matrix metalloproteinase-2 (MMP-2) cleavable peptide (GPLGVRGDK, pepA) (Fig. 2D).62 Matrix metalloproteinase-2 (MMP-2) is high-expressed in a variety of tumors and plays a key role in the invasion and metastasis of tumors, which is widely used in designing adaptive materials.37 The formed MA-pepA-Ce6 NPs are degraded by cleaving GPLGVRGDK at a specific site between glycine (G) and valine (V) by highly-expressed MMP-2 and exposing the VRGDK-Ce6 and MET-GPLG. The exposed VRGDK demonstrates good binding ability toward the integrin αvβ3 receptor to ensure sufficient accumulation, thus inducing efficient ablation of the primary tumor and eliciting a robust antitumor immunity response under laser irradiation. Moreover, the released MET-GPLG downregulates the PD-L1 expression and further improves the antitumor immune response initiated by PDT. Compared with nonresponsive peptide conjugated nanoparticles (MA-GplgvRGDK-Ce6, MApepa-Ce6 NPs), MA-pepA-Ce6 NPs could produce large amounts of ROS to induce more effective ICD with laser irradiation in vitro. Consistent with the in vitro experiment, MA-pepA-Ce6 NPs exhibit better suppression of tumor growth (Fig. 2E) and antitumor photoimmunotherapy by reducing the PD-L1 expression and promoting DC maturation (Fig. 2F). Similarly, Jiang et al. designed a self-assembled nanomedicine for immune checkpoint blockade combined photodynamic-immunotherapy by covalently conjugating anti-PD-L1 peptide (APP, NYSKPTDRQYHF-NH2) with a photosensitizer (IR780) linked by a MMP-2 cleavable peptide sequence.63 The introduction of MMP-2 responsive peptide sequences can not only consume MMP-2 in tumor tissues, but also accurately release the hydrophilic anti-PD-L1 peptide APP and trigger the generation of smaller sized photosensitizer nanoparticles in an enzyme-responsive manner, which can endow deep tumor penetration and increase tumor accumulation of IR780. Under 808 nm NIR laser irradiation, the smaller IR780 can eliminate primary tumors by generating singlet oxygen and stimulating a strong antitumor immune response, ultimately causing a prominent eradication effect to the metastatic tumors with the assistance of APP.
3.2 PTT-synergized immunotherapy
PTT is a rapidly developing antitumor therapy method that has emerged in recent years. This technique depends on the use of photothermal agents capable of converting light to heat upon laser irradiation. Local hyperpyrexia in the tumors accumulated with photothermal agents may lead to ablation of the pathological tissue.64,65 PTT can not only kill tumor cells by hyperthermia, but also elicit antitumor immune responses by various mechanisms including tumor cell damage, tumor surface molecule changes, heat shock proteins, exosomes release and through direct effects on immune cells.66 In addition, appropriate hyperthermia mediated by PTT can remodel an immunosuppressive tumor microenvironment to promote the infiltration of killer immune cells and downregulate the level of immune suppressive cells.67Compared with PDT, PTT has the following advantages: (1) PTT does not depend on endogenous oxygen. (2) Organic photothermal nanomaterials do not need the release of their monomers at the tumor site, and their aggregated form is more conducive to the light-to-heat conversion. (3) Supramolecular self-assembly provides a facile and potent method for the preparation of organic photothermal materials with high stability and photothermal conversion efficiency, which is coined the “supramolecular photothermal effect”.23 The stability, near infrared absorption capacity and photothermal conversion performance of the PIT agent were enhanced by simply regulating the non-covalent interaction between photosensitizers and peptides during supramolecular assembly. These advantages bring new hope for the treatment of some malignant tumors that are extremely hypoxic and difficult for drugs to enter, such as pancreatic cancer. Highly metastatic pancreatic cancer is an extremely fatal malignancy with a very low survival rate due to the high presence of dense pancreatic desmoplastic stroma. Hypoxia and poor blood transport of the stromal barrier block the entry of therapeutic agents into the tumor tissue and make pancreatic tumors clinically resistant to conventional treatment regimens.68,69 Therefore, PTT-synergized immunotherapy