Open Access Article
Open Access ArticleSynthesis of medium-ring lactams and macrocyclic peptide mimetics via conjugate addition/ring expansion cascade reactions†‡
Kleopas Y.
Palate
 ,
Zhongzhen
Yang
,
Adrian C.
Whitwood
,
Zhongzhen
Yang
,
Adrian C.
Whitwood
 and
William P.
Unsworth
and
William P.
Unsworth
 *
*
Department of Chemistry, University of York, Heslington, York, YO10 5DD, UK. E-mail: William.unsworth@york.ac.uk
First published on 9th February 2022
Abstract
A novel conjugate addition/ring expansion (CARE) cascade reaction sequence is reported that enables medium-sized ring and macrocyclic bis-lactams to be prepared from primary amines and cyclic imides. The reactions are simple to perform, generally high yielding, and very broad in scope, especially with respect to the primary amine component. CARE reactions can also be performed iteratively, enabling β-peptoid-based macrocyclic peptide mimetics to be ‘grown’ via well controlled, sequential 4-atom ring expansion reactions, with the incorporation of varied functionalised amines during each iteration.
Introduction
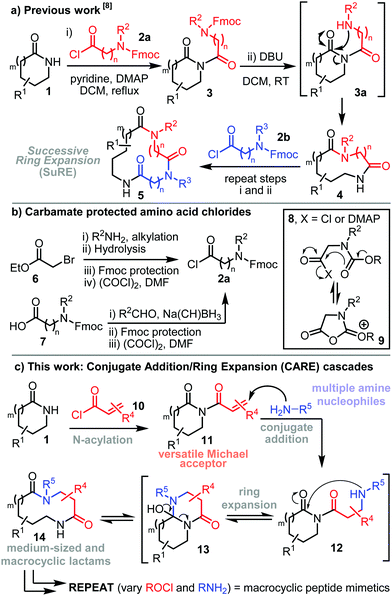
Cascade reaction sequences are widely used in synthetic chemistry to streamline the preparation of complex molecules.1,2 Performing multiple reaction steps in a single operation brings obvious benefits in terms of the overall brevity of synthetic routes and can obviate the need to directly handle reactive and/or toxic intermediates. This strategy can also lead to the development of synthetic cascades greater than the sum of their parts, whereby the overall cascade reaction proceeds more efficiently than the analogous stepwise process.3 Ring expansion reactions are also important in synthetic chemistry,4,5 especially for the synthesis of biologically relevant medium-sized rings and macrocycles,6 compounds that can be difficult to make using classical end-to-end cyclisation methods.7 This manuscript is focused on combining these two individually powerful approaches for the synthesis of macrocyclic peptide mimetics, using a novel Conjugate Addition/Ring Expansion (CARE) cascade reaction sequence.Previous work from our laboratory has established a robust method for the 3- and 4-atom ring expansion of lactams 1 upon reaction with acyl chlorides derived from Fmoc-protected amino acids (e.g.2a); following N-acylation of the lactam to form imide 3, reaction with base promotes Fmoc-cleavage (3 → 3a) and spontaneous ring expansion (3a → 4, Scheme 1a).8 The reactions typically proceed in good yield over two steps, and the lactams products 4 can themselves be expanded further by repeating the same two-step sequence (e.g.4 → 5), thus enabling Successive Ring Expansion (SuRE).8,9 However, there are limitations associated with the use of acid chlorides of the type 2a, most notably that a 3- or 4-step synthesis is typically needed to make them (6 or 7 → 2a, Scheme 1b), and that carbamate-mediated acid chloride deactivation/degradation (8 → 9, Scheme 1b box) can negatively affect the reactions in cases where lactam acylation is sluggish.10
Avoiding the use of protecting groups was therefore a key factor when designing the new method in this manuscript. We postulated that N-acylation of a lactam 1 using a simple acryloyl chloride derivative 10 would generate imide 11, and that this Michael acceptor could engage in a conjugate addition (11 → 12) ring expansion (12 → 13 → 14, Scheme 1c) cascade reaction sequence upon treatment with primary amine nucleophiles.11 The successful realisation of this approach is described herein. The method is extremely broad in scope, with 54 novel CARE reactions reported in this manuscript, demonstrated across a diverse array of functionalised lactams and amines. The reactions are easy to perform, they are insensitive to air and moisture, and work in a wide range of solvents, including water. CARE reactions can also be performed iteratively, to enable the synthesis of macrocyclic peptide mimetics, based on β-peptoid linkages,12 by performing sequential N-acylation and CARE reactions.
