Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pd-Catalysed oxidative carbonylation of α-amino amides to hydantoins under mild conditions†‡
Aleksandr
Voronov
a,
Vinayak
Botla
a,
Luca
Montanari
a,
Carla
Carfagna
 b,
Raffaella
Mancuso
c,
Bartolo
Gabriele
b,
Raffaella
Mancuso
c,
Bartolo
Gabriele
 c,
Giovanni
Maestri
c,
Giovanni
Maestri
 ad,
Elena
Motti
ad,
Elena
Motti
 ad and
Nicola
Della Ca
ad and
Nicola
Della Ca
 *ad
*ad
aDepartment of Chemistry, Life Sciences and Environmental Sustainability (SCVSA), University of Parma, Parco Area delle Scienze, 17/A, Parma 43124, Italy. E-mail: nicola.dellaca@unipr.it
bDepartment of Industrial Chemistry “T. Montanari”, University of Bologna, Bologna 40136, Italy
cLaboratory of Industrial and Synthetic Organic Chemistry (LISOC), Department of Chemistry and Chemical Technologies, University of Calabria, Via P. Bucci 12/C, Arcavacata di Rende 87036, Cosenza, Italy
dCIRCC (Interuniversity Consortium Chemical Reactivity and Catalysis), via Celso Ulpiani 27, Bari 70126, Italy
First published on 9th December 2021
Abstract
The first example of palladium-catalysed oxidative carbonylation of unprotected α-amino amides to hydantoins is described here. The selective synthesis of the target compounds was achieved under mild conditions (1 atm of CO), without ligands and bases. The catalytic system overrode the common reaction pathway that usually leads instead to the formation of symmetrical ureas.
Imidazolidine-2,4-dione (hydantoin) is a privileged structural unit in natural products and pharmaceuticals,1 such as Phenytoin, Ethotoin and Enzalutamide.2 Hydantoins display an array of utilities that include their usage as ligands,3 directing groups,4 organocatalysts,5 intermediates in organic synthesis,6 and functional moieties in specialty polymers.7 Traditional routes to this scaffold include the Bucherer–Bergs reaction,8 the Urech9 and the Read10 syntheses, and the Biltz reaction.11 Enantiopure hydantoins can provide additional key properties, which can be exploited in medicinal chemistry12 and organic chemistry.13 Besides the well-established Urech and Read syntheses, the most common strategy to chiral imidazolidine-2,4-diones relies on the use of optically pure building blocks.14 In this context, α-amino acid derivatives are converted into the corresponding ureido compounds and then annulated by reacting with isocyanates or other unfriendly acylating agents (i.e. phosgene, triphosgene and 1,1-carbonyldiimidazole (CDI)) and an amine.1a These stoichiometric strategies, despite their efficiency, suffer from limited atom-economy, and low generality and functional group tolerance. Alternatively, the optically enriched hydantoins can be accessed by elegant enantioselective transformations.15 Their general applicability is however weakened by the high cost of these catalytic systems, the limited availability of reagents or low enantioselectivity values. Therefore, catalytic protocols for the synthesis of chiral hydantoins featuring improved efficiency, atom-economy and versatility are still in high demand.16
Catalytic carbonylative approaches to hydantoins are highly attractive because they merge all of the above-mentioned issues. However, only a few examples have been reported so far.17 In 1994, Beller and co-workers reported a simple but remarkable synthesis of hydantoins by palladium-catalysed high-pressure carbonylation of aldehydes with urea derivatives (Scheme 1a).17a More recently, McElwee-White et al. reported the first example of carbonylation of primary amino amides to hydantoins by means of the W(CO)6 catalyst (Scheme 1b).17b Meanwhile, a high pressure of CO (80 atm) and a stoichiometric amount of the base (DBU) were essential. This is a unique example of the catalytic carbonylative synthesis of hydantoins from amino amides. This challenging goal is indeed usually inaccessible because the palladium catalysed carbonylation of primary amino acids via catalytic CO insertion leads exclusively to the formation of urea18 or benzolactam derivatives.19
Here, we describe the first example of palladium-catalysed carbonylative transformation of α-amino amides to hydantoins under mild conditions and low CO pressure (1 atm) (Scheme 1c). Competitive pathways to urea and benzolactam derivatives are successfully circumvented, and carbonylation takes place without racemisation of the chiral centre.