has unique advantages in the treatment of pancreatic tumors. Our group designed supramolecular nanofibrils (TP5-ICG NFs) by co-assembly of clinically approved NIR photosensitizer indocyanine green (ICG) and immunomodulatory thymopentin (TP5) for combinatorial NIR photothermal immunotherapy of pancreatic cancer (Fig. 3A).70 ICG is a clinically approved NIR contrast agent with good biocompatibility but extremely fast metabolism, which is difficult to be directly applied in photothermal therapy. TP5, as a common immunomodulator, can induce T cell differentiation and has abundant hydrogen bond binding sites to induce ICG to form long-range ordered filament nanostructures, which are suitable for localized injection with more obvious retention in tumor tissue. The resulting TP5-ICG NFs exhibited stability against the physiological environment and photodegradation, enabling their high photothermal conversion efficiency and excellent primary tumor ablation (Fig. 3B). The results confirmed that TP5-ICG NFs can ablate orthotopic pancreatic cancer through the effect of PTT under laser irradiation and trigger a subsequent immunomodulatory effect to promote DC maturation (Fig. 3C) and proliferation of cytotoxic T lymphocytes, thus inhibiting tumor recurrence and distal metastasis (Fig. 3D). This strategy and treatment model can be extended to other immunopeptides to enhance the immune response, creating opportunities for the treatment of other malignant tumors such as those that respond poorly to checkpoint blocking immunotherapy. Injectable supramolecular peptide hydrogels have been designed as promising photoimmunotherapeutic platforms for encapsulation and controlled release of multicomponent drugs. Our group recently fabricated collagen polypeptide hydrogels with incorporation of a photothermal drug methylene blue (MB) and immunological agent imiquimod (R837) for PTT-synergized immunotherapy (Fig. 3E).71 After intratumoral injection of supramolecular hydrogels, the high temperature generated by MB can effectively ablate the primary tumor and further produce tumor-associated antigens (TAA) under mild laser irradiation. Subsequently, TAA generated from PTT, together with R837 released in the hydrogel by a thermal effect, trigger the immune response for inhibiting distal tumor growth (Fig. 3F).
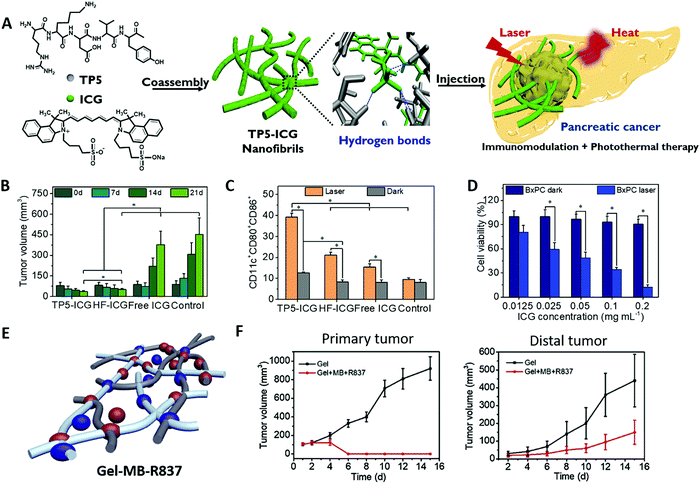 | ||
| Fig. 3 (A) The formation and application of thymopentin-based near-infrared photothermal immunomodulatory nanofibrils. (B) In vivo photothermal therapy inhibiting tumor growth. (C) CD11c+CD80+CD86+ cell frequencies among DC2.4 cells after different treatments in a transwell system. (D) Quantitative frequency of CD3+, CD3+CD4+, and CD3+CD8+ T cells in mouse splenocytes after different treatments by flow cytometry analysis. Adapted with permission from ref. 70. Copyright 2021 John Wiley and Sons. (E) Schematic illustration of the self-assembled collagen polypeptide hydrogel with encapsulation of MB and R837. (F) Volume growth curves of primary and distal tumors during photothermal immunotherapy in vivo. Adapted with permission from ref. 71. Copyright 2019 Elsevier. | ||
PTT-synergized immune checkpoint blockade therapy has been shown to promote the maturation of DCs and infiltration of cytotoxic T cells, which markedly improve tumor regression and prevent tumor recurrence and metastasis.5 One research which combined PTT and an IDO inhibitor for enhanced tumor penetration and cancer immunotherapy was performed by Ma and co-workers.72 A tumor microenvironment-responsive prodrug nanoplatform (mPEG-IDOi/ICG NPs) via co-assembly of a responsive peptide (PVGLIG) conjugated IDO inhibitor (Epacadostat) and ICG was employed as a photoimmunoagent, which can be translated into small sized two-drug complexes in the tumor microenvironment by MMP-2 mediated peptide chain breaking to enhance cellular uptake. C57BL/6 mice implanted with B16-F10 melanoma cells were injected intravenously with mPEG-IDOi/ICG NPs, then treated with an 808 nm laser. The results showed mPEG-IDOi/ICG NPs greatly promoted DC maturation and efficiently prevented the growth of both primary and distal tumors. In another study, Qian et al. constructed a Au@Pt nanosystem modified with a multifunctional peptide formed by three functional components, an antagonist of PD-L1, (DPPA-1), a