Results and discussion
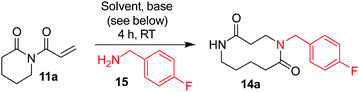
Reaction optimisation (Table 1) was performed using 6-membered ring imide 11a, itself prepared via the N-acylation of δ-valerolactam with acryloyl chloride.13p-Fluorobenzylamine 15 was chosen as a model primary amine as its fluorine group provided a convenient handle for reaction monitoring using 19F NMR. Initially, imide 11a (1 equiv.) and amine 15 (1.1 equiv.) were stirred in DCM (0.1 M) at RT with an excess of DBU (10 equiv.). These conditions were chosen to start as they were used to promote the ring expansion step in our published SuRE chemistry,8 and pleasingly, resulted in modest conversion (33%) into 10-membered lactam 14a (entry 1). Next, the reaction concentration was increased (0.1 M → 0.5 M, entry 2) as increasing the concentration has been shown to promote amine conjugate addition in related systems;14 this change did not improve conversion, but it had no significant negative impact either so was retained for further optimisation. We then questioned whether the base was necessary, and indeed, performing the same reaction without DBU led to improved conversion (entry 3).| Entry | Solvent | Conc. | Base | 14a/%a |
|---|---|---|---|---|
| a Imide 11a (1 equiv.) and amine 15 (1.1 equiv.) were stirred in the stated solvent at RT for 4 h unless stated, performed on a 0.5 mmol scale. 3,5-Bis(trifluoromethyl)bromobenzene (1 equiv.) was then added before an aliquot of the reaction mixture (ca. 0.2 mL) was taken, diluted with CDCl3 and analyzed directly by 19F NMR. Conversion to 14a was determined by the ratio of the 19F NMR resonance of 14a to that of the 3,5-bis(trifluoromethyl)bromobenzene internal standard. b 18 h reaction time. c isolated yield in parentheses, performed on a 5.0 mmol scale. | ||||
| 1 | DCMb | 0.1 M | DBU (10 equiv.) | 33 |
| 2 | DCMb | 0.5 M | DBU (10 equiv.) | 32 |
| 3 | DCM | 0.5 M | — | 42 |
| 4 | Toluene | 0.5 M | — | 48 |
| 5 | THF | 0.5 M | — | 52 |
| 6 | Et2O | 0.5 M | — | 41 |
| 7 | hexane | 0.5 M | — | 38 |
| 8 | DME | 0.5 M | — | 48 |
| 9 | MeCN | 0.5 M | — | 51 |
| 10 | DMSO | 0.5 M | — | 77 |
| 11 | DMF | 0.5 M | — | 77 |
| 12 | DMA | 0.5 M | — | 78 |
| 13 | NMP | 0.5 M | — | 80 |
| 14 | MeOH | 0.5 M | — | 82 (86)c |
| 15 | EtOH | 0.5 M | — | 80 |
| 16 | i-PrOH | 0.5 M | — | 71 |
| 17 | t-BuOH | 0.5 M | — | 73 |
| 18 | TFE | 0.5 M | — | 52 |
| 19 | HFIP | 0.5 M | — | 58 |
| 20 | H2O | 0.5 M | — | 69 |
A range of alternative solvents were then explored (entries 4–20); in total, 18 were tested and remarkably, all resulted in some conversion into 10-membered lactam 14a, including solvents compatible with biological systems, most notably water (entry 20). Overall, polar solvents tend to perform better, with alcohol solvents particularly effective (entries 14–17). Importantly, the conversion as measured using 19F NMR translates into a comparable synthetic yield, with 14a isolated in 86% following column chromatography, tested on a 5.0 mmol scale reaction using methanol as the solvent (entry 14). Methanol was therefore selected to take forward to the substrate scoping phase of the project, but the versatility of the reaction in terms of solvent is also notable and is important in scenarios where methanol is less effective (see later for examples).
