Structure and hydration of polyvinylpyrrolidone–hydrogen peroxide†‡
Luke I.
Chambers
a,
Dmitry S.
Yufit
a,
Mark A.
Fox
 a,
Osama M.
Musa
b and
Jonathan W.
Steed
a,
Osama M.
Musa
b and
Jonathan W.
Steed
 *a
*a
aDurham University, Department of Chemistry, Lower Mountjoy, Stockton Road, Durham, DH1 3LE, UK. E-mail: jon.steed@durham.ac.uk
bAshland LLC, 1005 Route 202/206, Bridgewater, NJ 08807, USA
First published on 1st December 2021
Abstract
The structure of the commercially important polyvinylpyrrolidone–hydrogen peroxide complex can be understood by reference to the co-crystal structure of a hydrogen peroxide complex and its mixed hydrates of a two-monomer unit model compound, bisVP·2H2O2. The mixed hydrates involve selective water substitution into one of the two independent hydrogen peroxide binding sites.
Hydrogen peroxide is a strong oxidizing agent, bleaching agent, and antiseptic used in a range of commercial applications including teeth whitening formulations.1–3 It oxidises the aromatic amino acids present in dentin phosphoprotein, decreasing the fluorescent intensity and lightening the colour of teeth.4,5 Pure hydrogen peroxide is unstable and easily decomposes in the presence of light or oxidizable compounds.6,7 This instability can be partially overcome with adducts such as urea–hydrogen peroxide8,9 that releases free hydrogen peroxide when dissolved in water, providing a more controlled application.10 However, the urea–hydrogen peroxide complex is unstable at 40 °C and decomposes on storage.11–13 Polyvinylpyrrolidone (PVP) forms a more stable complex with hydrogen peroxide called Peroxydone (PEX).14,15 PEX is a stable powder that releases hydrogen peroxide on contact with water or saliva.11,16 PEX was first described in 196717 and can be prepared using a variety of methods including dissolving the PVP with hydrogen peroxide and evaporating the solvent,18 spray drying18 and spraying an aqueous solution of hydrogen peroxide onto a fluidised bed of PVP.19,20 One of the main uses of PEX is in modern teeth whitening formulations.21–23 The structure of PEX is unknown but is thought to involve hydrogen bonding from the hydrogen peroxide to the pyrrolidone carbonyl group in either a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of hydrogen peroxide to PVP monomer.24 Infrared (IR) spectroscopy and ab initio calculations show14 that the interaction energy of a model monomer 1-ethyl-2-pyrrolidone with hydrogen peroxide is stronger than with water, which means the hydrogen peroxide should stay associated with pyrrolidones even in water. The calculations also reveal strong self-association between adjacent hydrogen peroxide units, which suggests the formation of extended ribbon structures in PEX.
2 ratio of hydrogen peroxide to PVP monomer.24 Infrared (IR) spectroscopy and ab initio calculations show14 that the interaction energy of a model monomer 1-ethyl-2-pyrrolidone with hydrogen peroxide is stronger than with water, which means the hydrogen peroxide should stay associated with pyrrolidones even in water. The calculations also reveal strong self-association between adjacent hydrogen peroxide units, which suggests the formation of extended ribbon structures in PEX.The structure of polymer materials can be complex to study due to the large molecular weights and polydispersity making analytical data difficult to interpret.25 To overcome this complexity, small molecule analogues of PVP can be used.25 For example the X-ray crystal structure of a two monomer PVP model compound (bisVP) showed that the structure of the WHO essential medicine povidone–iodine is better represented as involving intermolecular hydrogen bonding between separate polymer chains rather than an intramolecular hydrogen bond between adjacent units.26 Small molecule models analogues of PVP and polyvinylcaprolactam (PVCap) have also been used to help understand the interactions between polymeric amorphous solid dispersants and active pharmaceutical ingredients,27 and to understand the role of PVP and PVCap as clathrate hydrate inhibitors in the oilfield industry.28 The present work aims to improve the understanding of the interactions between PVP and hydrogen peroxide and the effects of hydration using IR spectroscopy, solid-state (SS) NMR spectroscopy, density functional theory (DFT) calculations and structure elucidation of bisVP-H2O2 model systems using single crystal X-ray diffraction.