MMP 2-resposive peptide (PLGVRG), and a tumor homing peptide LyP-1 (CGNKRTRGC).73 After they reached the tumor microenvironment, the nanosystem was broken down into two parts within the high expression of MMP-2. One part was LyP-1 peptide on Au@Pt that promoted the tumor enrichment and eliminated primary tumors by laser irradiation, and the other part was an anti-PD-L1 peptide that further activated cytotoxic T lymphocytes by PD-L1 immune checkpoint blockage, inhibiting tumor metastasis. AUNP-12, as an immune checkpoint modulator targeting the PD-1/PD-L1 pathway included in clinical studies, exhibited low toxicity and could inhibit the growth of various tumor cells such as B16F10 and 4T1.74 However, AUNP-12 is often limited by enzyme-induced rapid degradation when used alone in vivo. You et al. prepared AA@PN NPs by co-loading hollow gold nanoshells and AUNP-12 peptide into polylactic acid-hydroxyacetic acid (PLGA) nanoparticles, which could maintain a release period of up to 40 days for the AUNP-12 peptide. After the single intratumoral injection of AA@PN NPs and irradiation with a 980 nm laser at the tumor site, a good inhibitory effect on the primary tumor was achieved by the PTT. In addition, continuous release of AUNP-12 peptide triggered by a laser can block the PD-1/PD-L1 pathway to activate T cells, thus generating a systemic immune response. Similarly, Wang et al. designed an intelligent biomimetic nanoplatform for metastatic triple negative breast cancer via combined PTT with immune checkpoint blockade therapy with AUNP-12 peptides.75
Tumors arise from mutations, and the number of mutations determines the amount of neoantigens on the surface of tumor cells. Tumors that are impervious to immune checkpoint inhibitors usually have fewer mutations and are less likely to attract adequate tumor-related lymphocyte (TIL) cell infiltration, known as “cold” tumors or nonimmunogenic tumors.76 Peptide vaccine-synergized photothermal-immunotherapy may be an available strategy because the introduction of antigenic peptides can make up for the deficiency of tumor specific antigen (TSA) generated in situ by PTT and enhance immune responses with the assistance of immunoadjuvant. Wu et al. proposed a versatile photothermal vaccine consisting of photothermal agent ICG, immune adjuvant imiquimod (R837) and foreign cytotoxic T lymphocyte antigen peptide with the sequence of SIINFEKL that were co-encapsulated to acetylated dextran (AcDEX).77 According to experiments of various tumor models, a collaborative strategy combining PTT and a vaccine significantly enhanced infiltration of MHC-I+ cells and CD8+ T cells into tumors, slowed tumor growth and prolonged the survival time of mice.
In addition to low immunogenicity, most tumors generally possess immunosuppressive TME, which is saturated with abundant immunosuppressive cells, such as myeloid-derived suppressor cells (MDSCs), M2 phenotype tumor-associated macrophages (TAMs), regulatory T cells (Tregs), etc., resulting in an ineffective antitumor immune response and resistance to photoimmunotherapy.78 The tumor-associated macrophage (TAM) occupies a large proportion in the tumor-infiltrating lymphocytes (TILs), usually displaying an M2 phenotype, which can promote tumor growth and metastasis through tumor angiogenesis, invasion and T cell suppression.79 Based on this, a double targeted photothermal-immunotherapy nanoparticle (HA-AuNR/M-M2pep NP), composed of gold nanorods (HA-AuNRs) co-modified with MMP2-responsive M2pep fusion peptides (M-M2pep) and hyaluronic acid (HA), was designed to deplete M2-TAMs for enhancing the photothermal immunotherapy effect.80 At the tumor region, on the one hand, HA-AuNR/M-M2pep NPs released M2pep that can specifically bind M2-TAMs in response to over-expressed metalloproteinases in TME to selectively consume M2-TAMs and reshape immunosuppressive TME. On the other hand, HA-AuNR can actively target the CD44 receptor highly-expressed in tumor cells and achieve precise photothermal therapy (PTT) under 808 nm laser irradiation, which leads to ICD of tumor cells and initiation of an antitumor immune response. The collaborative strategy of immunosuppressive microenvironment regulation and PTT-induced immune activation effectively prevent the growth of tumors and prolong the survival time of mice.