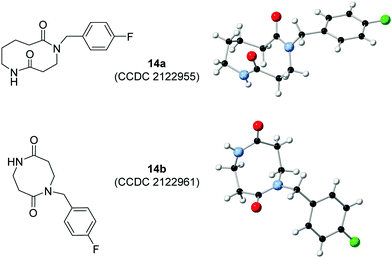
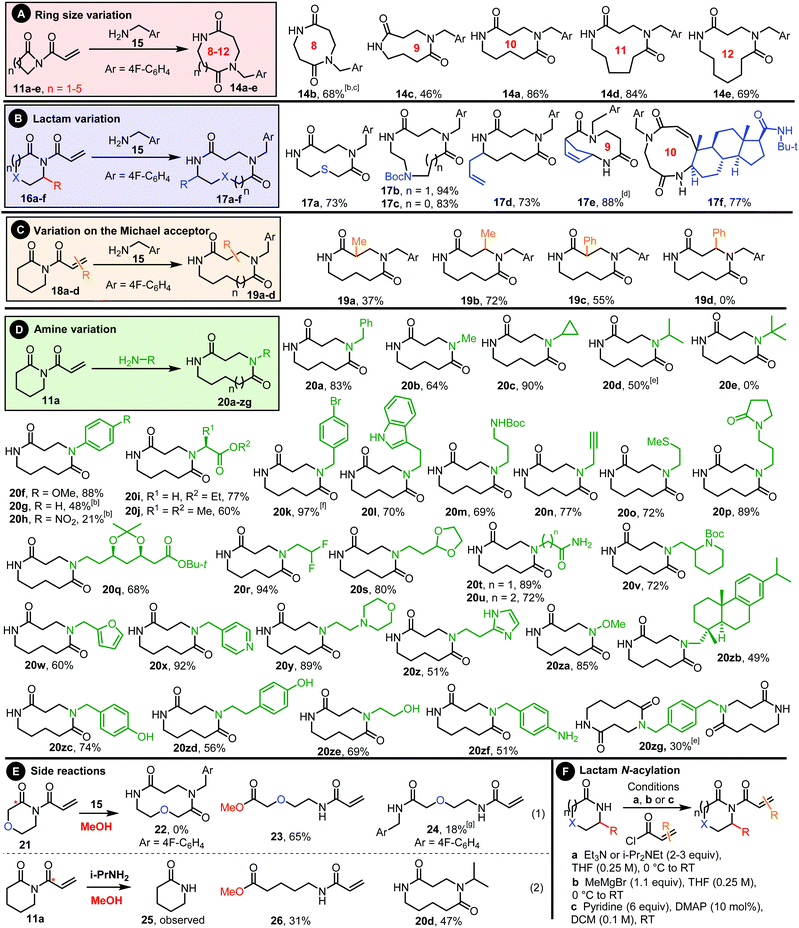
The scope with respect to lactam ring size was examined first, with 4–8-membered ring imides 11a–e prepared from the corresponding lactams and acryloyl chloride. All were reacted with p-fluorobenzylamine 15 and in all cases the desired ring-expanded products were formed (14a–e, Scheme 2A). The standard protocol (methanol, 0.5 M, 4 h) was used in all examples, except for the expansion of 4-membered imide 11b into 8-membered 14b. In this case, a complex mixture of products was formed when the reaction was done in methanol, with the only tractable products arising from unwanted ring opening of the β-lactam, both by the amine 15 and methanol (see ESI† for details). However, by performing the reaction in DCM instead of methanol and increasing the reaction time, the desired ring expanded product 14b could be isolated in 68% yield. This result highlights the value of the wide solvent compatibility of CARE in finding contingencies for substrates that have chemical functionality not compatible with the standard methanol conditions. The assigned structures of products 14a and 14b were both confirmed via X-ray crystallography (Fig. 1).15
Functionalised lactam starting materials (16a–f) were also examined, with the expected ring expanded products 17a–f all formed in good yields; this series includes sulfide-, carbamate- and alkene-containing lactams, as well as bicyclic and steroidal lactams (Scheme 2B). Substitution on the Michael acceptor is also tolerated both at the α- and β-position (19a–c, Scheme 2C), although a notable exception is cinnamoyl-derivative 18d (R = Ph at the β-position) which failed to react, presumably due to its lower electrophilicity as a Michael acceptor.
The ability to freely vary the primary amine coupling partner is arguably the most powerful feature of the CARE method (Scheme 2D). Aliphatic primary amines typically work well, provided they are not too bulky; the steric influence on the reaction yields is clear when comparing relatively unhindered cyclopropylamine (20c, 90%), moderately bulky iso-propylamine (20d, 50%) and bulky tert-butylamine (20e, 0%). Unsurprisingly, electronics also influence the CARE reactions, presumably by modulated the amine's nucleophilicity; for example, when comparing aniline derivatives, the electron-rich p-OMe substituted product 20f was formed in 83% using the standard protocol, but there was a drop in yield when moving to aniline itself (20g, 48%) and an electron-poor p-NO2 derivative (20h, 21%), even when using longer reaction times.