PEX K-30 and free PVP K-25 of similar molecular weight were characterised by FTIR spectroscopy (ESI,‡ Fig. S1) the νOH stretching band from the hydrogen peroxide occurs at 3226 cm−1 in PEX (Table S1, ESI‡). PVP is hygroscopic and also exhibits a νOH stretching band of lower intensity at 3458 cm−1 which is assigned to trace quantities of water.29 The νCO stretching band shifts from 1667 cm−1 for PVP to 1638 cm−1 for PEX, indicating that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond is weaker for PEX as a result of hydrogen bonding interactions between the C
O bond is weaker for PEX as a result of hydrogen bonding interactions between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of PVP and the OH groups of the hydrogen peroxide.30 Two other PVP and PEX pairs with different molecular weights were also characterised; a K-90 analogue and crosslinked XL-10. Both the K-90 and XL-10 samples show similar changes in the FTIR spectra upon hydrogen peroxide complexation. The PVP K-25 and PEX K-30 samples were also characterised by solid-state MAS 13C NMR spectroscopy (Fig. S3, ESI‡). The peak for the carbonyl carbon atom C1 shifts from 175.7 ppm for PVP to 177.8 ppm for PEX, consistent with the formation of hydrogen bonds between the carbonyl group and the hydrogen peroxide. The SS NMR spectra of PVP K-90 and PVP XL-10 are similar. The occurrence of a single peak for the carbonyl carbon atom indicates the majority of PVP carbonyl groups form the same number of hydrogen bonds with hydrogen peroxide molecules.
O of PVP and the OH groups of the hydrogen peroxide.30 Two other PVP and PEX pairs with different molecular weights were also characterised; a K-90 analogue and crosslinked XL-10. Both the K-90 and XL-10 samples show similar changes in the FTIR spectra upon hydrogen peroxide complexation. The PVP K-25 and PEX K-30 samples were also characterised by solid-state MAS 13C NMR spectroscopy (Fig. S3, ESI‡). The peak for the carbonyl carbon atom C1 shifts from 175.7 ppm for PVP to 177.8 ppm for PEX, consistent with the formation of hydrogen bonds between the carbonyl group and the hydrogen peroxide. The SS NMR spectra of PVP K-90 and PVP XL-10 are similar. The occurrence of a single peak for the carbonyl carbon atom indicates the majority of PVP carbonyl groups form the same number of hydrogen bonds with hydrogen peroxide molecules.
Potassium permanganate titration analysis indicates ratios of hydrogen peroxide to pyrrolidone monomer unit of between 0.68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 0.86
1 and 0.86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 depending on the PEX type while elemental analysis gives ratios of 0.77
1 depending on the PEX type while elemental analysis gives ratios of 0.77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 0.92
1 to 0.92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. These ratios of under 1
1. These ratios of under 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 suggest that some PVP monomer units are either not associated with hydrogen peroxide or that a single hydrogen peroxide molecule can bridge between two pyrrolidone monomers. The presence of adventitious moisture is also likely, and some carbonyl sites may interact with water instead of hydrogen peroxide.
1 suggest that some PVP monomer units are either not associated with hydrogen peroxide or that a single hydrogen peroxide molecule can bridge between two pyrrolidone monomers. The presence of adventitious moisture is also likely, and some carbonyl sites may interact with water instead of hydrogen peroxide.
The small molecule model bisVP was used for comparison with the polymer system and to obtain direct structural information. BisVP was dissolved in ethyl acetate and aqueous hydrogen peroxide (80 wt%) was added, and the solvent was removed to leave an amorphous material. The FTIR spectrum of this amorphous sample is similar to that of PEX K30 with both the νOH band and νCO bands shifting to lower wavenumber compared to PVP, suggesting that the bisVP behaves similarly to the polymer system. The lower wavenumbers observed for the bisVP model system (Table S1, ESI‡) compared to the polymer suggests the carbonyl group is more accessible for hydrogen bond formation in bisVP.
Cooling solutions of bisVP with varying amounts of hydrogen peroxide at −28 °C for one week resulted in the formation of three different crystalline samples. These materials are unstable and melt slowly at room temperature. The FTIR spectra of the crystalline materials (before melting) show considerably sharper peaks compared to the amorphous product (Fig. S2, ESI‡). The νOH and νCO stretching bands are shifted to a lower wavenumber compared to the amorphous material which indicates stronger hydrogen bonding is present. The νCO stretching bands of the crystalline solid shift to a lower wavenumber when a higher ratio of hydrogen peroxide is used indicating increasing hydrogen bonding strength with increasing hydrogen peroxide content.
