Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Prostate-specific membrane antigen (PSMA) targeted singlet oxygen delivery via endoperoxide tethered ligands†
Lei
Wang
,
Lei
Tang
,
Yingjie
Liu
,
Hao
Wu
,
Ziang
Liu
,
Jin
Li
,
Yue
Pan
* and
Engin U.
Akkaya
 *
*
State Key Laboratory of Fine Chemicals, Department of Pharmaceutical Science, Dalian University of Technology, 2 Linggong Road, Dalian, Liaoning 116024, China. E-mail: panyue0811@dlut.edu.cn; eua@dlut.edu.cn
First published on 14th January 2022
Abstract
Singlet oxygen is the primary agent responsible for the therapeutic effects of photodynamic therapy (PDT). In this work, we demonstrate that singlet oxygen release due to thermal endoperoxide cycloreversion can be targeted towards specific features of selected cancer cells, and this targeted singlet oxygen delivery can serve as an effective therapeutic tool. Thus, cytotoxic singlet oxygen can be delivered regioselectively into prostate specific membrane antigen (PSMA) overexpressing lymph node carcinoma (LNCaP) cells. However, unlike typical photodynamic processes, there is no need for light or oxygen. The potential of the approach is exciting, considering the limitations on the availability of light and oxygen in deep-seated tumors.
Singlet oxygen is a reactive oxygen species (ROS) which can be generated photochemically, chemically or enzymatically, both in vitro and in vivo.1 In aqueous solutions, the half-life of singlet oxygen is about 3.5 microseconds. It is believed to have a very short diffusion distance in vivo, as short as 10–20 nm, according to certain estimates.2 This short lifetime is mostly due to physical quenching with biomolecules,3 rather than water inside the cells. Thus, if the generation (or release) of singlet oxygen can be spatially controlled, a very precise therapeutic tool can be obtained.
In recent years, controlled release of singlet oxygen has attracted attention.4 Previously, we proposed4b,5 that chemically and biochemically triggered singlet oxygen release from endoperoxides may have important therapeutic implications (Targeted Singlet Oxygen Delivery, or TSOD) by essentially providing the benefits of PDT, without its limitations.
Prostate cancer (PCa) is the second-leading cause of cancer mortality in men.6 Prostate specific membrane antigen (PSMA) is a transmembrane protein and a metalloenzyme,7 which is highly expressed in the prostate, but is expressed up to 12-fold more in a cancerous prostate. PSMA levels correlate with the proliferation, migration, invasion, adhesion and survival characteristics of cancer cells.8 Thus, not surprisingly, this protein is targeted in a number of therapeutic schemes.9
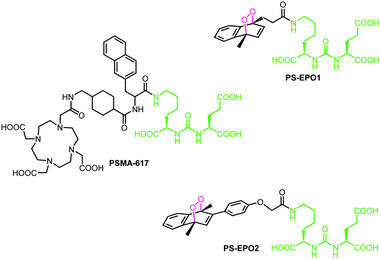
A large variety of ligands were tested for their affinity to PSMA.10 Typically, once the ligand binds to the target, the PSMA–ligand complex undergoes endocytosis and unloads the ligand, then it is recycled back to the membrane.11 Most of these ligands contain a particular unit abbreviated as KuE (lysine–urea–glutamate) which targets the active site of the protein. Among them, PSMA-617 was found to be highly promising, and making use of its chelator DOTA unit, the 177Lu3+ complex of PSMA-617 has undergone clinical tests against certain types of prostate cancers (Fig. 1).12
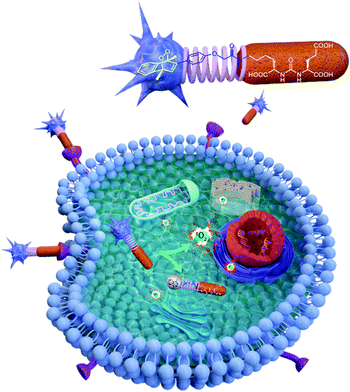
Here, in this work, we targeted a PCa associated protein, prostate specific membrane antigen (PSMA), with ligand–endoperoxide conjugates. The PSMA ligand (KuE) carries a naphthalene derived unit; thus, we surmised that the naphthalene endoperoxide structure to be substituted will only cause minimal structural changes, and thus not impact the affinity of the ligand for PSMA. The endocytosed ligand–endoperoxide conjugate would thermally release singlet oxygen into the cytosol of targeted cancer cells (Fig. 2).