The excellent functional group compatibility of CARE is exemplified by the range of functionalised amines used to make products (20i–zb); all were formed in good to excellent yields from amines containing a wide array of functional groups, including esters, halides, carbamates, terminal alkynes, sulfides, amides, acetals, furans, various aza-heterocycles, hydroxylamine derivatives and others. Notably, the primary amine motif can also out-compete other unprotected nucleophiles like phenols, alcohols and anilines (20zc–f), while the diamine-tethered bis-lactam 20zg was also made from p-xylylene diamine.
Thus, the CARE method has been demonstrated to work well across a wide range of substrates, with most reactions tested working well. However, as well as highlighting the successful cases, it is instructive to consider the relatively rare cases in which the reaction does not proceed in the typical way (Scheme 2E). The biggest challenge relates to chemoselectivity, specifically that the imide starting materials typically contain three electrophilic centres. One of these is the β-position of the Michael acceptor (i.e. the required site for conjugate addition), and based on our previous ring expansion work, which includes detailed DFT studies,8c we are confident that once conjugate addition has taken place the ring expansion step should be facile. However, both carbonyl groups of the imide are also electrophilic and can react competitively with the primary amine and/or nucleophilic solvent molecules. For example, when imide 21 was reacted under the standard conditions, none of the expected ring expanded product 22 was formed; instead linear products 23 and 24 were isolated, arising from nucleophilic ring opening through attack at the internal imide carbonyl (highlighted with a star) by methanol or p-fluorobenzylamine respectively (Scheme 2E, eqn (1)). Presumably, the cyclic ether oxygen increases the electrophilicity of the adjacent carbonyl and changes the typical kinetic preference for conjugate addition. This is a similar observation to that described earlier, during the CARE of the β-lactam-based imide 11b into 8-membered 14b (Scheme 2A).
Competing nucleophilic attack at the external imide carbonyl has also been observed in cases where the conjugate addition step is sluggish; for example, when imide 11a was reacted with relatively bulky i-propylamine under the standard conditions, de-acylated lactam 25 was formed in the reaction, presumably as a result of nucleophilic attack by methanol and/or the amine at the highlighted carbonyl, alongside ring-opened side product 26 and the desired ring expanded product 20d (Scheme 2E, eqn (2)). In situations like this, where the methanol solvent promotes side reactions, alternative solvents can be considered, and in this case a solvent switch to DMF resulted in a modest increase in yield of the ring expanded product 20d (50%, Scheme 2D).
The requisite imides used for all the CARE reactions in this manuscript were prepared using basic reaction conditions, using one of the three related N-acylation methods summarised in Scheme 2F, with full details for all imide preparations included in the ESI.‡
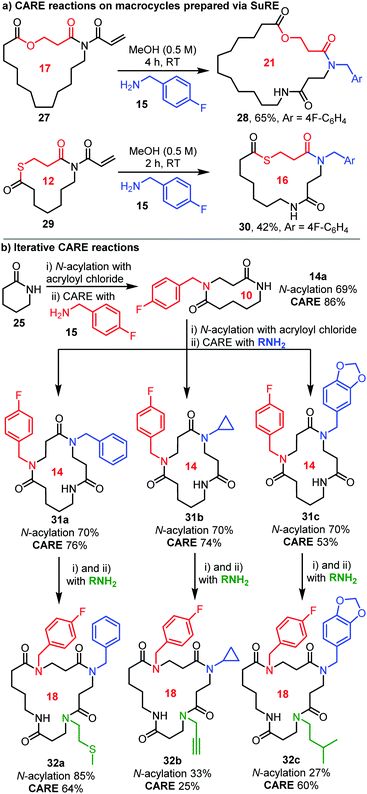
The products in Scheme 2 are all lactams that can potentially be used in CARE reactions themselves. Therefore, the possibility of using CARE in iterative ring expansion processes was explored. First, lactone- and thiolactone-containing macrocyclic imides 27 and 29 were tested, with the precursor lactams prepared using our published SuRE method (with the section inserted via the first ring expansion highlighted in red).8b,d Pleasingly, both imides were converted into the ring-expanded products 28 and 30 respectively using amine 15 and the standard conditions (Scheme 3a). It is also possible to perform CARE reactions iteratively (Scheme 3b). For example, starting from δ-valerolactam 25, N-acylation with acryloyl chloride followed by CARE with p-fluorobenzylamine 15 delivered 10-membered bis-lactam 14a. Then, another N-acylation/CARE sequence was performed starting from 14a using three different amines (shown in blue) to afford 14-membered products 31a–c in good yields. Each of compounds 31a–c were then expanded a third time in the same way (amine shown in green) to furnish 18-membered β-peptoid-based tetra-peptide mimetics 32a–c. This ability to use CARE to install different functionalised building blocks in sequence was a major driving force when developing the reaction. Sequence specific cyclic peptides have numerous important biochemical applications, for example arginine-glycine-aspartic acid (RGD) peptides, that have found wide utility in cell culture models and as targeted therapeutic agents.16 Of course, the CARE method can only be used to promote 4-atom ring expansion, and hence cannot be used to target cyclic peptides based on proteinogenic amino acids. Nonetheless, what is does offer is a versatile route to sequence specific β-peptoid-based macrocycles, that could become similarly useful in future biochemical studies.