These microcrystalline bisVP-H2O2 adducts were added as seeds to bisVP solutions with varying amounts of peroxide in ethanol and stored at −28 °C which resulted in single-crystal X-ray diffraction (SXRD) quality crystals for general formula bisVP·nH2O2·mH2O. A monohydrate-monohydrogenperoxide bisVP·H2O2·H2O was obtained from a peroxide deficient solution (bisVP to peroxide ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7). Increasing the amount of peroxide to 1
0.7). Increasing the amount of peroxide to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 gave a mixed material of formula bisVP·1.7H2O2·0.3H2O while excess peroxide (ratio 1
1.4 gave a mixed material of formula bisVP·1.7H2O2·0.3H2O while excess peroxide (ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
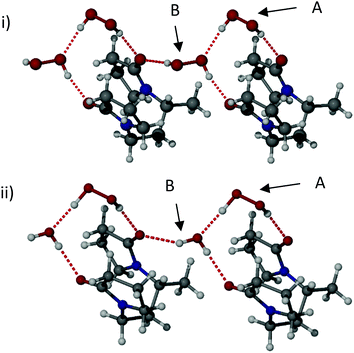
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) gave a bis(hydrogenperoxide) complex, bisVP·2H2O2. All three materials were characterised by SXRD (Fig. 1 and Fig. S5, ESI‡). The three structures are isomorphous. The two hydrogen peroxide molecules in bisVP·2H2O2 have very different supramolecular environments and different conformations. One of the hydrogen peroxide molecules (A) has a torsion angle HOOH of 83(3)° while the other molecule (B) has a torsion angle of 119(3)°. Both torsion angles represent a skew geometry.31 Molecule A is hydrogen bonded to one of the bisVP carbonyl groups with a relatively short O⋯O distance of 2.749(2) Å suggesting a strong hydrogen bond.31 Importantly, this molecule is also hydrogen bonded to one of the oxygen atoms of another hydrogen peroxide molecule B connecting the two hydrogen peroxide units. This hydrogen bond has a similar O⋯O distance of 2.759(2) Å. Hydrogen peroxide molecule B is hydrogen bonded to carbonyl groups of two different bisVP molecules resulting in a bridging interaction between two bisVP molecules (Fig. 1i, ESI‡). The O⋯O distances in these hydrogen bonds are remarkably short at 2.622(2) and 2.708(2) Å, suggesting they are considerably stronger than those formed by peroxide molecule A. The covalent O–O bond distance for hydrogen peroxide molecules A and B are 1.464(2) Å and 1.451(2) Å, respectively, both are close to the bond length in crystalline hydrogen peroxide (1.461(3) Å).32 The crystal structure also shows that the hydrogen peroxide units form discrete dimers linked by hydrogen bonds to the bisVP carbonyl oxygen atoms in this two-monomer model rather than the chain of peroxides suggested by Panarin et al.14
3) gave a bis(hydrogenperoxide) complex, bisVP·2H2O2. All three materials were characterised by SXRD (Fig. 1 and Fig. S5, ESI‡). The three structures are isomorphous. The two hydrogen peroxide molecules in bisVP·2H2O2 have very different supramolecular environments and different conformations. One of the hydrogen peroxide molecules (A) has a torsion angle HOOH of 83(3)° while the other molecule (B) has a torsion angle of 119(3)°. Both torsion angles represent a skew geometry.31 Molecule A is hydrogen bonded to one of the bisVP carbonyl groups with a relatively short O⋯O distance of 2.749(2) Å suggesting a strong hydrogen bond.31 Importantly, this molecule is also hydrogen bonded to one of the oxygen atoms of another hydrogen peroxide molecule B connecting the two hydrogen peroxide units. This hydrogen bond has a similar O⋯O distance of 2.759(2) Å. Hydrogen peroxide molecule B is hydrogen bonded to carbonyl groups of two different bisVP molecules resulting in a bridging interaction between two bisVP molecules (Fig. 1i, ESI‡). The O⋯O distances in these hydrogen bonds are remarkably short at 2.622(2) and 2.708(2) Å, suggesting they are considerably stronger than those formed by peroxide molecule A. The covalent O–O bond distance for hydrogen peroxide molecules A and B are 1.464(2) Å and 1.451(2) Å, respectively, both are close to the bond length in crystalline hydrogen peroxide (1.461(3) Å).32 The crystal structure also shows that the hydrogen peroxide units form discrete dimers linked by hydrogen bonds to the bisVP carbonyl oxygen atoms in this two-monomer model rather than the chain of peroxides suggested by Panarin et al.14
The partial hydrate structures are very similar to the bis(hydrogenperoxide) adduct except that just one of the two hydrogen peroxide sites, molecule B, is either partially or completely occupied by water. The potential for water molecules to selectively replace hydrogen peroxide is likely to be related to the sub-stoichiometric hydrogen peroxide content of PEX found by elemental analysis and manganate titration. The intramolecular distance between the two carbonyl oxygen atoms in the bisVP molecule varies across the three structures with bisVP·H2O2·H2O having a distance of 3.512(3) Å, bisVP·1.7H2O2·0.3H2O 3.436(3) Å, and bisVP·2H2O2 3.399(2) Å. Hence the binding site is somewhat flexible and exhibits its optimum geometry with H2O2 explaining the preference for hydrogen peroxide uptake over water in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 ratio solution.