Firstly, in the context of our work on the carbonylation of amines to ureas20 in the presence of the PdI2/KI catalytic system,21 we examined to what extent amino amides can be used as hydantoin precursors under palladium catalysis. The easily accessible amide of L-phenylalanine (S)-1a was selected as a model substrate.22 The initial attempts to direct the carbonylation of (S)-1a to imidazolidine-2,4-dione 2a were completely unsuccessful. In fact, the PdI2/KI catalytic system turned out to be as efficient in the synthesis of ureas as ineffective in the formation of hydantoins. Many experiments performed under various conditions, using different solvents and concentrations, afforded urea 3a in quantitative yields and no traces of hydantoin 2a were retrieved (see Table S1 in the ESI‡).
The formation of urea derivatives is also the most preferred pathway when primary amines, including unprotected amino esters, are carbonylated under milder palladium-based conditions.18 Exceptions to this behaviour include the palladium-catalysed oxidative carbonylation of N-protected amino acids to 3,4-dihydroisoquinolinones23 and the synthesis of benzolactams by the palladium-catalysed carbonylation of N-unprotected amino esters.19a
Inspired by the work of Garcia and Granell,19a who were successful in preventing the formation of urea in favour of benzolactam derivatives, we decided to test the model substrate (S)-1a under similar carbonylative conditions. The use of Pd(OAc)2 and benzoquinone (BQ) as oxidants, 1 atm of carbon monoxide (CO) and acetic acid as the solvent (Table 1) gratifyingly hindered the formation of both urea 3a and acetylamide 4a. Similarly, the formation of a benzolactam derivative via C–H activation19a was not detected, and hydantoin 2a was obtained in 74% NMR yield (Table 1, entry 1). Importantly, the absolute configuration of the chiral centre of 2a was found to be S (Fig. S1 and S2 of the ESI‡).24 Unfortunately, the ultimate purification of the target product was unsatisfactory due to the presence of traces of BQ derivatives. A possible adduct of BQ and the product25 were difficult to remove by chromatography. We were pleased to find that the yield increased on lowering the reaction temperature to 80 °C (Table 1, entry 2). Nonetheless, the purification issues persisted even though BQ was employed in a near stoichiometric amount (Table 1, entry 3). This led us to consider alternative oxidants. Methyl-p-benzoquinone (MBQ) and 2,6-dimethyl-p-benzoquinone (DMBQ) were however less efficient (Table 1, entries 4 and 5).
| Entry | Pd (mol%) | Oxidant (equiv.) | T (°C) | t (h) | Yield 2a (%) |
|---|---|---|---|---|---|
a Reaction conditions: (S)-1a (0.3 mmol), CO (balloon, 1 atm), Pd catalyst, oxidant, and acetic acid (0.1 M).
b Isolated yields.
c NMR yields.
d Impure of quinone.
e Compounds 3a and 4a were isolated in 15% and 27% yields, respectively.
f An atmosphere of CO and air (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were employed.
g Compound 4a was isolated in 11% yield and traces of 3a were detected. 1) were employed.
g Compound 4a was isolated in 11% yield and traces of 3a were detected.
|
|||||
| 1 | Pd(OAc)2 (10) | BQ (2.0) | 120 | 6 | 74,c 70bd |
| 2 | Pd(OAc)2 (10) | BQ (2.0) | 80 | 6 | 86,c 83bd |
| 3 | Pd(OAc)2 (10) | BQ (1.2) | 80 | 6 | 80,c 76bd |
| 4 | Pd(OAc)2 (10) | MBQ (1.2) | 80 | 6 | 19bd |
| 5 | Pd(OAc)2 (10) | DMBQ (1.2) | 80 | 6 | 23bd |
| 6 | Pd(OAc)2 (10) | Cu(OAc)2 (2.2) | 80 | 6 | 16be |
| 7 | Pd(OAc)2 (10) | Cu(OAc)2 (1.0)f | 80 | 6 | 64bg |
| 8 | Pd(OAc)2 (10) | AgOAc (2.2) | 80 | 6 | 81b |
| 9 | Pd(OAc) 2 (10) | TEMPO (2.2) | 80 | 2 | 84 |
| 10 | Pd(MeCN)2Cl2 (10) | TEMPO (2.2) | 80 | 2 | 77b |
| 11 | Pd(TFA)2 (10) | TEMPO (2.2) | 80 | 2 | 60b |
| 12 | Pd2(dba)3 (10) | TEMPO (2.2) | 80 | 2 | 68b |
| 13 | Pd(OAc)2 (5) | TEMPO (2.2) | 80 | 3 | 81b |
| 14 | Pd(OAc)2 (2) | TEMPO (2.2) | 80 | 5 | 74b |
| 15 | Pd(OAc)2 (10) | TEMPO (2.2) | 60 | 3 | 65b |
Copper(II) acetate, a common oxidant in palladium-catalysed reactions, gave satisfactory results only in combination with air (Table 1, entries 6 and 7). The purification of the final product was much easier, but compound 4a was formed in a higher amount, suggesting that the competitive acetylation of (S)-1a might be accelerated by a copper(II) species.26 High yield and selectivity were achieved when 2.2 equivalents of silver acetate were used (Table 1, entry 8). However, we aimed to find a more sustainable oxidation system despite its high efficiency. Pleasingly, TEMPO provided the highest yield of (S)-2a, which could be easily isolated in this case (Table 1, entry 9). Remarkably, the use of 2.2 equiv. of TEMPO (see Table S2 in the ESI‡) caused a significant reduction of the reaction time (from 6 to 2 hours), too. Moreover, the acetylated compound 4a was no longer observed. In all reactions carried out with TEMPO, the selectivity reached values close to 99%, as only a reduced amount of the starting material was recovered. Other palladium precursors behaved well but were less efficient (Table 1, entries 10–12). The reduction of Pd(OAc)2 from 10% to 5% and 2% was still acceptable in terms of yield, but longer reaction times were required (Table 1, entries 13 and 14). The optimal reaction temperature was 80 °C since at 60 °C lower performance was observed (Table 1, entry 15). Alternative polar solvents, such as MeCN and DMF, provided less satisfactory results and still required the combination with acetic acid (see Table S2 in the ESI‡).24 Then, to explore the reaction scope (Table 2), an array of α-amino amides were smoothly prepared from the corresponding esters (see the ESI‡) and submitted under the optimised reaction conditions (Table 1, entry 9).
Firstly, the catalytic process was successfully extended to the racemic phenylalanine amide (rac)-1a that was converted to (rac)-2a in 78% yield. Different R1 substituents were then explored. The methyl group on the amide nitrogen (R1 = Me) provided an excellent yield of compound 2b, while a longer alkyl chain (R1 = Hex) seems to decrease the efficiency of the reaction (2c, 71%). The allyl substituent is only partially tolerated with the corresponding hydantoin 2d being obtained in 56% yield. The benzyl moiety was particularly beneficial to this transformation and nearly 90% yield was achieved with both electron withdrawing and electron releasing groups (2e–g, 88–90%). Substrate 1h, bearing a phenylethyl unit, afforded the desired product 2h in 85% yield. Aryl amides were also compatible with this process and allowed 2-arylhydantoins 2i–k to be prepared in excellent yields (85–93%). However, two strong EWGs at the ring (CN group) were detrimental to the process (2l, 13%). The unsubstituted amide of (rac)-phenylalanine 1m was successfully converted to the hydantoin scaffold in a good yield (2m, 75%). Different natural amino acids were then considered. The hydroxyl function of tyrosine amides was well tolerated, delivering the expected carbonylated products 2n–p in good yields. A comparable outcome was observed for valine derivatives (2q–r, 72–88%) and alanine amide 1s (2s, 73%), while the N-butyl amide of tryptophan led to the desired product 2t in a poor 41% yield as the C–H activation at the C2 position of indole turned out to be a competitive pathway.27 Finally, dipeptide 1u gave the corresponding hydantoin 2u in 38% yield.
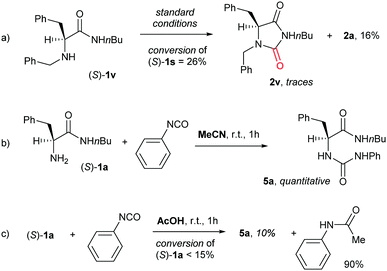
A few control experiments were performed (Scheme 2) to gain preliminary insights into the reaction mechanism. Firstly, an attempt to carbonylate the secondary amino amide (S)-1v was performed under the standard conditions. Surprisingly, the expected product (S)-2v was detected in traces only and hydantoin (S)-2a was isolated in a limited amount (Scheme 2a). This would suggest the intermediacy in the sequence of an isocyanate, which, in turn, could be generated from primary amines only.20c,28 Keeping this in mind, the reaction of (S)-1a with phenyl isocyanate was performed in MeCN and AcOH (Scheme 2b and c). Urea 5a was quantitatively obtained in MeCN. In contrast, 10% of 5a was observed in AcOH together with N-phenylacetamide in 90% yield.29 This is consistent with the complete suppression of urea 3 in reactions performed in acetic acid. Similarly, it could correlate with the formation of traces of acetamide 4, due to the reaction of an isocyanate intermediate with acetic acid,29 under suboptimal conditions (Table 1).
Summarising, two reaction pathways are in principle possible. The first one involves an isocyanate intermediate (Scheme 3, pathway a) and the other a palladacycle one (Scheme 3, pathway b). Initially, Pd(OAc)2 coordinates the free amino group leading to complex I. Insertion of CO leads to intermediate II, which can evolve to isocyanate A (pathway a). Compound 4 can be generated by the reaction of intermediate A with acetic acid.29 Alternatively, it can derive directly from the free amino group30 under acetate/acetic acid conditions. Product 2 can be formed from A by an intramolecular attack of the amide moiety to isocyanate.31 The formation of 3, which is usually the preferred product in conventional reaction media (Scheme 2b),1a,32 is completely prevented here mainly thanks to the acidic medium (Scheme 2c). Furthermore, the reaction of the amine with the isocyanate to afford 3 might be disfavoured in acidic media because 1 should be mostly in its protonated form. The alternative pathway b, where II leads to palladacycle III, which then undergoes reductive elimination to hydantoin 2, cannot be ruled out. In both pathways a and b, the Pd(0) species are promptly re-oxidised by TEMPO to Pd(OAc)2.
In conclusion, we have developed a new palladium-catalysed carbonylative protocol to access hydantoins from α-amino amides with excellent yields under very mild conditions. Importantly, the common formation of urea derivatives under palladium catalysis is completely circumvented here. The key features of the method include the use of largely available starting materials (α-amino amides and CO) and acetic acid as an environmentally friendly and cheap chemical. Moreover, the catalytic method does not require bases and ligands. Target compounds retain the chiral information of the reagent.
This work was benefited from the equipment and framework of the COMP-HUB Initiative, funded by the “Departments of Excellence” program of MIUR, 2018-2022. V. B. and N. D. are grateful to receive funding from the Horizon 2020 research and innovation programme under the MSCA-IF (METACYL, grant no. 894026).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) L. Konnert, F. Lamaty, J. Martinez and E. Colacino, Chem. Rev., 2017, 117, 13757 CrossRef CAS; (b) M. Meusel and M. Gütschow, Org. Prep. Proced. Int., 2004, 36, 391 CrossRef CAS; (c) E. Ware, Chem. Rev., 1950, 46, 403 CrossRef CAS.
- S. Cho, S.-H. Kim and D. Shin, Eur. J. Med. Chem., 2019, 164, 517 CrossRef CAS PubMed.
- (a) S. J. Sabounchei, M. Sayadi, M. S. Hashemi, A. Hashemi, D. Nematollahi, E. Salahifar and R. W. Gable, Appl. Organomet. Chem., 2017, 31, e3716 CrossRef; (b) M. Ruiz-Castañeda, A. M. Rodríguez, A. H. Aboo, B. R. Manzano, G. Espino, J. Xiao and F. A. Jalón, Appl. Organomet. Chem., 2020, 34, e6003 CrossRef.
- J. A. Leitch, H. P. Cook, Y. Bhonoah and C. G. Frost, J. Org. Chem., 2016, 81, 10081 CrossRef CAS.
- M. Kheirabadi, N. Çelebi-Ölçüm, M. F. L. Parker, Q. Zhao, G. Kiss, K. N. Houk and C. E. Schafmeister, J. Am. Chem. Soc., 2012, 134, 18345 CrossRef CAS.
- (a) V. C. Oliveira, J. M. Oliveira, V. H. Menezes da Silva, I. U. Khan and C. R. D. Correia, Adv. Synth. Catal., 2020, 362, 3395 CrossRef; (b) F. Fernández-Nieto, J. Mas Roselló, S. Lenoir, S. Hardy and J. Clayden, Org. Lett., 2015, 17, 3838 CrossRef; (c) M. Beller, M. Eckert, W. A. Moradi and H. Neumann, Angew. Chem., Int. Ed., 1999, 38, 1454 CrossRef CAS.
- (a) V. Ravichandran, R. K. Rai, V. Kesavan and A. Jayakrishnan, J. Biomater. Sci. Polym. Ed., 2017, 28, 2131 CrossRef CAS PubMed; (b) R. K. Rai and A. Jayakrishnan, New J. Chem., 2019, 43, 3778 RSC.
- M. Kalník, P. Gabko, M. Bella and M. Koóš, Molecules, 2021, 26, 4024 CrossRef.
- F. Urech, Justus Liebigs Ann. Chem. Pharm., 1873, 165, 99 CrossRef.
- W. T. Read, J. Am. Chem. Soc., 1922, 44, 1746 CrossRef CAS.
- H. Biltz, Ber. Dtsch. Chem. Ges., 1908, 41, 1379 CrossRef CAS.
- S. Hotha, J. C. Yarrow, J. G. Yang, S. Garrett, K. V. Renduchintala, T. U. Mayer and T. M. Kapoor, Angew. Chem., Int. Ed., 2003, 42, 2379 CrossRef CAS PubMed.
- (a) F. Chen, C.-F. Lu, J.-Q. Nie, G.-C. Yang, Z.-X. Chen and N.-G. Dong, Tetrahedron: Asymmetry, 2015, 26, 180 CrossRef CAS; (b) G.-J. Lu, J.-Q. Nie, Z.-X. Chen, G.-C. Yang and C.-F. Lu, Tetrahedron: Asymmetry, 2013, 24, 1331 CrossRef CAS.
- (a) L. Konnert, L. Gonnet, I. Halasz, J.-S. Suppo, R. M. de Figueiredo, J.-M. Campagne, F. Lamaty, J. Martinez and E. Colacino, J. Org. Chem., 2016, 81, 9802 CrossRef CAS; (b) H. Liu, Z. Yang and Z. Pan, Org. Lett., 2014, 16, 5902 CrossRef CAS PubMed; (c) N. Declas, F. Le Vaillant and J. Waser, Org. Lett., 2019, 21, 524 CrossRef CAS.
- (a) J. Song, Z.-J. Zhang, S.-S. Chen, T. Fan and L.-Z. Gong, J. Am. Chem. Soc., 2018, 140, 3177 CrossRef CAS PubMed; (b) J. Izquierdo, J. Etxabe, E. Duñabeitia, A. Landa, M. Oiarbide and C. Palomo, Chem. – Eur. J., 2018, 24, 7217 CrossRef CAS; (c) R. C. Atkinson, F. Fernández-Nieto, J. Mas Roselló and J. Clayden, Angew. Chem., Int. Ed., 2015, 54, 8961 CrossRef CAS PubMed; (d) K. Tomohara, T. Yoshimura, R. Hyakutake, P. Yang and T. Kawabata, J. Am. Chem. Soc., 2013, 135, 13294 CrossRef CAS PubMed.
- For selected catalytic routes to chiral hydantoins, see: (a) J. F. Briones and G. S. Basarab, Chem. Commun., 2016, 52, 8541 RSC; (b) A. Kondoh, Y. Ota, T. Komuro, F. Egawa, K. Kanomata and M. Terada, Chem. Sci., 2016, 7, 1057 RSC; (c) T. Mashiko, N. Kumagai and M. Shibasaki, J. Am. Chem. Soc., 2009, 131, 14990 CrossRef CAS PubMed.
- (a) M. Beller, M. Eckert, W. A. Moradi and H. Neumann, Angew. Chem., Int. Ed., 1999, 38, 1454 CrossRef CAS; (b) S. M. Dumbris, D. J. Díaz and L. McElwee-White, J. Org. Chem., 2009, 74, 8862 CrossRef CAS.
- K. Orito, M. Miyazawa, T. Nakamura, A. Horibata, H. Ushito, H. Nagasaki, M. Yuguchi, S. Yamashita, T. Yamazaki and M. Tokuda, J. Org. Chem., 2006, 71, 5951 CrossRef CAS.
- (a) J. Albert, X. Ariza, T. Calvet, M. Font-Bardia, J. Garcia, J. Granell, A. Lamela, B. López, M. Martinez, L. Ortega, A. Rodriguez and D. Santos, Organometallics, 2013, 32, 649 CrossRef CAS; (b) L. Zeng, H. Li, J. Hu, D. Zhang, J. Hu, P. Peng, S. Wang, R. Shi, J. Peng, C.-W. Pao, J.-L. Chen, J.-F. Lee, H. Zhang, Y.-H. Chen and A. Lei, Nat. Catal., 2020, 3, 438 CrossRef CAS.
- (a) R. Mancuso, D. S. Raut, N. Della Ca’, F. Fini, C. Carfagna and B. Gabriele, ChemSusChem, 2015, 8, 2204 CrossRef CAS; (b) N. Della Ca’, P. Bottarelli, A. Dibenedetto, M. Aresta, B. Gabriele, G. Salerno and M. Costa, J. Catal., 2011, 282, 120 CrossRef; (c) B. Gabriele, G. Salerno, R. Mancuso and M. Costa, J. Org. Chem., 2004, 69, 4741 CrossRef CAS.
- R. Mancuso, N. Della Ca’, L. Veltri, I. Ziccarelli and B. Gabriele, Catalysts, 2019, 9, 610 CrossRef.
- The aminoamide L-1a was prepared from the corresponding L-amino acid methyl ester (see ESI‡ for details).
- L. Zhang, C. Wang, J. Han, Z.-B. Huang and Y. Zhao, J. Org. Chem., 2016, 81, 5256 CrossRef CAS.
- See ESI‡ for details.
- S. Bittner, Amino Acids, 2006, 30, 205 CrossRef CAS PubMed.
- S. Yamada, S. Terashima and M. Wagatsuma, Tetrahedron Lett., 1970, 11, 1501 CrossRef.
- (a) H. Han, S.-D. Yang and J.-B. Xia, J. Org. Chem., 2019, 84, 3357 CrossRef CAS; (b) J. Ying, Q. Gao and X.-F. Wu, J. Org. Chem., 2019, 84, 14297 CrossRef CAS.
- B. Gabriele, R. Mancuso, G. Salerno and M. Costa, Chem. Commun., 2003, 486 RSC.
- W. R. Sorenson, J. Org. Chem., 1959, 24, 978 CrossRef CAS.
- H. S. Prasad, G. R. Srinivasa and D. Channe Gowda, Synth. Commun., 2005, 35, 1189 CrossRef CAS.
- J. S. Nowick, D. L. Holmes, G. Noronha, E. M. Smith, T. M. Nguyen and S.-L. Huang, J. Org. Chem., 1996, 61, 3929 CrossRef CAS.
- Y. Chen, L. Su, X. Yang, W. Pan and H. Fang, Tetrahedron, 2015, 71, 9234 CrossRef CAS.
Footnotes |
| † The authors dedicate this manuscript to Prof. Mirco Costa on the occasion of his 80th birthday. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cc04154a |
| This journal is © The Royal Society of Chemistry 2022 |