Conclusions
In summary, a practical and versatile iterative conjugate addition/ring expansion sequence is described for the synthesis of medium-sized/macrocyclic lactams and peptide mimetics based on β-peptoid linkages. The imide precursors that undergo the CARE cascade can react with a wide array of functionalised amines without the need for protecting groups. The products can be thought of as macrocyclic peptide/peptoid mimetics, a compound class with significant potential for use in medicinal and biological chemistry applications.17 The iterative nature of the CARE reactions will be of value when optimising the properties of the macrocyclic products (e.g. in structure activity relationship studies), and its operational simplicity and wide scope should ensure that the CARE method is well-used, both by specialist synthetic chemists, and by researchers working in more applied fields.The demonstrated wide solvent compatibility of CARE should also have important implications. For example, CARE reactions have been successfully applied in solvents like DMSO and water, that are commonly used to handle biologically relevant molecules like peptides or proteins. This, coupled with the demonstrated high selectivity for reaction on primary amines in the presence of a wide array of other functional groups, provides encouragement that CARE reactions based on the selective functionalisation amines in complex macromolecules (e.g. lysine residues in peptides/proteins) could emerge over time.
Author contributions
The project was conceived by WPU and KYP. Initial method development and optimisation was done by KYP. Reaction scope and further method development was done by KYP and ZY. The manuscript was written through contributions from all authors. X-ray crystallography data acquisition, processing and analysis was done by ACW. The project was directed and managed by WPU.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the University of York for the provision of an Eleanor Dodson Fellowship (to W. P. U.) and for supporting K. Y. P. with a PhD studentship, and the China Scholarship Council for funding (Z. Y.). Special thanks to Claudia Flandoli for preparing the graphical abstract artwork.Notes and references
- For reviews of tandem/cascade reactions, see: (a) R. J. K. Taylor, M. Reid, J. Foot and S. A. Raw, Acc. Chem. Res., 2005, 38, 851 CrossRef CAS PubMed; (b) K. C. Nicolaou and J. S. Chen, Chem. Soc. Rev., 2009, 38, 2993 RSC; (c) B. Prabagar, N. Ghosh and A. K. Sahoo, Synlett, 2017, 2539 CAS; (d) J. M. Sperl and V. Sieber, ACS Catal., 2018, 8, 2385 CrossRef CAS; (e) H.-M. Huang, M. Garduño-Castro, C. Morrill and D. J. Procter, Chem. Soc. Rev., 2019, 48, 4626 RSC.
- For recent examples from our group, see: (a) A. Lawer, J. A. Rossi-Ashton, T. C. Stephens, B. J. Challis, R. G. Epton, J. M. Lynam and W. P. Unsworth, Angew. Chem., Int. Ed., 2019, 58, 13942 CrossRef CAS PubMed; (b) J. A. Rossi-Ashton, A. K. Clarke, R. J. K. Taylor and W. P. Unsworth, Org. Lett., 2020, 22, 1175 CrossRef CAS PubMed; (c) N. Inprung, M. J. James, R. J. K. Taylor and W. P. Unsworth, Org. Lett., 2021, 23, 2063 CrossRef CAS PubMed.
- For an excellent recent example, see: S. Biwas, B. F. Van Steijvoort, M. Waeterschoot, N. R. Bheemireddy, G. Evano and B. U. W. Maes, Angew. Chem., Int. Ed., 2021, 60, 21988 CrossRef PubMed.