1.4 ratio solution.
DFT calculations were performed on multimolecular models of bisVP and PVP molecules and their H2O2 and H2O complexes to investigate the preferred binding modes for water and H2O2 in both bisVP and oligomer segments of PEX. Calculations using an array of 6 bisVP and 12 H2O2 molecules derived from the crystal structure of bisVP·2H2O2 as the starting geometry retained the overall packing arrangement (Fig. S13, ESI‡) after full optimisation with the averaged intramolecular distance between the two carbonyl oxygen atoms of 3.51 Å – a difference of 0.03 Å compared to the corresponding experimental value. The simulated IR spectrum from a frequency calculation on this optimised geometry revealed peaks at 3312–3175 and 1636 cm−1 corresponding to νOH and νCO stretching bands, respectively. The agreement with the corresponding observed values of 3253 and 1625 cm−1 gives confidence in the accuracy of the multimolecular model at the B3LYP/6-31G(d)/GD3BJ level used here.
Replacement of the hydrogen peroxide with water molecules in this same starting geometry showed that water can replace both types of hydrogen peroxide in this structure with simulated νOH and νCO stretching bands of 3441 and 1643 cm−1, respectively. These values are consistent with the experimental values observed for ‘wet’ bisVP of 3448 cm−1 and 1668 cm−1, although the experimental values are highly dependent on water content.
The crystal structure of bisVP·H2O2·H2O reveals a clear preference for water molecules to occupy the B positions rather than the A positions. Geometry optimisations of the AB type structures containing 6 bisVP, 6 H2O2 and 6 H2O molecules at the A and B positions (Fig. S14 and S15, ESI‡) confirm this strong preference for the water molecules to localise at the B positions with a lower Gibbs free energy of 10.0 kcal mol−1 (298.15 K, 1 atm). The averaged intramolecular distance between the two carbonyl oxygen atoms in the more stable optimised model bisVP·H2O2·H2O is 3.44 Å – a difference of 0.04 Å with respect to experimental data.
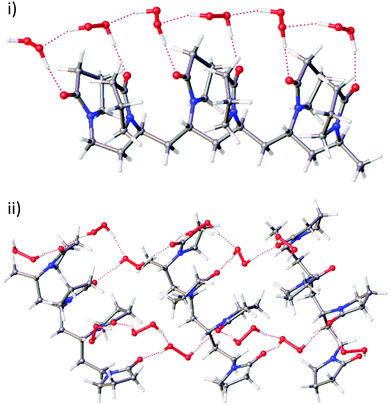
In order to extend the bisVP model to longer PVP fragments, geometry optimisations of four and six monomer fragments of PVP were examined. Starting geometries of PVP tetramers were generated from the bisVP geometries. These initial models were optimised to establish whether AB and all-A motifs are possible in PVP materials with hydrogen peroxide and/or water molecules present. All motifs proved to be feasible and the DFT study does not rule out either the AB or all-A hydrogen bonding motifs in PEX materials. The DFT optimised structure of three VP tetramers binding 12 H2O2 molecules in an AB cross-linked fashion is shown in Fig. 2ii and represents one possible idealised model for PEX. This geometry does not seem to tolerate water substitution while retaining an ordered cross-linked structure. Optimisation of a longer six-monomer fragment starting with a chain of alternating A and B hydrogen peroxide molecules results in a regular geometry with only one type of hydrogen bonding motif resembling the A-type found in the single crystal structure (Fig. 2i). This implies that the distances between carbonyl groups along the PVP chain tolerate an extended chain of H2O2 molecules of type A but are not appropriate for H2O2 AB type motifs. If AB type motifs are present in PEX then they would result in cross-linking across PVP chains instead of hydrogen bonding along a single polymer chain.
Calculated 13C NMR chemical shifts based on the optimised geometries of uncomplexed PVP tetramer and both types of PVP tetramer-hydrogen peroxide models (A-type or AB-type) gave carbonyl carbon atom chemical shifts of 173.7, 178.1 and 177.8 ppm, respectively in very good agreement with observed resonances 175.7 and 177.8 ppm for PVP-K25 and PEX K-30.
The crystallographic and DFT results suggest two potential structural models (Fig. 3). One possibility involves a structure in which the hydrogen peroxide molecules are in a single environment A-type motif forming a repeating chain along a single polymer strand. Alternatively, an AB-type motif may result in crosslinking between different polymer chains. The greater stability of H2O2 in the A-type site suggests that the single site model shown in Fig. 3a is more likely, although the cross-linking in the model shown in Fig. 3b is likely to result in greater crystallinity and greater water tolerance and the real PEX material may involve regions of both types of structure depending on crystallinity and water content. The experimental ratio of less than one H2O2 molecule per pyrrolidone unit in PEX can be explained by the occurrence of water defects as exemplified by the partial substitution of peroxide by water in the crystal structures of bisVP·nH2O2·mH2O.
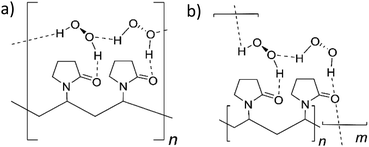 | ||
| Fig. 3 The two proposed structures of PEX (a) single type of hydrogen peroxide hydrogen bonded chain (A-type). (b) Hydrogen peroxide cross-linking between PVP molecules (AB-type). | ||
In summary, co-crystal structures of bisVP with varying amounts of hydrogen peroxide were used as model compounds for peroxydone to understand the bonding between PVP and hydrogen peroxide. FTIR data shows that this model system is closely comparable to peroxydone and in the ideal structure that the peroxydone carbonyl is hydrogen bonded to one hydrogen peroxide molecule. The H2O2 molecules in the co-crystal of bisVP·2H2O2 form AB dimeric pairs hydrogen bonding between carbonyl oxygen atoms. This structure undergoes selective replacement of bridging B-type hydrogen peroxide molecules with water at low peroxide concentration. DFT calculations show that a regular chain of hydrogen-bonded hydrogen peroxide molecules along the PVP chain is likely to be the most stable arrangement (Fig. 3a) but cross linking between PVP chains may also occur depending on crystallinity and the amount of water present.
We thank Ashland LLC and the Engineering and Physical Sciences Research Council for funding, through the Soft Matter and Functional Interfaces Centre for Doctoral Training. We thank Dr David Apperley for his assistance with SS NMR analysis.
Conflicts of interest
Ashland LLC is a commercial producer of peroxydone.Notes and references
- W. T. Hess, Kirk-Othmer Encyclopedia of Chemical Technology, Wiley, New York, 5th edn, 2000, vol. 13, pp. 470–485 Search PubMed.
- M. Sulieman, M. Addy, E. MacDonald and J. S. Rees, J. Dent., 2004, 32, 295–299 CrossRef CAS PubMed.
- M. V. Marshall, L. P. Cancro and S. L. Fischman, J. Periodontol., 1995, 66, 786–796 CrossRef CAS PubMed.
- H. Eimar, R. Siciliano, M.-N. Abdallah, S. A. Nader, W. M. Amin, P.-P. Martinez, A. Celemin, M. Cerruti and F. Tamimi, J. Dent., 2012, 40, e25–e33 CrossRef CAS.
- T. Jiang, Y. R. Guo, X. W. Feng, Y. Sa, X. Yang, M. Wang, P. Li and Y. N. Wang, J. Dent. Res., 2018, 97, 1339–1345 CrossRef CAS.
- J. H. Baxendale and J. A. Wilson, Trans. Faraday Soc., 1957, 53, 344–356 RSC.
- E. S. Shanley and F. P. Greenspan, Ind. Eng. Chem., 1947, 39, 1536–1543 CrossRef CAS.
- C.-S. Lu, E. W. Hughes and P. A. Giguère, J. Am. Chem. Soc., 1941, 63, 1507–1513 CrossRef CAS.
- C. M. Carey, J. Evidence-Based Complementary Altern. Med., 2014, 14, 70–76 CrossRef.
- A. C. Peixoto, S. C. Vaez, N. A. R. Pereira, C. N. D. S. Santana, K. D. A. Soares, A. C. T. R. Romão, L. F. Ferreira, P. R. S. Martins-Filho and A. L. Faria-E-Silva, J. Appl. Oral Sci., 2018, 26, e20170573 Search PubMed.
- ISP Investments LLC, US Pat., 5206385, 1993 Search PubMed.
- C. D. M. Bonesi, L. S. Ulian, P. Balem and V. W. Angeli, Braz. J. Pharm. Sci., 2011, 47, 719–724 CrossRef CAS.
- S. Okonogi, A. Kaewpinta, T. Rades, A. Müllertz, M. Yang, S. Khongkhunthian and P. Chaijareenont, Pharmaceuticals, 2020, 13, 381 CrossRef CAS PubMed.
- E. F. Panarin, K. K. Kalninsh and D. V. Pestov, Eur. Polym. J., 2001, 37, 375–379 CrossRef CAS.
- D. Modhave, B. Barrios and A. Paudel, Pharmaceutics, 2019, 11, 457 CrossRef CAS.
- G. K. Surya Prakash, A. Shakhmin, K. E. Glinton, S. Rao, T. Mathew and G. A. Olah, Green Chem., 2014, 16, 3616–3622 RSC.
- GAF Corp., US Pat., 3376110A, 1968 Search PubMed.
- GAF Corp., US Pat., 3480557, 1969 Search PubMed.
- ISP Investments LLC, WO Pat., 1992017158A1, 1992 Search PubMed.
- ISP Investments LLC, US Pat., 5008093, 1991 Search PubMed.
- ISP Investments LLC, US Pat., 8137658B2, 2012 Search PubMed.
- ISP Investments LLC, US Pat., 2007/0183988A1, 2007 Search PubMed.
- Colgate-Palmolive Company, US Pat., 2015/0366766A1, 2015 Search PubMed.
- ISP Investments LLC, US Pat., 9920146B2, 2018 Search PubMed.
- J. R. Davenport, O. M. Musa, M. J. Paterson, M.-O. M. Piepenbrock, K. Fucke and J. W. Steed, Chem. Commun., 2011, 47, 9891–9893 RSC.
- M. J. Goodwin, B. W. Steed, D. S. Yufit, O. M. Musa, D. J. Berry and J. W. Steed, Cryst. Growth Des., 2017, 17, 5552–5558 CrossRef CAS.
- M. J. Goodwin, O. M. Musa, D. J. Berry and J. W. Steed, Cryst. Growth Des., 2018, 18, 701–709 CrossRef CAS.
- A. Perrin, M. J. Goodwin, O. M. Musa, D. J. Berry, P. Corner, K. Edkins, D. S. Yufit and J. W. Steed, Cryst. Growth Des., 2017, 17, 3236–3249 CrossRef CAS.
- S. Fitzpatrick, J. F. McCabe, C. R. Petts and S. W. Booth, Int. J. Pharm., 2002, 246, 143–151 CrossRef CAS PubMed.
- A. Perrin, M. J. Goodwin, S. Callear, A. K. Soper, O. M. Musa and J. W. Steed, J. Phys. Chem. B, 2018, 122, 4901–4912 CrossRef CAS PubMed.
- P. V. Prikhodchenko, A. G. Medvedev, T. A. Tripol'skaya, A. V. Churakov, Y. Wolanov, J. A. K. Howard and O. Lev, CrystEngComm, 2011, 13, 2399–2407 RSC.
- J. M. Savariault and M. S. Lehmann, J. Am. Chem. Soc., 1980, 102, 1298–1303 CrossRef CAS.
Footnotes |
| † Dedicated to Prof. Peter C. Junk on the occasion of his 60th Birthday. |
| ‡ Electronic supplementary information (ESI) available: Sample preparation, IR and SS NMR spectra, analysis and X-ray crystallographic data. CCDC 2088109, 2088110 and 208811. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc06047c |
| This journal is © The Royal Society of Chemistry 2022 |