With these considerations, naphthalene precursors of PS-EPO1 and PS-EPO2 (compounds 7 and 11, shown in Fig. 3) were synthesized from commercially available materials in good yields (see the ESI†). With these two precursors in hand, we synthesized the corresponding endoperoxides PS-EPO1 and PS-EPO2 by irradiation of compounds 7 and 11, respectively, in the presence of methylene blue using a 630 nm LED array in D2O. After completion of the reactions, photosensitizer methylene blue was then removed using a cation exchange resin.
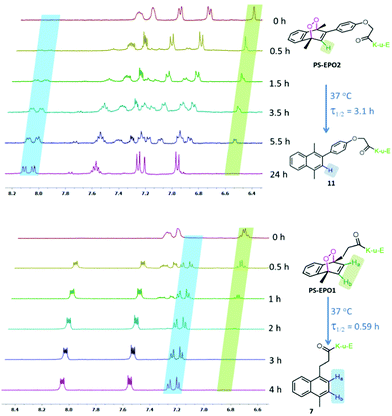 | ||
| Fig. 3 Temporal evolution of partial 1H NMR spectra of PS-EPO2 (top) and PS-EPO1 (bottom) in D2O at 37 °C. | ||
Our choice of the two structurally different naphthalene endoperoxides PS-EPO1 and PS-EPO2 was guided by an understanding of the effects of substitution on the rates of cycloreversion reactions.13 It was reported13b that phenyl substitution at the 2 position of the endoperoxide slows down the rate up to 10-fold compared to the parent compound (1,4-dimethyl-1,4-endoperoxide). The rate of singlet oxygen release may be a more important parameter than the total amount of singlet oxygen released, because of the dynamic nature of the cells, with many interlinked non-equilibrium processes.14
We studied the rate of cycloreversion of both compounds PS-EPO1 and PS-EPO2 using 1H NMR, and the results are shown in Fig. 3. The NMR spectra in the aromatic region have relatively isolated peaks, which allow us to follow the reaction. The NMR spectra show the cycloreversion of PS-EPO1. The peaks between 6.8 and 6.9 ppm are from the two protons in the endoperoxide ring (Ha and Hb) and the newly generated peaks between 7.2 and 7.3 ppm belong to its naphthalene precursor (7). This result clearly indicated that endoperoxide PS-EPO1 was converted to its naphthalene precursor 7 within 4 hours. A similar result was obtained when PS-EPO2 was tested by the same procedure. When incubated at 37 °C in D2O, the half-life of PS-EPO1 is 0.59 h, whereas PS-EPO2 has a half-life of 3.1 h. As expected, the endoperoxide with a phenyl substitution at the 2-position (PS-EPO2) has a longer half-life compared to PS-EPO1. NMR data also showed that cycloreversion to naphthalene precursors proceeds without any undesirable by-products, with molecular oxygen as the only other product.
In order to confirm that the release of oxygen is in the excited state (singlet oxygen) and at different rates with these two endoperoxides, we monitored the reaction using the singlet oxygen probe SOSG (Singlet Oxygen Sensor Green), following the spectral changes (Fig. 4). The cycloreversion rate differences are reflected in the rate of increase of fluorescence changes of SOSG in buffered solutions. While PS-EPO1 releases singlet oxygen much faster compared to PS-EPO2, the total amount of singlet oxygen trapped with the probe is comparable.
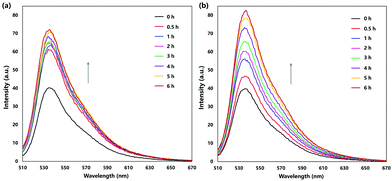 | ||
| Fig. 4 Thermal singlet oxygen release by (a) PS-EPO1 and (b) PS-EPO2 as monitored using the SOSG probe. Emission spectra were acquired by excitation at 488 nm. | ||
The binding affinities of the endoperoxides were determined by an ELISA (Fig. 5 and ESI†). As expected, both compounds showed strong PSMA affinity with equilibrium dissociation constants near 20 nM.
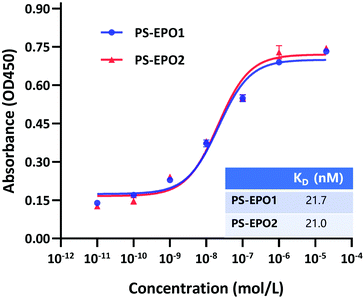 | ||
| Fig. 5 PSMA-based ELISA to determine the binding affinity of PS-EPO1 and PS-EPO2 for the PSMA protein. The KD data were calculated using GraphPad Prism. | ||
Before we tested the activity of the two PSMA-targeting endoperoxides, we wanted to demonstrate enhanced expression of PSMA in LNCaP cells compared to two other prostate cells with known lower expression of PSMA (PC3, DU145). This was confirmed by western blot analysis for the PSMA which showed larger PSMA expression in the cell extracts and membrane enriched extracts of LNCaP cells, as expected, compared to the other two prostate cancer cell lines (PC3 and DU145) (Fig. 6). β-Actin is the internal reference protein. In addition, we carried out immunostaining imaging, showing the expression levels of PMSA (ESI,† Fig. S17).
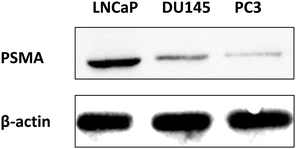 | ||
| Fig. 6 Western blot analysis for detection of PSMA expression in LNCaP, DU145, and PC3 cell membranes, in comparison to internal reference β-actin. | ||
MTT assays of cell viability also produce results confirming the validity of our approach. First, the endoperoxide precursor compounds 7 and 11 show negligible cytotoxicity towards all three cell lines tested. However, endoperoxide PS-EPO1 and especially PS-EPO2 discriminate between PSMA overexpressing LNCaP cell lines and the others (Fig. 7 and ESI†). As expected, the cytotoxicity is more pronounced towards LNCaP, and the difference in cell death is 20% more than PC3 cells. Among the endoperoxides PS-EPO1 and PS-EPO2, the latter compound seems to be more effective, possibly due to its longer half-life. The IC50 of PS-EPO2 was determined to be 60 μM with LNCaP cells, whereas for PC3 cells it is larger than 200 μM.
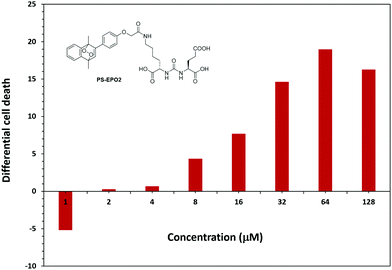 | ||
| Fig. 7 Percentage cell death difference (LNCaP-PC3) due to PS-EPO2 as determined by MTT assays with LNCaP and PC3. The cell death is larger in LNCaP cells except for the 1 μM data point. | ||
In order to eliminate any inhibitory effects as part of the PSMA mediated toxicity, we compared the activities of the precursor naphthalene compound 11 and PS-EPO2 (Fig. 8). For all cell types, cytotoxicity is mostly due to singlet oxygen, but the singlet oxygen toxicity is the most effective for PSMA overexpressing LNCaP cells.
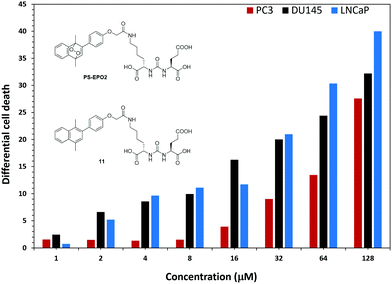 | ||
| Fig. 8 The difference in cell death as determined by MTT assays between the endoperoxide compound PS-EPO2 and the precursor naphthalene 11 at the indicated concentrations. | ||
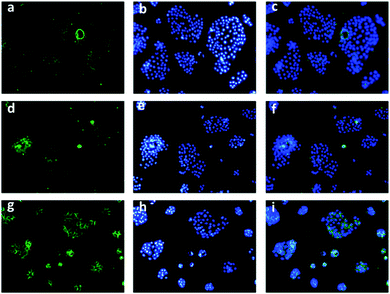
To track the generation of singlet oxygen in living cells, an imaging experiment was conducted with a commonly used ROS indicator, 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), by CLSM. When PC3 cells which express lower PSMA were treated with DCFH-DA and PS-EPO2 accordingly, weak fluorescence emission was detected, indicating the existence of endogenous singlet oxygen (Fig. 9). Inspired by this result, DU145 and LNCaP cells were tested according to the same procedure. As expected, LNCaP cells with enhanced expression of PSMA showed significant green fluorescence compared to the other two cell lines. It was indicated that a larger amount of singlet oxygen is trapped by the probe when the LNCaP cells are incubated with PS-EPO2 compared to the other cell types. This is in accordance with our expectations of enhanced PSMA-mediated endocytosis for the singlet oxygen releasing compound.
Also, in the absence of endoperoxides, all three cell types showed no or hardly discernable emission following ROS probe incubation (ESI,† Fig. S24), further confirming that the sources of the ROS (singlet oxygen) are the endoperoxide compounds.
In conclusion, we developed a PSMA-targeted singlet oxygen delivery system. The endoperoxide modified ligand shows good inhibition towards PSMA overexpressing LNCaP cells. Comprehensive design of endoperoxide structures allows us to control the release ratio of singlet oxygen and it is clear that the rate of singlet oxygen delivery and the exact location of the singlet oxygen release can be important parameters in optimizing the effectiveness of the proposed method. We demonstrated that Targeted Singlet Oxygen Delivery (TSOD) can be an effective tool for cancer therapy, and our work in establishing this approach is in progress.
The authors acknowledge financial support from the LiaoNing Revitalization Talents Program (E. U. A.: XLYC1902001, L. W.: XLYC1907021) and the National Natural Science Foundation of China (L. W.: 22007008, Y. P.: 81803024).
Conflicts of interest
There are no conflicts to declare.Notes and references
- Y. You, Org. Biomol. Chem., 2018, 16, 4044–4060 RSC.
- (a) P. Di Mascio, G. R. Martinez, S. Miyamoto, G. E. Ronsein, M. H. G. Medeiros and J. Cadet, Chem. Rev., 2019, 119, 2043–2086 CrossRef CAS PubMed; (b) P. Ogilby, Photochem. Photobiol. Sci., 2010, 9, 1543–1560 CrossRef CAS PubMed; (c) M. Bregnhøj, M. Westberg, F. Jensen and P. R. Ogilby, Phys. Chem. Chem. Phys., 2016, 18, 22946–22961 RSC.
- A. Baker and J. R. Kanofsky, Photochem. Photobiol., 1992, 55, 523–528 CrossRef CAS PubMed.
- (a) W. Fudickar and T. Linker, ChemPhotoChem, 2018, 2, 548–558 CrossRef CAS; (b) E. Ucar, D. Xi, O. Seven, C. Kaya, X. J. Peng, W. Sun and E. U. Akkaya, Chem. Commun., 2019, 55, 13808–13811 RSC; (c) M. Qu, N. Wu, W. Q. Jiang, L. Wang, M. S. Akkaya and E. U. Akkaya, RSC Adv., 2021, 11, 19083–19087 RSC.
- (a) S. Kolemen, T. Ozdemir, D. Lee, G. M. Kim, T. Karatas, J. Yoon and E. U. Akkaya, Angew. Chem., Int. Ed., 2016, 55, 3606–3610 CrossRef CAS PubMed; (b) I. S. Turan, D. Yildiz, A. Turksoy, G. Gunaydin and E. U. Akkaya, Angew. Chem., Int. Ed., 2016, 55, 2875–2878 CrossRef CAS PubMed; (c) S. Ayan, G. Gunaydin, N. Yesilgul-Mehmetcik, M. E. Gedik, O. Seven and E. U. Akkaya, Chem. Commun., 2020, 55, 14793–14796 RSC.
- F. Bray, J. Ferlay, I. Soerjomataram, R. L. Siegel, L. A. Torre and A. Jemal, CA-Cancer J. Clin., 2018, 68, 394–424 CrossRef PubMed.
- R. G. Lapidus, C. W. Tiffany, J. T. Isaacs and B. S. Slusher, Prostate, 2000, 45, 350–354 CrossRef CAS PubMed.
- (a) Y. Zhang, Z. Guo, T. Du, J. Chen, W. Wang, K. Xu, T. Lin and H. Huang, Prostate, 2013, 73, 835–841 CrossRef CAS PubMed; (b) J. S. Ross, C. E. Sheehan, H. A. G. Fisher, R. P. Kaufman Jr., P. Kaur, K. Gray, I. Webb, G. S. Gray, R. Mosher and B. V. S. Kallakury, Clin. Cancer Res., 2003, 9, 6357–6362 CAS.
- (a) A. P. Kiess, S. R. Banerjee, R. C. Mease, S. P. Rowe, A. Rao, C. A. Foss, Y. Chen, X. Yang, S. Y. Cho, S. Nimmagadda and M. G. Pomper, Q. J. Nucl. Med. Mol. Imaging, 2019, 59, 241–268 Search PubMed; (b) A. Cimadamore, M. Cheng, M. Santoni, A. Lopez-Beltran, N. Battelli, F. Massari, A. B. Galosi, M. Scarpelli and R. Montironi, Front. Oncol., 2018, 8, 653 CrossRef PubMed.
- M. Eiber, W. P. Fendler, S. P. Rowe, J. Calais, M. S. Hofman, T. Maurer, S. M. Schwarzenboeck, C. Kratowchil, K. Herrmann and F. L. Giesel, J. Nucl. Med., 2017, 58, 67S–76S CrossRef CAS PubMed.
- (a) J. Roy, T. X. Nguyen, A. K. Kanduluru, C. Venkatesh, W. Lv, P. V. Reddy, P. S. Low and M. Cushman, J. Med. Chem., 2015, 58, 3094–3103 CrossRef CAS PubMed; (b) H. Liu, A. K. Rajasekaran, P. Moy, Y. Xia, S. Kim, V. Navarro, R. Rahmati and N. H. Bander, Cancer Res., 1998, 58, 2674–2681 Search PubMed.
- M. J. Morris, J. S. De Bono, K. N. Chi, K. Fizazi, K. Herrmann, K. Rahbar, S. T. Tagawa, L. T. Nordquist, N. Vaishampayan, G. El-Haddad, C. H. Park, T. M. Beer, W. J. Pérez-Contreras, M. Desilvio, E. E. Kpamegan, G. Gericke, R. A. Messmann, B. J. Krause and A. O. Sartor, J. Clin. Oncol., 2021, 39, LBA4–LBA4 Search PubMed.
- (a) H. H. Wasserman, K. B. Wiberg, D. L. Larsen and J. Parr, J. Org. Chem., 2005, 70, 105–109 CrossRef CAS PubMed; (b) M. Klapper and T. Linker, Chem. – Eur. J., 2015, 21, 8569–8577 CrossRef PubMed.
- S. Ornes, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 423–424 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data of compounds, copies of NMR spectra for the synthesized compounds and biological data. See DOI: 10.1039/d1cc05810j |
| This journal is © The Royal Society of Chemistry 2022 |