- For reviews of ring expansion chemistry, see: (a) M. Hesse, Ring Enlargement in Organic Chemistry, Wiley-VCH, Weinheim, 1991 Search PubMed; (b) W. P. Unsworth and J. R. Donald, Chem. – Eur. J., 2017, 23, 8780 CrossRef PubMed; (c) K. Prantz and J. Mulzer, Chem. Rev., 2010, 110, 3741 CrossRef CAS PubMed; (d) T. C. Stephens and W. P. Unsworth, Synlett, 2020, 133 CAS; (e) A. K. Clarke and W. P. Unsworth, Chem. Sci., 2020, 11, 2876 RSC.
- For selected recent examples, see reference 2a and: (a) B. Zhou, L. Li, X.-Q. Zhu, J.-Z. Yan, Y.-L. Guo and L.-W. Ye, Angew. Chem., Int. Ed., 2017, 56, 4015 CrossRef CAS PubMed For related ring expansion methods to make medium-sized rings using 3,3-sigmatropic rearrangement, see: ; (b) X. Gao, M. Xia, C. Yuan, L. Zhou, W. Sun, C. Li, B. Wu, D. Zhu, C. Zhang, B. Zheng, D. Wang and H. Guo, ACS Catal., 2019, 9, 1645 CrossRef CAS; (c) R. Mendoza-Sanchez, V. B. Corless, Q. N. N. Nguyen, M. Bergeron-Brlek, J. Frost, S. Adachi, D. J. Tantillo and A. K. Yudin, Chem. – Eur. J., 2017, 23, 13319 CrossRef CAS PubMed; (d) A. Osipyan, A. Sapegin, A. S. Novikov and M. Krasavin, J. Org. Chem., 2018, 83, 9707 CrossRef CAS PubMed; (e) D. R. Loya, A. Jean, M. Cormier, C. Fressigné, S. Nejrotti, J. Blanchet, J. Maddaluno and M. De Paolis, Chem. – Eur. J., 2018, 24, 2080 CrossRef CAS PubMed; (f) A. Dierks, J. Tönjes, M. Schmidtmann and J. Christoffers, Chem. – Eur. J., 2019, 25, 14912 CrossRef CAS PubMed; (g) J. E. Hall, J. V. Matlock, J. W. Ward, K. V. Gray and J. Clayden, Angew. Chem., Int. Ed., 2016, 55, 11153 CrossRef CAS PubMed; (h) R. Costil, Q. Lefebvre and J. Clayden, Angew. Chem., Int. Ed., 2017, 56, 14602 CrossRef CAS PubMed; (i) R. A. Bauer, T. A. Wenderski and D. S. Tan, Nat. Chem. Biol., 2013, 9, 21 CrossRef CAS PubMed; (j) T. Guney, T. A. Wenderski, M. W. Boudreau and D. S. Tan, Chem. – Eur. J., 2018, 24, 13150 CrossRef CAS PubMed; (k) Z.-L. Li, X.-H. Li, N. Wang, N.-Y. Yang and X.-Y. Liu, Angew. Chem., Int. Ed., 2016, 55, 15100 CrossRef CAS PubMed; (l) L. Li, Z.-L. Li, F.-L. Wang, Z. Guo, Y.-F. Cheng, N. Wang, X.-W. Dong, C. Fang, J. Liu, C. Hou, B. Tan and X.-Y. Liu, Nat. Commun., 2016, 7, 13852, DOI:10.1038/ncomms13852; (m) Y. Xia, S. Ochi and G. Dong, J. Am. Chem. Soc., 2019, 141, 13038 CrossRef CAS PubMed; (n) Y. Yuan, Z. Guo, Y. Mu, Y. Wang, M. Xu and Y. Li, Adv. Synth. Catal., 2020, 362, 1298 CrossRef CAS; (o) J. Shang, V. J. Thombare, C. L. Charron, U. Wille and C. Hutton, Chem. – Eur. J., 2021, 26, 1620–1625 CrossRef PubMed.
- For medium-sized rings and macrocycles in medicinal chemistry, see: (a) K. R. Romines, K. D. Watenpaugh, P. K. Tomich, W. J. Howe, J. K. Morris, K. D. Lovasz, A. M. Mulichak, B. C. Finze, J. C. Lynn, M.-M. Horng, F. J. Schwende, M. J. Ruwart, G. L. Zipp, K.-T. Chong, L. A. Dolak, L. N. Toth, G. M. Howard, B. D. Rush, K. F. Wilkinson, P. L. Possert, R. J. Dalga and R. R. Hinshaw, J. Med. Chem., 1995, 38, 1884 CrossRef CAS PubMed; (b) T. P. Majhi, B. Achari and P. Chattopadhyay, Heterocycles, 2007, 71, 1011 CrossRef CAS; (c) F. Kopp, C. F. Stratton, L. B. Akella and D. S. Tan, Nat. Chem. Biol., 2012, 8, 358 CrossRef CAS PubMed; (d) R. A. Bauer, T. A. Wenderski and D. S. Tan, Nat. Chem. Biol., 2013, 9, 21 CrossRef CAS PubMed; (e) C. Zhao, Z. Ye, Z.-X. Ma, S. A. Wildman, S. A. Blaszczyk, L. Hu, I. A. Guizei and W. Tang, Nat. Commun., 2019, 10, 4015, DOI:10.1038/s41467-019-11976-2; (f) E. M. Driggers, S. P. Hale, J. Lee and N. K. Terrett, Nat. Rev. Drug Discovery, 2008, 7, 608 CrossRef CAS PubMed; (g) E. Marsault and M. L. Peterson, J. Med. Chem., 2011, 54, 1961 CrossRef CAS PubMed; (h) F. Giordanetto and J. Kihlberg, J. Med. Chem., 2014, 57, 278 CrossRef CAS PubMed; (i) A. K. Yudin, Chem. Sci., 2015, 6, 30 RSC; (j) M. D. Cummings and S. Sekharan, J. Med. Chem., 2019, 62, 6843 CrossRef CAS PubMed.
- For important insight into the efficiency of large ring cyclisation reactions, see: (a) G. Illuminati and L. Mandolini, Acc. Chem. Res., 1981, 14, 95 CrossRef CAS; (b) F. Fastrez, J. Phys. Chem., 1989, 93, 2635 CrossRef; (c) J. C. Collins and K. James, Med. Chem. Commun., 2012, 3, 1489 RSC; (d) H. Kurouchi and T. Ohwada, J. Org. Chem., 2020, 85, 876 CrossRef CAS PubMed.
- (a) T. C. Stephens, M. Lodi, A. Steer, Y. Lin, M. Gill and W. P. Unsworth, Chem. – Eur. J., 2017, 23, 13314 CrossRef CAS PubMed; (b) T. C. Stephens, A. Lawer, T. French and W. P. Unsworth, Chem. – Eur. J., 2018, 24, 13947 CrossRef CAS PubMed; (c) A. Lawer, R. G. Epton, T. C. Stephens, K. Y. Palate, M. Lodi, E. Marotte, K. J. Lamb, J. K. Sangha, J. Lynam and W. P. Unsworth, Chem. – Eur. J., 2020, 26, 12674 CrossRef CAS PubMed; (d) K. Y. Palate, R. G. Epton, A. C. Whitwood, J. M. Lynam and W. P. Unsworth, Org. Biomol. Chem., 2021, 19, 1404 RSC.
- For SuRE reactions based on the expansion of cyclic β-ketoesters, see: (a) C. Kitsiou, J. J. Hindes, P. I’Anson, P. Jackson, T. C. Wilson, E. K. Daly, H. R. Felstead, P. Hearnshaw and W. P. Unsworth, Angew. Chem., Int. Ed., 2015, 54, 15794 CrossRef CAS PubMed; (b) L. G. Baud, M. A. Manning, H. L. Arkless, T. C. Stephens and W. P. Unsworth, Chem. – Eur. J., 2017, 23, 2225 CrossRef CAS PubMed.
- L. A. Carpino and M. Bienert, J. Org. Chem., 1991, 56, 2635 CrossRef CAS.
- For a conceptually related cascade Michael/lactamisation process, see: G. J. Noordzij and C. H. R. M. Wilsens, Front. Chem., 2019, 729 CrossRef CAS PubMed.
- (a) O. Roy, S. Faure, V. Thery, C. Didierjean and C. Taillefumier, Org. Lett., 2008, 10, 921 CrossRef CAS PubMed; (b) E. De Santis, A. A. Edwards, B. D. Alexander, S. J. Holder, A.-S. Biesse-Martin, B. V. Nielsen, D. Mistry, L. Waters, G. Siligardi, R. Hussain, S. Faure and C. Taillefumier, Org. Biomol. Chem., 2016, 14, 11371 RSC; (c) C. Caumes, T. Hjelmgaard, O. Roy, M. Reynaud, D. Servent, C. Taillefumier and S. Faure, Med. Chem. Commun., 2012, 3, 1531 RSC.
- For the N-acylation methods used in the manuscript, see: refe. 8a and: (a) D. P. Curran and M.-H. Yoon, Tetrahedron, 1997, 53, 1971 CrossRef CAS; (b) M. Li, V. Carreras, A. Jalba and T. Ollevier, Org. Lett., 2018, 20, 995 CrossRef CAS PubMed.
- B. C. Ranu and S. Banerjee, Tetrahedron Lett., 2007, 48, 141 CrossRef CAS.
- CCDC 2122955 (14a) and 2122961 (14b) contain the crystallographic data for compounds‡.
- (a) P. J. LeValley, E. M. Ovadia, C. A. Bresette, L. A. Sawicki, E. Maverakis, S. Baic and A. M. Kloxin, Chem. Commun., 2018, 54, 6923 RSC; (b) T. G. Kapp, F. Rechenmacher, S. Neubauer, O. V. Maltsev, E. A. Cavalcanti-Adam, R. Zarka, U. Reuning, J. Notni, H. J. Wester, C. Mas-Moruno, J. Spatz, B. Geiger and H. Kessler, Sci. Rep., 2017, 7, 39805 CrossRef CAS PubMed; (c) W. Xiao, Y. Wang, E. Y. Lau, J. Luo, N. Yao, C. Shi, L. Meza, H. Tseng, Y. Maeda, P. Kumaresan, R. Liu, F. C. Lightstone, Y. Takada and K. S. Lam, Mol. Cancer Ther., 2010, 9, 2714 CrossRef CAS PubMed; (d) J. Zhu, C. Tang, K. Kottke-Marchant and R. E. Marchant, Bioconjugate Chem., 2009, 20, 333 CrossRef CAS PubMed; (e) E. Lieb, M. Hacker, J. Tessmar, L. A. Kunz-Schughart, J. Fiedler, C. Dahmen, U. Hersel, H. Kessler, M. B. Schulz and A. Gopferich, Biomaterials, 2005, 26, 2333 CrossRef CAS PubMed.
- For biologically important cyclic peptides/peptoids and mimetics, see references 6 and: (a) R. H. Kohli, C. T. Walsh and M. D. Burkart, Nature, 2002, 418, 658 CrossRef CAS PubMed; (b) J. Gavenonis, B. A. Sheneman, T. R. Siegert, M. R. Eshelman and J. A. Kritzer, Nat. Chem. Biol., 2014, 10, 716 CrossRef CAS PubMed; (c) E. A. Villar, D. Beglov, S. Chennamadhavuni, J. A. Porco Jr, D. Kozakov, S. Vajda and A. Whitty, Nat. Chem. Biol., 2014, 10, 723 CrossRef CAS PubMed; (d) W. Xu, Y. H. Lau, G. Fischer, Y. S. Tan, A. Chattopadhyay, M. de la Roche, M. Hyvönen, C. Verma, D. R. Spring and L. S. Itzhaki, J. Am. Chem. Soc., 2017, 139, 2245 CrossRef CAS PubMed; (e) Y. H. Lau, P. de Andrade, Y. Wu and D. R. Spring, Chem. Soc. Rev., 2015, 44, 91 RSC; (f) S. B. Y. Shin, B. Yoo, L. J. Todaro and K. Kirshenbaum, J. Am. Chem. Soc., 2007, 129, 3218 CrossRef CAS PubMed; (g) A. M. Webster and S. L. Cobb, Tetrahedron Lett., 2017, 58, 1010 CrossRef CAS; (h) O. R. Maguire, B. Taylor, E. M. Higgins, M. Rees, S. L. Cobb, N. S. Simpkins, C. J. Hayes and A. C. O'Donoghue, Chem. Sci., 2020, 11, 7722 RSC; (i) S. Roesner, G. J. Saunders, I. Wilkening, E. Jayawant, J. V. Geden, P. Kerby, A. M. Dixon, R. Notman and M. Shipman, Chem. Sci., 2019, 10, 2465 RSC.
Footnotes |
| † This manuscript is dedicated to the memory of Prof Eric Marsault, who helped us greatly with scientific advice and encouragement when starting our research into biologically important macrocycles. |
| ‡ Electronic supplementary information (ESI) available. CCDC 2122955 and 2122961. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cb00245g |
| This journal is © The Royal Society of Chemistry 2022 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif)