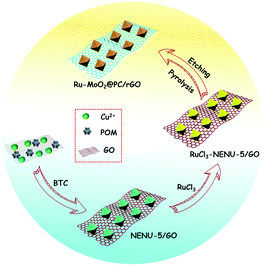
MOF-derived ruthenium-doped amorphous molybdenum dioxide hybrid for highly efficient hydrogen evolution reaction in alkaline media†
Ji-Yuan
Han
,
Sheng-Hao
Cai
,
Ji-Yu
Zhu
,
Shuang
Yang
and
Ji-Sen
Li
 *
*
Department of Chemistry and Chemical Engineering, Jining University, Shandong 273155, China. E-mail: senjili@sina.com
First published on 29th November 2021
Abstract
Ruthenium-doped amorphous molybdenum dioxide coupled with a reduced graphene oxide hybrid (Ru-MoO2@PC/rGO) is synthesized using polyoxometalate-based MOFs/GO as a precursor. Benefitting from the synergistic effect of numerous exposed active sites, Ru dopants and the introduction of GO, the designed catalyst shows exceptional electrocatalytic performance toward the HER in alkaline media.
To fulfill the sustainable development needs of society, exploring clean and renewable energies, which can overcome the energy crisis and environmental issues, is of great significance. Hydrogen with eco-friendly characteristics and zero-carbon emissions is considered as an ideal alternative to traditional fossil fuels.1 Electrochemical water splitting has been recognized as one of the technologies with the most potential for hydrogen generation.2 In particular, alkaline electrolysis is a technically feasible and well developed technology for the industrial production of hydrogen.3 Nevertheless, the reaction kinetics for the alkaline hydrogen evolution reaction (HER) are much more sluggish than those under acidic conditions.4 To overcome this dilemma, the construction of highly effective and stable HER catalysts in alkaline solutions is necessary, but remains a challenge. Currently, although Pt-based compounds are the benchmark electrocatalysts toward the HER, their scarcity and high cost severely hamper their large-scale commercial application.5 As a result, it is highly desirable to develop effective HER electrocatalysts with lower precious metal loading or using non-noble metals.
Recently, transition metal oxide (TMO) electrocatalysts have been widely explored due to their abundance and diverse crystal structures.6,7 Among them, molybdenum dioxide (MoO2), which possesses high electrical conductivity, rich active sites and remarkable durability, has served as a promising non-precious metal catalyst toward water electrolysis.8–11 Nonetheless, MoO2 nanoparticles usually suffer from inevitable aggregation during heating treatment, which can lead to limited active sites and eventually a dramatic decrease of catalytic performance. In addition, few reported MoO2-based nanomaterials show HER electroactivity competitive with that of Pt-based catalysts, especially in alkaline media.12,13 To this end, enormous effort has been dedicated to boosting the catalytic performance of metal oxides by various approaches,8,16 for instance, phase engineering, metal doping and so on. As reported, the phase engineering of catalysts plays a pivotal role in the improvement of electrocatalytic behavior. In comparison with their crystalline counterpart, amorphous TMOs can provide abundant active sites, unsaturated electronic structure and reassembly capacity, which are conducive to electrocatalysis.14,15 Regrettably, their electrical conductivity is markedly decreased. To compensate the demerits, coupling amorphous TMOs with conductive substrates, such as carbon cloth,16 graphene oxide (GO)17 and so on,11,18 is proposed as an effective strategy. As far as we know, amorphous TMO-based catalysts for the HER have been rarely reported to date.19 Besides, metal doping by introducing second metals into TMOs is also identified as a viable approach to modulate the intrinsic electronic structure of electrocatalysts.11,20 In this regard, ruthenium (Ru), as the cheapest Pt-group metal, holds great promise for the HER, benefitting from Pt-like Ru–H bonding strength.21–28
Metal–organic frameworks (MOFs), as a kind of porous material, have been actively investigated in recent years.29,30 Particularly, polyoxometalate (POM)-based MOFs (POMOFs), integrating the merits of POMs and MOFs, have drawn immense attention and are helpful in the in situ synthesis of highly dispersed Mo (W, V)-based materials through carbonization treatment.31,32 On the other hand, porous carbon derived from organic ligands of MOFs can not only effectively prevent metal nanoparticles from agglomerating and corroding, but also promote electron/mass transfer, further enhancing catalytic performance. By virtue of the above-mentioned points, if Ru-doped POMOFs are elaborately fabricated, they are expected to be employed as precursors to synthesize porous carbon supported Ru-doped Mo (W, V)-based oxides.
Herein, we report a design of a novel hybrid, namely Ru-doped amorphous MoO2 embedded in porous carbon supported on reduced graphene oxide (Ru-MoO2@PC/rGO) by a feasible multi-step strategy (Scheme 1). The resultant nanocomposite combines several attractive merits such as enriched active sites, high intrinsic catalytic activity and prominent conductivity. As expected, when evaluated as a catalyst for the HER in an alkaline electrolyte, the designed Ru-MoO2@PC/rGO exhibits extraordinary electrochemical activity with a lower overpotential (126 mV at 10 mA cm−2) and a smaller Tafel slope (43.5 mV dec−1), as well as long-term stability, even outperforming the commercial Pt/C catalyst.
According to previous literature,12,23 the synthesized RuCl3-NENU-5/GO precursor was characterized by scanning electron microscopy (SEM) and powder X-ray diffraction (PXRD), shown in Fig. S1 (ESI†). For comparison, NENU-5, RuCl3-NENU-5 and NENU-5/GO were also synthesized by a similar method, and their structures and morphologies were measured by PXRD and SEM, respectively (Fig. S2–S5, ESI†).
As revealed in Fig. S6a (ESI†), the PXRD pattern of Ru-MoO2@PC/rGO exhibits a broad and weak peak at approximately 25° which is ascribed to the carbon substrate. Unexpectedly, no characteristic peaks of Ru and MoO2 species can be distinguished, implying that the crystallinity is much poorer.18 In Fig. S6b (ESI†), the resulting Ru-MoO2@PC/rGO presents a typical IV isotherm with a hysteresis loop, suggesting the formation of rich mesopores4 on the basis of the Brunauer–Emmett range of 2–30 nm (inset of Fig. S6b, ESI†). Such porous architecture can endow the designed sample with a larger accessible surface area, numerous exposed active sites and faster reaction rate and gas diffusion, further boosting the electrocatalytic behavior.20
In Fig. 1a and b, the SEM and transmission electron microscopy (TEM) images depict that the as-prepared Ru-MoO2@PC/rGO retains the morphological features. Of note, it is clearly observed that Ru-MoO2@PC/rGO possesses hollow nanostructures with loading nanoparticles (Fig. 1c), which are favorable to promoting gas release and electrolyte penetration.8,12 As exhibited in the high-resolution TEM (HRTEM) image (Fig. 1d), lots of highly dispersed ultrafine nanoparticles are encapsulated by the carbon layer. Surprisingly, no distinct lattice fringes could be intuitively discerned. The selected-area electron diffraction (SAED) image, as shown in the inset of Fig. 1d, displays a broad and diffuse halo-like ring, undoubtedly validating the amorphous nature of Ru-MoO2@PC/rGO,10 which is consistent with the results of the PXRD analysis. From Fig. 1e, the STEM and corresponding element mapping images reflect the uniform distribution of C, Mo, O, Ru and P on the designed Ru-MoO2@PC/rGO and this can be corroborated by energy dispersive X-ray spectroscopy (EDS, Fig. S7, ESI†).
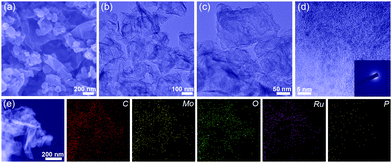 | ||
| Fig. 1 (a) SEM, (b and c) TEM and (d) HRTEM images and (e) STEM and corresponding element mapping images of Ru-MoO2@PC/rGO for C, Mo, O, Ru and P. Inset of (d) is a SAED pattern. | ||
In addition, MoO2@PC, MoO2@PC/rGO, and Ru-MoO2@PC were also synthesized under analogous experimental conditions for comparison. At the same time, the corresponding morphological and structural characteristics were also analyzed by SEM and PXRD in detail, shown in Fig. S8–S11 (ESI†). Interestingly, the amorphous Ru-MoO2 hybrid could be prepared when a RuCl3 solution was introduced. By contrast, without adding RuCl3, the resultant samples were metallic MoO2 nanocomposites. Accordingly, it is reasonable to conclude that the existence of RuCl3 is crucial for the formation of the amorphous hybrid.
For the Mo 3d XPS spectrum in Fig. 2a, two doublets could be observed, assigned to Mo4+ (228.4/231.9 eV) and Mo6+ (232.7/235.8 eV), originating from the inevitable surface oxidation.8,12 The Ru 3p spectrum in Fig. 2b exhibits two peaks at 463.3/485.6 eV, which can be attributed to Ru 3p3/2 and Ru 3p1/2 of Ru0.22 In the P 2p spectrum (Fig. 2c), the peak centered at 133.4 eV is ascribed to P–C, implying the successful integration of P into the carbon matrix. On the other hand, the signal at 134.2 eV belongs to a phosphate species, which confirms that partial oxidation occurs on the surface of Ru-MoO2@PC/rGO.8Fig. 2d shows the O 1s XPS spectrum, where the binding energies at 530.7, 531.8 and 533.3 eV are attributed to O–Mo, O–H and adsorbed H2O, respectively.5,13
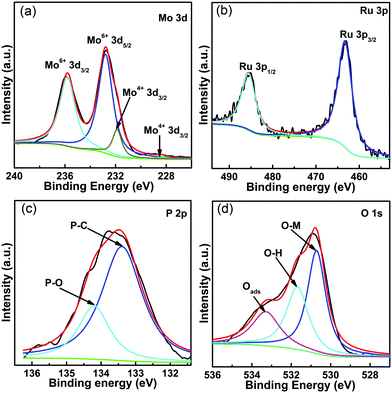 | ||
| Fig. 2 (a–d) High-resolution spectra of Mo 3d, Ru 3p, P 2p and O 1s of Ru-MoO2@PC/rGO, respectively. | ||
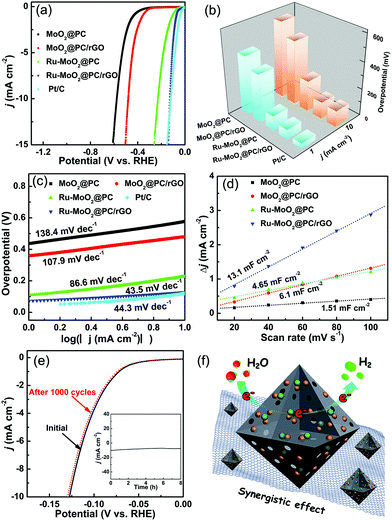
The electrochemical HER performances of the designed products were examined in Ar-saturated 1.0 M KOH solution utilizing a representative three-electrode apparatus. For comparison, commercial Pt/C (20 wt%) was also evaluated. Fig. 3a exhibits the linear sweep voltammetry curves (LSVs) of the samples with 95% iR compensation. As expected, commercial Pt/C possesses outstanding catalytic performance. The resultant Ru-MoO2@PC/rGO displays substantially improved catalytic activity with overpotentials of 74 and 126 mV to derive current densities of 1 and 10 mA cm−2 (Fig. 3a and b), respectively, which are lower than those of MoO2@PC (439 and 576 mV), MoO2@PC/rGO (359 and 479 mV), and Ru-MoO2@PC (111 and 230 mV). Impressively, when the current density is beyond 12 mA cm−2, the designed Ru-MoO2@PC/rGO even exceeds the commercial Pt/C catalyst, evidencing that the hybrid has superior Pt-like electroactivity for the HER in an alkaline electrolyte. Additionally, the influence of the amount of GO on the catalytic activity was examined simultaneously (Fig. S12, ESI†). To assess the reaction kinetics for the HER, Tafel plots were obtained from the corresponding LSVs (Fig. 3c). Strikingly, the designed Ru-MoO2@PC/rGO reveals a Tafel slope of merely 43.5 mV dec−1, which is slightly lower than that of commercial Pt/C (44.3 mV dec−1), implying that the efficient HER pathway on the Ru-MoO2@PC/rGO surface follows the Volmer–Heyrovsky mechanism (Note S1, ESI†).8,10 In sharp contrast, the other calculated Tafel slopes increase in the trend of Ru-MoO2@PC (86.6 mV dec−1) < MoO2@PC/rGO (107.9 mV dec−1) < MoO2@PC (138.4 mV dec−1). Such an extraordinary electrocatalytic activity of Ru-MoO2@PC/rGO is comparable, or even superior, to most efficient alkaline HER catalysts reported so far (Table S1, ESI†).3,8,9 As a consequence, this result strongly manifests that the synergistic contribution of the amorphous porous structure, metal doping and highly conductive substrate in Ru-MoO2@PC/rGO can effectively expedite the electrochemical performance toward the HER. Additionally, the electrochemical active surface area (ECSA) of the as-synthesized samples can be measured according to the double layer capacitance (Cdl),5,21 which is achieved by cyclic voltammetry (CV) technology at different scan rates (Fig. S13, ESI†). As shown in Fig. 3d, the Cdl value of Ru-MoO2@PC/rGO is determined to be 13.1 mF cm−2, which is about 2.2, 3.1 and 8.7 times higher than those of MoO2@PC/rGO, Ru-MoO2@PC and MoO2@PC, respectively. This unambiguously proves that the designed Ru-MoO2@PC/rGO enables more accessible active sites to be exposed and this is responsible for the enhancement of electrocatalytic HER behavior. Furthermore, the current density of the resultant catalyst was normalized by its Cdl (Fig. S14, ESI†). Remarkably, Ru-MoO2@PC/rGO still presents the best catalytic performance toward the HER in comparison with contrast catalysts, indicative of the highest intrinsic activity. Apart from outstanding catalytic activity, the durability of an electrocatalyst is also important for the practical application of water splitting. As depicted in Fig. 3e, it is found that the LSV curve after 1000 cycles could almost overlap the initial one, signifying its prominent stability. The inset of Fig. 3e exhibits that the time-dependent current density (i–t) measurement was carried out at a constant overpotential of 126 mV. During continuous operation for 10 h, Ru-MoO2@PC/rGO can still retain 82% of its initial current density, reconfirming the robust durability toward the HER (Fig. 3e, inset). Remarkably, Ru-MoO2@PC/rGO still shows excellent stability even at a high current density of 150 mA cm−2 (Fig. S15, ESI†). Moreover, the SEM, TEM, PXRD and XPS results after the stability test powerfully attest the durable nature of the as-fabricated Ru-MoO2@PC/rGO (Fig. S16 and S17, ESI†). Therefore, both the electrocatalytic properties and structural characterization strongly testify that Ru-MoO2@PC/rGO shows excellent electrocatalytic performance and long-term stability toward catalyzing alkaline hydrogen production. Considering the aforementioned results, the exceptional electrocatalytic HER performance can be attributed to the combined effect of the following factors, as elucidated in Fig. 3f. (1) Compared to crystalline counterparts, the Ru-doped amorphous MoO2 hybrid can provide higher intrinsic activity and more accessible active centers, thereby leading to enhanced catalytic performance.14 (2) As is well-known, Ru is regarded as an ideal HER catalyst owing to its Pt-like activity.23 The introduction of Ru may regulate the electronic structure of MoO2 to improve the electrical conduction, as supported by the density functional theory (DFT) calculations.6,8,9,22,27,28 (3) Numerous hierarchical pores are expected to maximize the contact with active sites and facilitate mass/charge transfer during the catalytic process. Meanwhile, the confinement effect of the porous structure is helpful to prevent nanoparticles from aggregating throughout thermal treatment.10 (4) The P-dopant can produce plenty of active centers, further resulting in remarkable enhancement of the catalytic performance for the HER.21 In parallel, the rGO support not only offers a larger surface area to immobilize nanoparticles, but greatly promotes the electrical conductivity.12 All in all, the combination of these reasons is responsible for the superior catalytic performance toward the HER in basic media, which is also supported by the related DFT calculations (Fig. S18 and S19, ESI†).
In summary, for the first time, we have successfully fabricated a novel Ru-doped amorphous MoO2 catalyst utilizing Ru-POMOFs/GO as a precursor. The elaborate design can expose numerous active sites, boost intrinsic catalytic activity and accelerate charge/mass transfer during the electrolytic process. Owing to the advantages of the unique composition and structure, the resultant catalyst shows unprecedented electrochemical activity and robust cycle stability for the HER in alkaline conditions, ranking among the best HER electrocatalysts reported to date. More importantly, this work not only offers a highly efficient catalyst toward HER, but also presents a new concept in rationally designing and exploring high-performance catalysts for energy conversion and storage.
This work was financially supported by the National Natural Science Foundation of China (No. 21971086) and the Natural Science Foundation of Shandong Province (No. ZR2019MB013).
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. A. Turner, Science, 2004, 305, 972–974 CrossRef CAS PubMed.
- J.-S. Li, S. Zhang, J.-Q. Sha, H. Wang, M.-Z. Liu, L.-X. Kong and G.-D. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 17140–17146 CrossRef CAS.
- L. Wang, J. Fan, Y. Liu, M. Chen, Y. Lin, H. Bi, B. Liu, N. Shi, D. Xu, J. Bao and M. Han, Adv. Funct. Mater., 2021, 31, 2010912 CrossRef CAS.
- Y. Jin, H. Wang, J. Li, X. Yue, Y. Han, P. K. Shen and Y. Cui, Adv. Mater., 2016, 28, 3785–3790 CrossRef CAS PubMed.
- P. Zhai, M. Xia, Y. Wu, G. Zhang, J. Gao, B. Zhang, S. Cao, Y. Zhang, Z. Li, Z. Fan, C. Wang, X. Zhang, J. T. Miller, L. Sun and J. Hou, Nat. Commun., 2021, 12, 4587 CrossRef CAS PubMed.
- Y. Zhu, Q. Lin, Y. Zhong, H. A. Tahini, Z. Shao and H. Wang, Energy Environ. Sci., 2020, 13, 3361–3392 RSC.
- T. Soltani, X. Zhu, A. Yamamoto, S. P. Singh, E. Fudo, A. Tanaka, H. Kominami and H. Yoshida, Appl. Catal., B, 2021, 286, 119899 CrossRef CAS.
- G. Yang, Y. Jiao, H. Yan, Y. Xie, A. Wu, X. Dong, D. Guo, C. Tian and H. Fu, Adv. Mater., 2020, 32, 2000455 CrossRef CAS.
- F. Gong, M. Liu, S. Ye, L. Gong, G. Zeng, L. Xu, X. Zhang, Y. Zhang, L. Zhou, S. Fang and J. Liu, Adv. Funct. Mater., 2021, 31, 2101715 CrossRef CAS.
- D. Wu, D. Chen, J. Zhu and S. Mu, Small, 2021, 17, 2102777 CrossRef CAS.
- H. Zeng, S. Chen, Y. Q. Jin, J. Li, J. Song, Z. Le, G. Liang, H. Zhang, F. Xie, J. Chen, Y. Jin, X. Chen and H. Meng, ACS Energy Lett., 2020, 5, 1908–1915 CrossRef CAS.
- Y.-J. Tang, M.-R. Gao, C.-H. Liu, S.-L. Li, H.-L. Jiang, Y.-Q. Lan, M. Han and S.-H. Yu, Angew. Chem., Int. Ed., 2015, 54, 12928–12932 CrossRef CAS.
- P. Jiang, Y. Yang, R. Shi, G. Xia, J. Chen, J. Su and Q. Chen, J. Mater. Chem. A, 2017, 5, 5475–5485 RSC.
- S. Anantharaj and S. Noda, Small, 2020, 16, e1905779 CrossRef PubMed.
- C. Guo, Y. Shi, S. Lu, Y. Yu and B. Zhang, Chin. J. Catal., 2021, 42, 1287–1296 CrossRef CAS.
- Q. Li, Z. Xing, D. Wang, X. Sun and X. Yang, ACS Catal., 2016, 6, 2797–2801 CrossRef CAS.
- A. K. Sharma, H. Joshi, K. Ojha and A. K. Singh, Chem. Commun., 2019, 55, 2186–2189 RSC.
- Z. Dong, F. Lin, Y. Yao and L. Jiao, Adv. Energy Mater., 2019, 9, 1902703 CrossRef CAS.
- J. Xu, C. Zhang, H. Liu, J. Sun, R. Xie, Y. Qiu, F. Lü, Y. Liu, L. Zhuo, X. Liu and J. Luo, Nano Energy, 2020, 70, 104529 CrossRef CAS.
- J. Su, R. Ge, K. Jiang, Y. Dong, F. Hao, Z. Tian, G. Chen and L. Chen, Adv. Mater., 2018, 30, 1801351 CrossRef PubMed.
- J.-S. Li, M.-J. Huang, Y.-W. Zhou, X.-N. Chen, S. Yang, J.-Y. Zhu, G.-D. Liu, L.-J. Ma, S.-H. Cai and J.-Y. Han, J. Mater. Chem. A, 2021, 9, 12276–12282 RSC.
- D. Chen, R. Lu, Z. Pu, J. Zhu, H.-W. Li, F. Liu, S. Hu, X. Luo, J. Wu, Y. Zhao and S. Mu, Appl. Catal., B, 2020, 279, 119396 CrossRef CAS.
- M. Yang, L. Jiao, H. Dong, L. Zhou, C. Teng, D. Yan, T.-N. Ye, X. Chen, Y. Liu and H.-L. Jiang, Sci. Bull., 2021, 66, 257–264 CrossRef CAS.
- J. Yang, B. Chen, X. Liu, W. Liu, Z. Li, J. Dong, W. Chen, W. Yan, T. Yao, X. Duan, Y. Wu and Y. Li, Angew. Chem., Int. Ed., 2018, 57, 9495–9500 CrossRef CAS.
- F. Li, G.-F. Han, H.-J. Noh, I. Ahmad, I.-Y. Jeon and J.-B. Baek, Adv. Mater., 2018, 30, 1803676 CrossRef.
- A. Salah, H.-D. Ren, N. Al-Ansi, F.-Y. Yu, Z.-L. Lang, H. Tan and Y.-G. Li, J. Mater. Chem. A, 2021, 9, 20518–20529 RSC.
- L. Zhang, Z.-L. Lang, Y.-H. Wang, H. Tan, H.-Y. Zang, Z. Kang and Y.-G. Li, Energy Environ. Sci., 2019, 12, 2569–2580 RSC.
- G. Chen, T. Wang, J. Zhang, P. Liu, H. Sun, X. Zhuang, M. Chen and X. Feng, Adv. Mater., 2018, 30, 1706279 CrossRef.
- X. F. Lu, Y. Fang, D. Luan and X. W. D. Lou, Nano Lett., 2021, 21, 1555–1565 CrossRef CAS PubMed.
- Z. Liang, T. Qiu, S. Gao, R. Zhong and R. Zou, Adv. Energy Mater., 2018, 30, 2003410 Search PubMed.
- Y. Zhang, J. Liu, S.-L. Li, Z.-M. Su and Y.-Q. Lan, Energy Chem., 2019, 1, 100021 CrossRef.
- C. Zhang, P. Wang, W. Li, Z. Zhang, J. Zhu, Z. Pu, Y. Zhao and S. Mu, J. Mater. Chem. A, 2020, 8, 19348–19356 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cc05683b |
| This journal is © The Royal Society of Chemistry 2022 |