3.3 Combination of PIT with chemotherapy
Combinatorial therapy may further improve the therapeutic effect of monotherapy. As a first-line clinical treatment, chemotherapy can effectively prevent the proliferation, invasion and metastasis of cancer cells. It has been shown that certain chemotherapeutic agents, such as doxorubicin (DOX) and docetaxel (DTX) administered in appropriate doses, may mediate ICD to initiate a specific antitumor immune response.81,82 Therefore, combining accurately targeted phototherapy with chemotherapy may induce higher levels of ICD to increase tumor immunogenicity.No selectivity to tumors is the most serious side effect of chemotherapy. Chemotherapeutic agents kill not only tumor cells, but also normal cells and white blood cells, thereby weakening the body's immune response. Kim and co-workers proposed a visible-light-triggered prodrug strategy to potentiate checkpoint blockade therapy by reversing an immunosuppressive tumor microenvironment into a highly immunogenic microenvironment in combination with chemotherapy and PDT. They designed a photosensitizer–peptide–drug conjugate (VPF–FRRG–DOX), in which a commercially available photosensitizer verteporfin (VPF) is covalently conjugated to the chemotherapeutic drug doxorubicin (DOX) through a cathepin B-specific cleavable peptide (FRRG), and self-assembled it into nanoparticles (LT-NPs) in aqueous solution through intermolecular π–π stacking and hydrophobic interactions (Fig. 4A).42 The formed prodrug LT-NPs remain silent in the bloodstream, and once they reached tumor cells with highly-expressed cathepsin B, LT-NPs are specifically cleaved to VPF and DOX, inducing cancer-targeted cytotoxicity and ICD under light irradiation. Compared with the DOX group and VPF (+) group, the LT-NPs (+L) group significantly promoted maturation of DC cells and activation of T cells (Fig. 4B), while the proportion of immunosuppressive cells was considerably downregulated (Fig. 4C), which demonstrates the synergistic immune effect of chemotherapy combined with PDT. Furthermore, combined with a PD-L1 blockade, the LT-NPs group greatly inhibited colon tumor growth and lung metastasis by generating an immune-favorable tumor microenvironment and initiating a strong antitumor immune response.
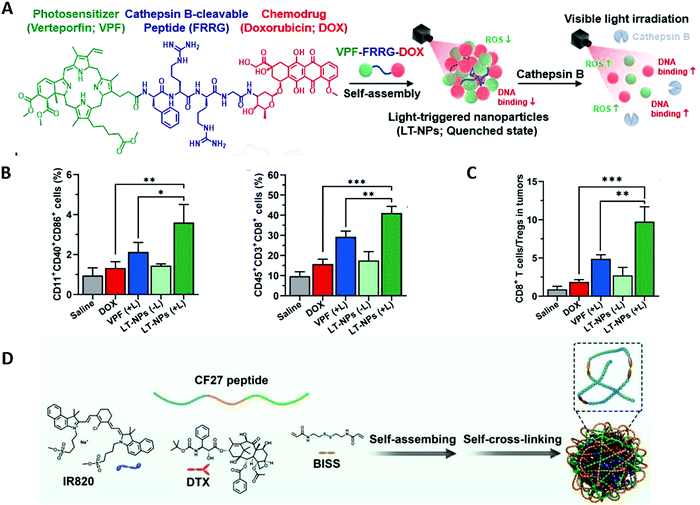 | ||
| Fig. 4 (A) LT-NPs are prepared by self-assembly of a cathepsin B specific cleavable prodrug of verteporfin (VPF), cathepsin B-specific cleavable peptide (FRRG), and doxorubicin conjugate (VPFFRRG-DOX). (B) Tumor-infiltrating matured dendritic cells and cytotoxic T cells on day 7 after treatments (n = 5). (C) The ratio of CD8+ T cells to Tregs in tumor tissues. Adapted with permission from ref. 42. Copyright 2021 American Chemical Society. (D) The supramolecular assembly and self-cross-linking formation of NIR dye/drug/peptide hybrid nanoparticles. Adapted with permission from ref. 81. Copyright 2021 John Wiley and Sons. | ||
Hyperthermia including PTT has been shown to further improve the efficacy of chemotherapy.83 Synergistic PTT and chemotherapy has been shown to induce a higher level of ICD, promote tumor infiltration of CD8+ T cells, and achieve a robust immune effect.84 Qian et al. designed a drug-dye-peptide nano-assembly (DTX–IR820–CF27 NPs) by near infrared dye (IR820) that modulated the supramolecular assembly of chemodrugs (docetaxel, DTX) and subsequently introduced a peptide (CF27) to induce self-crosslinking of drug-dye nanoparticles (Fig. 4D).81 The CF27 was a predesigned peptide with 27 amino acid units composed of tumor targeting peptide (CGNKRTRGC), MMP-responsive peptide (PLGVRG) and anti-PD-L1 peptide (NYSKPTDRGYHF). The obtained nano-assemblies possess MMP/GSH dual responsive characteristics and are conducive to the penetration of the IR820 and DTX inside tumor tissue and precise release of anti-PD-L1 peptide. DTX–IR820–CF27 NPs showed an excellent photothermal effect and ablation of in situ tumor owing to the enhanced cumulation of DTX–IR820–CF27 in the tumor region. This example shows that chemotherapy combined with PTT is effective in the treatment of primary tumors. Compared with DTX–IR820 NPs, DTX–IR820–CF27 NPs resulted in a much better inhibitory effect to secondary tumors and improved the proliferation of favored effector T cells and the expression of IFN-γ, demonstrating that chemotherapy and PTT combined PD-1/PD-L1 blockage treatment initiates a powerful immune response. Similarly, Tian and co-workers designed SP94-PB-SF-Cy5.5 NPs for highly malignant hepatocellular carcinoma therapy by photothermal-chemotherapy combined with an immune checkpoint blockade.85
Conclusions and future perspectives
Photoimmunotherapy has been clinically proven to be effective in the treatment of some cancers, and the combinations with peptide therapeutics or peptide materials also exhibited strong promising preclinical potential in treating cancers. In this review, we provided an overview of recent advances in peptide-based supramolecular PIT agents for combinational photoimmunotherapy against cancer, and demonstrated that this strategy has great advantages in enhancing tumor accumulation of PIT agents, amplifying PIT therapeutic effects, and preventing tumor recurrence and metastasis from the view point of precise design of multipurpose peptide-based PIT agents. The introduction of peptides not only regulates the properties of photosensitizers to endow them with self-delivery, targeting and precise release capabilities, but also enables the combination of multiple therapies including PIT-synergized immune checkpoint blockades therapy, PIT-synergized tumor vaccine therapy, PIT-synergized immune modulator therapy, PIT-synergized immune cells therapy and PIT-synergized chemotherapy. Therapeutics combined with PIT provoke the immune response by enhancing immunogenicity, promoting the maturation of DCs and infiltration of cytotoxic T cells at the tumor site, and reversing the immunosuppressive tumor microenvironment.However, with all the advances described above, photoimmunotherapy still has some challenges that hinder its clinical progression in cancer treatments. First of all, the main challenge of phototherapy is the limited penetration depth of lasers especially for non-superficial tumors. Therefore, more and more attention has been paid to designing a peptide sequence to adjust the stacking of photosensitizers. This may be an effective method to regulate the stacking of photosensitizers through amino acid encoding and then tuning the photophysical and photochemical properties of the assemblies, such as a strong absorption capacity in NIR-II (900–1700), to meet the needs of phototherapy penetration depth, high photothermal conversion capacity or reactive oxygen species yielding. In addition, DAMPs and various heat shock proteins are proven to be released within a suitable temperature range (about 39 to 45 °C), but when the temperature is too high, over 60 °C, the protein is prone to degenerate causing fast cell necrosis, which is not conducive to immune activation. Therefore, the immune stimulation by PTT requires precise control of thermal dose. Lastly, the lymph node is the site where tumor antigens are first exposed to initiate antitumor immunity, and plays a key role in stimulating an antitumor immune response and resisting tumor metastasis. However, lymph nodes are also the earliest and most common sites of metastasis, and lymphatic metastasis is traditionally considered to be the early stage of distant metastasis. Therefore, the delivery, immune activation, and negative feedback mechanism of lymph nodes should be considered for the design of PIT drugs, so as to achieve an efficient cascade immune response between tumor and lymph node sites.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Project No. 22102188 and 22025207), the Innovation Research Community Science Fund of China (No. 21821005), the National Natural Science Fund BRICS STI Framework Programme (No. 51861145304) and the Chinese Postdoctoral Science Foundation (Grand No. 2020M680674).References
- J. Xu, J. Lv, Q. Zhuang, Z. Yang and Z. Liu, Nat. Nanotechnol., 2020, 15, 1043–1052 CrossRef CAS PubMed.
- K. Shao, S. Singha, X. Clemente-Casares, S. Tsai, Y. Yang and P. Santamaria, ACS Nano, 2015, 9, 16–30 CrossRef CAS PubMed.
- D. H. Kang, M. T. Weaver, N. J. Park, B. Smith, T. Mcardle and J. Carpenter, Nurs. Res, 2009, 58, 105–114 CrossRef PubMed.
- M. Abbas, Q. Zou, S. Li and X. Yan, Adv. Mater., 2017, 29, 1605021 CrossRef PubMed.
- C. W. Ng, J. Li and K. Pu, Adv. Funct. Mater., 2018, 28, 1804688 CrossRef.
- X. Li, G. L. Ferrel, M. C. Guerra, T. Hode, J. A. Lunn, O. Adalsteinsson, R. E. Nordquist, H. Liu and W. R. Chen, Photochem. Photobiol. Sci., 2011, 10, 817–821 CrossRef CAS PubMed.
- X. Li, M. F. Naylor, H. Le, R. E. Nordquist, T. K. Teague, C. A. Howard, C. Murray and W. R. Chen, Cancer Biol. Ther., 2010, 10, 1081–1087 CrossRef PubMed.
- Y. Maruoka, H. Wakiyama, P. L. Choyke and H. Kobayashi, Ebiomedicine, 2021, 70, 103501 CrossRef PubMed.
- T. Kato, H. Wakiyama, A. Furusawa, P. L. Choyke and H. Kobayashi, Cancers, 2021, 13, 2535 CrossRef CAS PubMed.
- P. Xu and F. Liang, Int. J. Nanomed., 2020, 15, 9159–9180 CrossRef CAS PubMed.
- Y. Li, X. Li, F. Zhou, A. Doughty, A. R. Hoover, R. E. Nordquist and W. R. Chen, Cancer Lett., 2019, 442, 429–438 CrossRef CAS PubMed.
- C. Yuan, W. Ji, R. Xing, J. Li, E. Gazit and X. Yan, Nat. Rev. Chem., 2019, 3, 567–588 CrossRef CAS.
- M. Cao, R. Xing, R. Chang, Y. Wang and X. Yan, Coord. Chem. Rev., 2019, 397, 14–27 CrossRef CAS.
- L. Li, Z. Qiao, L. Wang and H. Wang, Adv. Mater., 2018, 31, 1804971 CrossRef PubMed.
- S. Li, W. Zhang, H. Xue, R. Xing and X. Yan, Chem. Sci., 2020, 11, 8644–8656 RSC.
- R. Chang and X. Yan, Small Struct., 2020, 1, 2000068 CrossRef.
- M. Li, X. Zhao, J. Dai and Z. Yu, Sci. China: Mater., 2019, 62, 1759–1781 Search PubMed.
- S. Li, Q. Zou, R. Xing, T. Govindaraju, R. Fakhrullin and X. Yan, Theranostics, 2019, 9, 3249–3261 CrossRef CAS PubMed.
- R. Xing, Q. Zou, C. Yuan, L. Zhao, R. Chang and X. Yan, Adv. Mater., 2019, 31, 1900822 CrossRef PubMed.
- M. Abbas, R. Xing, N. Zhang, Q. Zou and X. Yan, ACS Biomater. Sci. Eng., 2018, 4, 2046–2052 CrossRef CAS PubMed.
- S. Li, L. Zhao, R. Chang, R. Xing and X. Yan, Chem. – Eur. J., 2019, 25, 13429–13435 CrossRef CAS PubMed.
- S. Li, Q. Zou, Y. Li, C. Yuan, R. Xing and X. Yan, J. Am. Chem. Soc., 2018, 140, 10794–10802 CrossRef CAS PubMed.
- L. Zhao, S. Li, Y. Liu, R. Xing and X. Yan, CCS Chem., 2019, 1, 173–180 CAS.
- R. Chang, Q. Zou, R. Xing and X. Yan, Adv. Therap., 2019, 2, 1900048 CrossRef.
- J. Wang, K. Liu, R. Xing and X. Yan, Chem. Soc. Rev., 2016, 45, 5589–5604 RSC.
- Y. Liu, G. Shen, L. Zhao, Q. Zou, T. Jiao and X. Yan, ACS Appl. Mater. Interfaces, 2019, 11, 41898–41905 CrossRef CAS PubMed.
- Y. Liu, L. Zhao, R. Xing, T. Jiao, W. Song and X. Yan, Chem. – Asian J., 2018, 13, 3526–3532 CrossRef CAS PubMed.
- H. Hong, Q. Zou, Y. Liu, S. Wang, G. Shen and X. Yan, ChemMedChem, 2021, 16, 2381–2385 CrossRef CAS PubMed.
- S. Li, Y. Liu, R. Xing and X. Yan, ChemBioChem, 2019, 20, 555–560 CrossRef CAS PubMed.
- F. Zhao, G. Shen, C. Chen, R. Xing, Q. Zou, G. Ma and X. Yan, Chem. – Eur. J., 2014, 20, 6880–6887 CrossRef CAS PubMed.
- X. Ren, Q. Zou, C. Yuan, R. Chang, R. Xing and X. Yan, Angew. Chem., Int. Ed., 2019, 58, 5872–5876 CrossRef CAS PubMed.
- Q. Zhang, D. Zheng, X. Dong, P. Pan, S. Zeng, F. Gao, S. Cheng and X. Zhang, J. Am. Chem. Soc., 2021, 143, 5127–5140 CrossRef CAS PubMed.
- Y. Liu, E. Naumenko, F. Akhatova, Q. Zou and X. Yan, Chem. Eng. J., 2021, 424, 130348 CrossRef CAS.
- J. S. Rudra, Y. F. Tian, J. P. Jung and J. H. Collier, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 622–627 CrossRef CAS PubMed.
- R. Xing, S. Li, N. Zhang, G. Shen, H. Mohwald and X. Yan, Biomacromolecules, 2017, 18, 3514–3523 CrossRef CAS PubMed.
- Y. Qian, H. Jin, S. Qiao, Y. Dai, C. Huang, L. Lu, Q. Luo and Z. Zhang, Biomaterials, 2016, 98, 171–183 CrossRef CAS PubMed.
- A. Gao, B. Chen, J. Gao, F. Zhou and H. Yu, Nano Lett., 2019, 20, 353–362 CrossRef PubMed.
- Q. Zou, M. Abbas, L. Zhao, S. Li, G. Shen and X. Yan, J. Am. Chem. Soc., 2017, 139, 1921–1927 CrossRef CAS PubMed.
- W. Li, J. Yang, L. Luo, M. Jiang and J. You, Nat. Commun., 2019, 10, 3349 CrossRef PubMed.
- R. Raheleh, N. L. Syn and R. Maryam, Front. Immunol., 2017, 8, 1320 CrossRef PubMed.
- W. Song, J. Kuang, C. X. Li, M. Zhang, D. Zheng, X. Zeng, C. Liu and X. Zhang, ACS Nano, 2018, 12, 1978–1989 CrossRef CAS PubMed.
- J. Choi, M. K. Shim, S. Yang, H. S. Hwang and K. Kim, ACS Nano, 2021, 15, 12086–12098 CrossRef CAS PubMed.
- P. Giorgio, C. Chiara, D. Piero, M. Roberta, R. Licia, F. M. Marincola and A. Andrea, J. Natl. Cancer Inst., 2002, 94, 805–818 CrossRef PubMed.
- M. Black, A. Trent, Y. Kostenko, J. Lee, C. Olive and M. Tirrell, Adv. Mater., 2012, 24, 3845–3849 CrossRef CAS PubMed.
- L. Zhang, Y. Huang, A. R. Lindstrom, T.-Y. Lin, K. S. Lam and Y. Li, Theranostics, 2019, 9, 7807–7825 CrossRef CAS PubMed.
- L. Chen, Y. Teng and W. Xu, Curr. Med. Chem., 2011, 18, 964–976 CrossRef CAS PubMed.
- K. Guzik, M. Tomala, D. Muszak, M. Konieczny, A. Hec, U. Baszkiewicz, M. Pustua, R. Butera, A. Dmling and T. A. Holak, Molecules, 2019, 24, 2071 CrossRef CAS PubMed.
- M. Mitsunaga, M. Ogawa, N. Kosaka, L. T. Rosenblum, P. L. Choyke and H. Kobayashi, Nat. Med., 2011, 17, 1685–1691 CrossRef CAS PubMed.
- Y. Liu, K. Ma, T. Jiao, R. Xing, G. Shen and X. Yan, Sci. Rep., 2017, 7, 42978 CrossRef CAS PubMed.
- X. Li, J. Lovell, J. Yoon and X. Chen, Nat. Rev. Clin. Oncol., 2020, 17, 657–674 CrossRef PubMed.
- M. Wang, J. Rao, M. Wang, X. Li, K. Liu, M. F. Naylor, R. E. Nordquist, W. R. Chen and F. Zhou, Theranostics, 2021, 11, 2218–2231 CrossRef CAS PubMed.
- D. R. Green, T. Ferguson, L. Zitvogel and G. Kroemer, Nat. Rev. Immunol., 2009, 9, 353–363 CrossRef CAS PubMed.
- E. Moldoveanu, A. Oros, F. L. Halalau and L. M. Popescu, Rom. J. Morphol. Embryol., 1996, 42, 41–51 CAS.
- C. Zhang, F. Gao, W. Wu, W. Qiu and X. Zhang, ACS Nano, 2019, 13, 11249–11262 CrossRef CAS PubMed.
- M. M. Mahmoodi, D. Abate-Pella, T. J. Pundsack, C. C. Palsuledesai, P. C. Goff, D. A. Blank and M. Distefano, J. Am. Chem. Soc., 2016, 138, 5848–5859 CrossRef CAS PubMed.
- D. Chen and I. Mellman, Immunity, 2013, 39, 1–10 CrossRef CAS PubMed.
- H. Cheng, G. Fan, J. Fan, R. Zheng, L. Zhao, P. Yuan, X. Zhao, X. Yu and S. Li, Macromol. Biosci., 2019, 19, 1800410 CrossRef PubMed.
- E. I. Buchbinder and A. Desai, Am. J. Clin. Oncol., 2015, 39, 98–106 CrossRef PubMed.
- Z. Zhou, J. Pang, X. Wu, W. Wu and M. Kong, Nano Res., 2020, 13, 1509–1518 CrossRef CAS.
- M. M. Gubin, X. Zhang, H. Schuster, E. Caron, J. P. Ward, T. Noguchi, Y. Ivanova, J. Hundal, C. D. Arthur, W.-J. Krebber, G. E. Mulder, M. Toebes, M. D. Vesely, S. S. K. Lam, A. J. Korman, J. P. Allison, G. J. Freeman, A. H. Sharpe, E. L. Pearce, T. N. Schumacher, R. Aebersold, H.-G. Rammensee, C. J. M. Melief, E. R. Mardis, W. E. Gillanders, M. N. Artyomov and R. D. Schreiber, Nature, 2014, 515, 577–581 CrossRef CAS PubMed.
- X. Li, S. Lee and J. Yoon, Chem. Soc. Rev., 2018, 47, 1174–1188 RSC.
- C. Hu, X. He, Y. Chen, X. Yang, L. Qin, T. Lei, Y. Zhou, T. Gong, Y. Huang and H. Gao, Adv. Funct. Mater., 2021, 31, 2007149 CrossRef CAS.
- N. Wang, Y. Zhou, Y. Xu, X. Ren and Y. Luan, Chem. Eng. J., 2020, 400, 125995 CrossRef CAS.
- L. Zhao, Y. Liu, R. Xing and X. Yan, Angew. Chem., Int. Ed., 2020, 59, 3793–3801 CrossRef CAS PubMed.
- L. Zhao, Y. Liu, R. Chang, R. Xing and X. Yan, Adv. Funct. Mater., 2019, 29, 1806877 CrossRef.
- S. Toraya-Brown and S. Fiering, Int. J. Hyperthermia, 2014, 30, 531–539 CrossRef CAS PubMed.
- Q. Hu, Z. Huang, Y. Duan, Z. Fu and B. Liu, Bioconjugate Chem., 2020, 31, 1268–1278 CrossRef CAS PubMed.
- H. Han, D. Valdepérez, Q. Jin, B. Yang, Z. Li, Y. Wu, B. Pelaz, W. J. Parak and J. Ji, ACS Nano, 2017, 11, 1281–1291 CrossRef CAS PubMed.
- A. N. Hosein, R. A. Brekken and A. Maitra, Nat. Rev. Gastroenterol. Hepatol., 2020, 17, 487–505 CrossRef PubMed.
- S. Li, W. Zhang, R. Xing, C. Yuan, H. Xue and X. Yan, Adv. Mater., 2021, 33, 2103733 CrossRef CAS PubMed.
- E. Mei, S. Li, J. Song, R. Xing, Z. Li and X. Yan, Colloids Surf., A, 2019, 577, 570–575 CrossRef CAS.
- Y. Liu, Y. Lu, X. Zhu, C. Li and G. Ma, Biomaterials, 2020, 242, 119933 CrossRef CAS PubMed.
- Q. Yang, J. Peng, K. Shi, Y. Xiao and Z. Qian, J. Controlled Release, 2019, 308, 29–43 CrossRef CAS PubMed.
- P. G. Sasikumar, L. K. Satyam, R. K. Shrimali, K. Subbarao and M. Ramachandra, Cancer Res., 2012, 72, 2850 Search PubMed.
- L. Luo, C. Zhu, H. Yin, M. Jiang, J. Zhang, B. Qin, Z. Luo, X. Yuan, J. Yang, W. Y. Du and J. You, ACS Nano, 2018, 12, 7647–7662 CrossRef CAS PubMed.
- M. Olza, B. N. Rodrigo, S. Zimmermann and G. Coukos, Lancet Oncol., 2020, 21, e419–e430 CrossRef.
- Y. Gao, Q. Zhao, M. Xiao, X. Huang and X. Wu, Biomaterials, 2021, 273, 120792 CrossRef CAS PubMed.
- Y. Liu and X. Cao, J. Mol. Med., 2015, 94, 509–522 CrossRef PubMed.
- J. Condeelis and J. W. Pollard, Cell, 2006, 124, 263–266 CrossRef CAS PubMed.
- D. Tian, F. Qin, H. Zhao, C. Zhang, H. Wang, N. Liu and Y. Ai, Colloids Surf., B, 2021, 202, 111681 CrossRef CAS PubMed.
- C. Xu, Y. Yu, Y. Sun, L. Kong, C. Yang, M. Hu, T. Yang, J. Zhang, Q. Hu and Z. Zhang, Adv. Funct. Mater., 2019, 29, 1905213 CrossRef CAS.
- J. Peng, Q. Yang, Y. Xiao, K. Shi, Q. Liu, Y. Hao, F. Yang, R. Han and Z. Qian, Adv. Funct. Mater., 2019, 29, 1900004 CrossRef.
- J. Liao, W. Li, J. Peng, Q. Yang, L. He, Y. Wei, X. Zhang and Z. Qian, Theranostics, 2015, 5, 345–356 CrossRef PubMed.
- Y. Wen, X. Chen, X. Zhu, Y. Gong and J. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 43393–43408 CrossRef CAS PubMed.
- T. Zhou, X. Liang, P. Wang, Y. Hu, Y. Qi, Y. Jin, Y. Du, C. Fang and J. Tian, ACS Nano, 2020, 14, 12679–12696 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |