Open Access Article
Open Access ArticleHydrogels for the treatment of oral and maxillofacial diseases: current research, challenges, and future directions
Mingshu
Huang†
 a,
Yisheng
Huang†
a,
Yisheng
Huang†
 a,
Hongyu
Liu†
a,
Hongyu
Liu†
 a,
Zhengming
Tang
a,
Yuanxin
Chen
a,
Zhijie
Huang
a,
Shuaimei
Xu†
*d,
Jianzhong
Du†
a,
Zhengming
Tang
a,
Yuanxin
Chen
a,
Zhijie
Huang
a,
Shuaimei
Xu†
*d,
Jianzhong
Du†
 *bc and
Bo
Jia†
*bc and
Bo
Jia†
 *a
*a
aDepartment of Oral and Maxillofacial Surgery, Stomatological Hospital, Southern Medical University, Guangzhou, Guangdong, China. E-mail: dentist-jia@163.com
bGynaecology and Obstetrics, Shanghai Fourth People's Hospital, School of Medicine, Tongji University, Shanghai 200434, China. E-mail: jzdu@tongji.edu.cn
cDepartment of Polymeric Materials, School of Materials Science and Engineering, Tongji University, 4800 Caoan Road, Shanghai 201804, China
dDepartment of Endodontics, Stomatological Hospital, Southern Medical University, Guangzhou, Guangdong, China. E-mail: xushuaimei@126.com
First published on 24th August 2022
Abstract
Oral and maxillofacial diseases such as infection and trauma often involve various organs and tissues, resulting in structural defects, dysfunctions and/or adverse effects on facial appearance. Hydrogels have been applied in the treatment of oral diseases and defect repair due to their three-dimensional network structure. With their biocompatible structure and unique stimulus-responsive property, hydrogels have been applied as an excellent drug-delivery system for treatments that mainly include oral mucosal diseases, wounds, periodontitis and cancer therapy. Hydrogels are also ideal scaffolds in regenerative engineering of dentin–pulp complex, periodontal tissue, bone and cartilage. This review discusses the fundamental structure of hydrogels in brief and then focuses on the characteristics and limitations in current research and applications of hydrogels. Finally, potential future directions are proposed.
1. Introduction
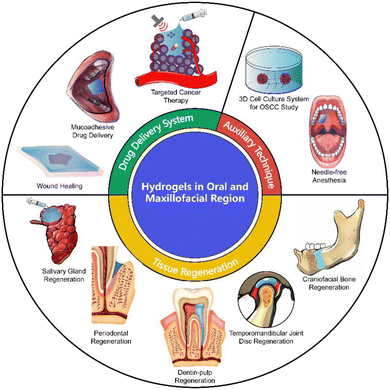
The oral and maxillofacial region is a complex system with various anatomical structures, e.g., teeth, periodontal tissue, skin and mucosa, and other organs or tissues, thus bears various physiological and social functions including mastication, speech, breathing and facial appearance. Owing to this complexity, there is a series of important challenges associated with treatments of oral and maxillofacial diseases. Specific oral and maxillofacial diseases such as periapical periodontitis, periodontitis and cancer often lead to irreversible structural defects and dysfunction of related anatomical structures including periodontal tissue, skin and mucosa, jawbone, salivary glands, and temporomandibular joint (TMJ). Moreover, the impact on appearance due to structural defects will further influence the physical and mental health of patients and reduce their quality of life (Fig. 1).1,2Another challenge is the complexity of drug delivery related to the complex anatomical structure of the oral and maxillofacial region. The oral and maxillofacial region is located at the beginning of the respiratory tract and digestive tract, and is associated with various tissues, sinus and space. Infectious diseases and secondary infection caused by trauma or radiotherapy often affect multiple organs and tissues simultaneously and are difficult to control. The key to achieving infection control is to sustain effective and long-term therapeutic drug concentration at the infection site in situ.3 Moreover, in the therapeutic process for head and neck squamous cell carcinoma (HNSCC), the dynamic anatomical structure and complex vascular system often lead to inaccurate release and limited absorption efficiency of chemotherapy and immunotherapy drugs. Therefore, an on-demand and in situ drug-delivery system is also essential.4
Generally, the treatments for oral and maxillofacial diseases are essential for overcoming the challenges of drug-delivery system (DDS) and defect repair, requiring the application of suitable biomaterials as the treatment platform. Among a wide range of biomaterials, hydrogels have shown promising potential to achieve effective therapeutic effect at the lesion sites or promote repair of structural defects simultaneously.4,5 In 1960, O. Wichterle and D. Lim discovered hydrogel material (i.e., hydrophilic gels), suggesting that this biomaterial could be an outstanding biocompatible material.6 Hydrogels are characterized by their stable three-dimensional structure and ability to retain large amounts of water or other bio-liquids. Since their inception, hydrogels have shown great potential and prospects in the field of biochemistry and biomedicine. Hydrogels do not themselves treat oral diseases or repair defects, but act as an outstanding carrier or platform that transfers various components (e.g., drugs, cells, and inorganic minerals), providing space and microenvironments to facilitate the function of the loaded components.7 Hydrogels can encapsulate drugs and other therapeutic ingredients and deliver them to the infection site, providing long-term and effective therapeutic effects through in situ osmotic administration.9 In addition, hydrogels have a structure and biocompatibility similar to natural extracellular matrix (ECM), enabling them to encapsulate and culture diverse stem cells or cytokines in tissue regenerative engineering.8 With a porous structure and degradable property, hydrogels constitute scaffolds and space for cell proliferation and differentiation, and subsequently degrade without residue.10
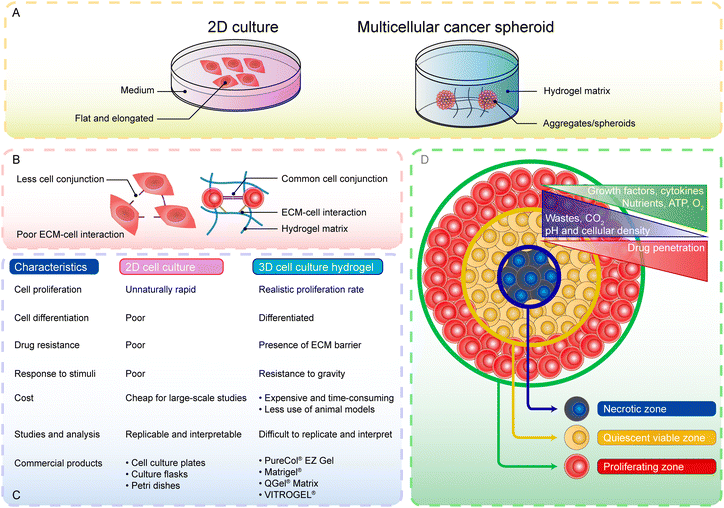
Currently, hydrogels have been applied in various fields of experimental study and clinical translation, including topical anesthesia,11 infection control,12 wound healing,13 tissue regenerative engineering,14–18 3D cell culture systems19 and anticancer therapy.20 The composition and structure endow hydrogels with flexible and multifunctional application forms such as injection,21 dressing,22 scaffold,23 3D bio-printing and bio-ink.24 Moreover, hydrogels provide auxiliary therapeutic techniques including needle-free anesthesia for non-invasive pain management25 and a 3D cell culture system for cancer study.19 Therefore, hydrogels have been a prospective candidate for the treatment of oral and maxillofacial diseases.
Most of the previous reviews have demonstrated the structure, preparation process, functionalization, and properties of diverse types of hydrogels as well as their clinical translations in other fields such as regenerative medicine.5,26 Preliminary literature reviews show that there are few reviews on the research and application of hydrogels in the field of oral and maxillofacial diseases. On the other hand, most research has been concentrated at a single certain point, but has not been systematically integrated. Therefore, our work aims to combine the mechanisms that have been studied with the latest research results on hydrogels in the oral and maxillofacial fields and summarize their latest achievements. In this review, we provide a brief overview of the components and structure of hydrogels, and of how hydrogels achieve these diverse functional properties. This review focuses on hydrogels in the treatment of diverse oral and maxillofacial diseases through infection control and defect repair, and discusses their characteristics and limitations. Finally, we discuss the existing shortcomings and limitations of clinical application of hydrogels, and possible directions for development are proposed.
2. Functionalization of hydrogels
2.1. Definition
Hydrogels are three-dimensional structures with an insoluble crosslinked network of hydrophilic copolymers, which endows them with the ability to adsorb and retain large amount of water in their swollen states.27 Water or biological fluid acts as a dispersion medium in the polymer networks.28 Their mechanical strength enables hydrogels to maintain a stable 3D structure, mainly referring to the strength intensity (which derives from the density of polymer molecules and crosslinking nature) and viscoelasticity (which derives from the deformation caused by polymer segment rearrangement).29 Their unique ability to retain water mainly derives from hydrophilic groups in the polymer chains including amino group, carboxyl group, and hydroxyl group. Other factors associated with water retention include the physical crosslinking structure, crosslinking density, composition of solution and synthesis technique of the co-polymer chains.30,31 Polymer networks also physically interact with water through capillaries, osmotic forces and hydration forces. These factors decide the inherent properties of hydrogels including mechanical strength, internal transport and diffusion properties8,32 that enable the chain networks to swell.28 Therefore, the fundamental structure and water-retaining function of hydrogels can be designed on-demand by controlling the composition and crosslinking approach of the hydrogel polymers.302.2. Classification
Hydrogels are mainly classified by sources of polymers, cross-linking methods, and synthetic methods. Classified by the source of co-polymers constitution, hydrogels can be defined as natural, synthetic, or hybrid.8 Naturally occurring polymers e.g., cellulose, chitosan (CS), alginate, silk, hyaluronic acid (HA), and extracellular matrix (ECM)33,34 usually exhibit biocompatibility, biodegradability, and pre-functionalization with integrin binding sites allowing for adhesion and coordinated cellular responses, while being limited by significant batch-to-batch variability and potential immunogenicity within foreign hosts.35 With stable composition, controllable structure, and low immunogenicity, synthetic hydrogels are applied for on-demand design in novel hydrogel preparation strategies.36,37 Some researchers have proposed combining these hydrogels in the form of co-polymers or inter-penetrating networks (IPNs) for precise modification to highlight the optimal properties of each of their constituent components.According to the synthetic methods, the polymers of hydrogels are classified as chemically crosslinking and physically crosslinking.30 Chemical crosslinking forms larger co-polymer molecules by covalent bonds between the precursors’ functional groups by grafting, radical polymerization, click chemistry, enzymatic reactions, thermo-gelation, and radiation crosslinking.27,31 Covalent bonds have been utilized to conjunct polymer molecules, making chemical crosslinking more resistant to mechanical forces than physical crosslinking.5 Chemical crosslinking shapes hydrogels by click-type chemistries including Schiff base coupling, hydrazide–aldehyde coupling, Diels–Alder linkage, and azide–alkyne cycloaddition.38 These reactions are commonly triggered by chemical crosslinking agents, enzymes, or stimuli38 with high conversion.5,39 Compared with chemically crosslinked hydrogels, physically crosslinked hydrogels express easy production and synthesis protocols due to the absence of crosslinking agents.40 Physical crosslinking mainly comprises non-covalent interactions e.g., ionic, electrostatic, hydrophobic interactions, crystallization, hydrogen bonds, chain entanglements and strong van der Waals interactions between chains.5,41 Physical crosslinking provides hydrogels with three-dimensional structure, mechanical strength and physical integrity.27 The structure of hydrogels strongly depends on the type and proportion of polymer monomers, crosslinking agents, synthesis and fabrication process, and the degradative and mechanical history. The characterization of hydrogels can be flexibly modified into amorphous, semicrystalline, H-bonded, supramolecular, or hydrocolloidal aggregates.42
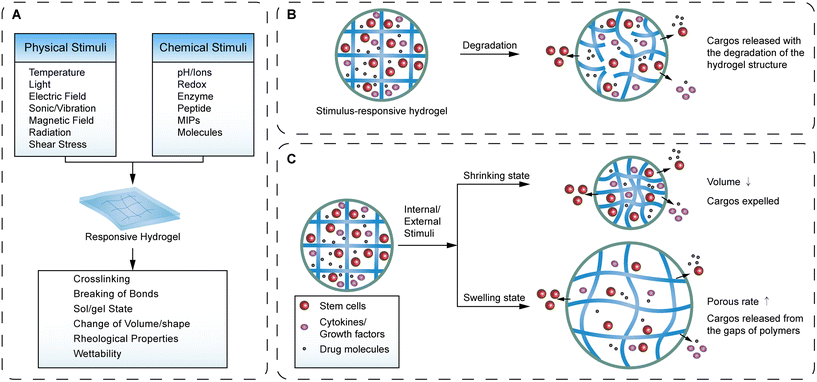
Hydrogels can also be classified into conventional and stimulus-responsive hydrogels. Most conventional hydrogels simply exhibit change of swelling state and free diffusion of components. Stimulus-responsive hydrogels can change their cross-linking structure in response to endogenous or exogenous stimuli e.g., temperature, light, and pH.27,43 This property endows hydrogels with multiple functions and expands their application range in the treatment of oral and maxillofacial diseases (Fig. 2).
2.3. Properties
The biocompatibility of hydrogels mainly comes from the polymer molecules that are similar to ECM and high degradability. Although hydrogels can be prepared directly by decellularized extracellular matrix (dECM), allogeneic dECM hydrogels are often difficult to put into clinical translation because of significant immunogenicity, batch-to-batch variation, and potential carcinogenic properties (e.g., Matrigel).44 Polymers present in ECM such as CS, alginate, HA, collagen, and gelatin are commonly extracted to produce natural hydrogels.10 Synthetic hydrogels such as poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA), and poly(methyl methacrylate) (PMMA) also have high biocompatibility, stability, and uniformity of composition, yet they usually require surface modification of Arg–Gly–Asp (RGD) peptide and integrins due to lacking functionalized surface markers.35 The biodegradability of hydrogels is characterized by the hydrolysis or enzymolysis of covalent bonds and the weakening of non-covalent interactions influenced by ionic strength, pH value, and temperature.45,46 Subsequently, the structure of hydrogels is destroyed and the content decreased, while the originally occupied space is gradually replaced by the newly formed tissue.47 The dissolved polymers and their degradable wastes are mainly eliminated by the liver, gallbladder, and spleen with a modest inflammatory response.46The polymers in the cross-linking network of hydrogels interact with the solute, so the hydrogel can be defined into relaxed states and swollen states. The relaxed state refers to the polymer chain itself before swelling, while the swollen state refers to the equilibrium state of the polymer after exposure to the solute. The porosity of hydrogels is larger in the swollen state, and the solute can be transported or diffused outward in the hydrogel matrix.42 In addition, pH, temperature and ion content are factors affecting solute diffusion.42,48 Therefore, the solute transport capacity of hydrogels can be regulated by adjusting the above factors.
The adhesive property of some hydrogels is the basis for applications in oral mucoadhesive drug delivery49 and wound dressing with hemostasis and self-healing.50 Inspired by mussel adhesive protein, the adhesion at moist sites derives from the side group of catechol on the dopamine (DOPA) molecule. In the presence of water, polydopamine hydrogel forms non-covalent bonds and strong covalent bonds between contact interfaces, providing hydrogels with sustained and stable adhesion.51,52 According to this strategy, adhesive hydrogels were optimized by covalently grafting amine- or thiol-terminated and glycosaminoglycan HA onto polydopamine, achieving more applications in angiogenesis, hematopoietic stem cell commitment and homing, and tumor metastasis.53 However, traditional viscous hydrogels often show short-term viscosity, poor mechanical properties, and a lack of antibacterial ability due to the peroxidation of catechol groups.54 Therefore, understanding how to prevent the oxidation of hydrogels is an important challenge. A novel redox hydrogel based on Ag-Lignin nanoparticles (Ag-Lignin NPs) can continuously produce free catechol groups and keep catechol side groups from oxidation for a long time, giving hydrogels long-term and repeatable adhesion. The phenolic hydroxyls or methoxy groups on the lignin can be converted to redox-active quinone/hydroquinone, and consequently to semiquinone radicals by a comproportionation reaction. The phenol or methoxy groups in lignin can reduce silver ions (Ag+) to Ag nanoparticles (AgNPs), while the redox products (quinone/hydroquinone) are converted into catechol groups in the presence of photogenerated electrons. Ag-Lignin NPs generate reactive oxygen species (ROS), resulting in a trigger to self-gelation of the hydrogel and an additional improvement in antibacterial ability.55 Of note, anti-inflammatory ability at the wound site is an essential requirement for hydrogel dressings in wound management. Some natural hydrogels such as HA with hyaluronidases exert outstanding properties of regulating inflammation by creating a barrier against exogenous microbiota and absorbing exudates containing inflammatory cytokines and chemokines.56 Hydrogels can also preserve and constantly release non-steroidal anti-inflammatory drugs (NSAIDs) such as nimesulide and aspirin, providing adjustable drug delivery for anti-inflammatory purposes.57,58
One of the most prominent properties of hydrogel is the ability to respond to external stimuli, including temperature, pH, light, magnetic field, chemical molecules, electric field or shear stress of the environment (Table 1).42,59 Hydrogels often show response to hydrogen ions or charges, undergoing changes in structure, swelling state and porous rate in response to changes of pH and electric field. The ionizable side groups in the hydrogel polymer chain can ionize hydrogen ions or bind hydrogen ions and exert a change of charge on the polymer chain, resulting in electrostatic repulsion or attraction, thus controlling the relaxed states and swollen states of the hydrogels.42,60 The fixed charges on the polymer chain enable the hydrogels to specifically adsorb some proteins or drugs, which is one of the fundamental bases for hydrogels to become multifunctional cargo carriers.61 The pH-responsive behavior enables hydrogels to swell and release drugs in the acidic environment in the stomach, thus protecting the bioactivity of proteins and drug molecules from enzyme degradation and hydrolysis in gastrointestinal delivery.60 The responsive property to the electric field may derive from the fixed charge on the hydrogel polymer skeletons and the conductive liquid or components that hydrogels carry. When an external electric field is applied, the ions and counter-ions in the charged network are attracted to the opposite direction by the electrophoretic force, thus forming a change in the swelling state.42
| Stimulus | Hydrogel | Structural property | Main cargo | Application | Form | Characteristic of hydrogel | Ref. |
|---|---|---|---|---|---|---|---|
| Abbreviations: PLGA, poly(lactic-co-glycolic acid); PIC, poly(isocyanopeptide); DOX: doxycycline, PNIPAM; poly(N-isopropylacrylamide); HCl, hydrochloride; CA4, combretastatin A4 disodium phosphate; HPMC, hydroxypropyl methylcellulose; BAG, bioactive glasses; p-TNTs, titanium dioxide nanotubes; VD3, 1α,25-dihydroxyvitamin D3; PNAGA, poly(N-acryloylglycinamide); KPS, potassium persulfate; PTH/PTHrP, parathyroid hormone/parathyroid hormone-related protein; RGD, arginine–glycine–aspartic; SCOS, sulfated chitooligosaccharides; aFGF, acidic fibroblast growth factor; Fu, furan; AMI, maleimide; Fmoc, fluorenylmethoxycarbonyl; FF, diphenylalanine; MIP, molecularly imprinted polymer; MNP, magnetic nanoparticle; rhBMP4, recombinant human bone morphogenetic protein 4; PBM, photobiomodulation; MC, methylcellulose; MSN, mesoporous silica nanoparticle; PDA, poly(dopamine); GO, graphene oxide; PAM, poly(acrylamide); QCSP, quaternized CS-g-polyaniline; PEGS–FA, poly(ethylene glycol)-co-poly(glycerol sebacate); TGF-β, transforming growth factor-β; CMC-OCS, chitosan-oxidized chondroitin sulfate; EGF, epidermal growth factor; KGN, kartogenin; ADV, acoustic droplet vaporization; FT, N-fluorenylmethoxycarbonyl-L-tryptophan; PAAm/PVA, poly acrylamide/poly(vinyl alcohol); HA–PBA, phenylboronic acid grafted hyaluronic acid; MIP, molecularly imprinted polymer; BISS, N,N′-bis(acryloyl)cystine; DA reaction, Diels–Alder reaction; CTC, circulating tumor cell; PVCL–Lys, poly(vinylcaprolactam)–lysine; MAX, maleic anhydride modified xylan; PAEMA, poly(2-azepane ethyl methacrylate). | |||||||
| Temperature | PLGA–PIC | Adjustable mass ratio of acid- and ester-capped PLGA microspheres | • DOX | • Drug delivery | Injection | • Exhibit long-term structural stability | 270 |
| • Lipoxin A4 | • Treatment of periodontitis | • Exert on-demand release without inflammatory response in vivo | |||||
| Pectin–CHO | Stay stable through hydrazinolysis | • DOX·HCl | • Drug delivery | Injection | • Obtain long-term antitumor efficacy | 271 | |
| PNIPAM | • CA4 | • Treatment of cancer | • Degrade at body temperature without leftover | ||||
| Pluronic F127-HPMC | Amide bonds split at 37 °C | BAG | Dentin and bone regeneration | Injection | • Gel and harden at body temperature | 272 | |
| • Rapid setting and homogenized dispersion exerted with an ultrasonic scaler | |||||||
| F-127 | Amide bonds split at 37 °C | • p-TNTs | • Enhancement of early osseointegration | • Coating | • F-127 coating possesses outstanding VD3 loading and controlled release capacity | 273 | |
| • VD3 | • Drug delivery | • Bio-cap | • VD3 improves the osteogenic differentiation and early osseointegration | ||||
| PNIPAM/PNAGA | Dual temperature response behaviors of UCST and LCST | KPS (thermal initiator) | • Electronic skin | Film | Exert adjustable, stretchable and self-healable properties and transparency variation under different temperatures | 274 | |
| • Wearable device | |||||||
| • Actuators | |||||||
| PEG–PCL–PEG | Gelation at 37 °C due to the hydrolytic degradation of poly(ε-caprolactone) | PTH/PTHrP | Enhancement of orthodontic tooth movement | Injection | Provide sustainable and stable release of PTH and PTHrP | 275 | |
| PIC/PIC–RGD | Characteristic stiffening response at high strains | RGD-peptide | Wound repair | Dressing | • Exert painless application and removal | 276 | |
| • Adheres well to wound as a physical barrier | |||||||
| • Recruit immune cells and myofibroblasts | |||||||
| Light (including NIR) | Pluronic® F-127 | Gelation at 37 °C and self-assembly in micelles | • rhBMP4 | PBM therapy for tissue healing and regeneration | Injection | Direct and accelerate hard tissue bio-engineering | 277 |
| • MSCs | |||||||
| Methylcellulose | Hydroxyl groups are substituted with methyl or hydroxypropyl groups | • DOX | OSCC treatment | Injection | • Controlled drug delivery and release of DOX | 265 | |
| • MSN | • Induce synergistic photothermal effects against OSCC | ||||||
| Cyclic O-nitrobenzyl modified HA | Employs imine anchoring to connect to host mucosa through S-nitrosylation coupling reaction | — | • Mucoadhesive drug delivery | Light-curing adhesive | • The adhesive was anchored to oral mucosa in 5 s with a dental light curing | 75 | |
| • Treatment of oral mucosal defect | • Perform thin, elastic, and degradable properties without discomfort | ||||||
| Electric | PDA–pGO–PAM | Hydrogen-bonding and cation–π interaction endowed with self-healability | • GO | Implantable and flexible bioelectronics | • Cell stimulator | • High conductivity and stretchability | 278 |
| • rGO | • Wearable electronic | • Mechanical and electrical self-healability | |||||
| • Self-adhesiveness without additional adhesive | |||||||
| • QCSP | 4-Formylbenzoic acid was grafted onto the PEGS by esterification reaction | • EGF | • Wound healing | Dressing | • Similar conductivity to skin and muscle | 21 | |
| • PEGS–FA | • TGF-β | • Drug delivery | • Able to transfer bioelectrical signals for promoting wound healing process | ||||
| • VEGF | |||||||
| Ultrasound | • CMC-OCS | CMC react with oxidized chondroitin sulfate through a Schiff base reaction | KGN, PLGA microsphere | Cartilage regeneration | Injection | Upon exposure to ultrasound, KGN showed burst release | 279 |
| • Perfluorocarbon double emulsion | ADV increased 22-fold in shear stiffness | Basic fibroblast GFs | Non-invasive and on-demand GFs release | Injection | Release more functional and bioactive bFGF | 280 | |
| • Fibrin | |||||||
| Shear stress | • FT–PAAm/PVA | Gel–sol transition was triggered by destroyed balance of H-bond and π–π stacking | — | • Cartilage substitution | Lubricating surface, supporting scaffold | • Supramolecular gelation by shear force performs lubricating function | 204 |
| • Artificial lubricating joint | • FT–PAAm/PVA network physically supports increased mechanical property | ||||||
| • HA–PBA | Stabilized through a secondary cross-linking between the acrylate moiety and thiol groups | rMSCs | • Cartilage regeneration | 3D printing bioink | • Significantly protect rMSCs with shear thinning properties of the copolymer | 281 | |
| • PVA | • Treatment of degenerative joint diseases | • Improve handling and 3D printability of the bioink | |||||
| pH | Fu–CS/AMI–CS | Synthesized through DA reaction | Chloramphenicol | Targeted-drug delivery of nerves | Injection | • Maintain pH-sensitivity and stable antibacterial activity of CS | 282 |
| • Prevent premature solubilization and burst release | |||||||
| Magnet | Fmoc-FF/Fmoc-RGD | Self-assembling amphiphilic dipeptides formed physical hydrogels | MNPs | Minimally invasive surgical biomaterial | Injection | Mobilization and stiffening in response to noncontact magnetic forces | 283 |
| MIP | Sodium alginate | The guluronate block of both alginate chain for crosslinking and gelation | Dopamine | Ti-implant surface modification | Coating | Continuously and steadily released dopamine | 284 |
| • Temperature | Anionic protein/CS | Imide covalent and electrostatic interactions endowed the hydrogel with injectability and structural stability | • Ag3AuS2 NPs | • Treatment of tongue cancer | Injection | Stably increased the photothermal efficiency by condensing NPs and reducing the heat loss | 264 |
| • Light | • CAL-27 | • Photothermal therapy | |||||
| • Temperature | PNIPAM-BISS | Degraded by GSH and H2O2 | • BISS | Drug storage | Micro-capsule | • Perform sensitively to temperature and pH | 285 |
| • pH | • DOX | • Degraded by reducing and oxidizing agent | |||||
| • Redox reaction | |||||||
| • Temperature | PNIPAM | Create sialic acid-imprinted sites on thermo-responsive hydrogel layer | Sialic acid | CTCs detection and collection | Layer | Non-invasive and specific recognition of sialic acid for selective capture and release of CTCs | 286 |
| • MIP | |||||||
| • Magnet | • PEG-b-PAEMA | Control the movement of microrobot through magnetic propulsion | • DOX | • Microcargo operation | Magnetic actuated microrobot | • Exert complex encapsulation and release of micro-/nanocargo | 287 |
| • pH | • PVP | • Fe3O4 NPs | • Drug release | • Controlled motion by magnetic propulsion | |||
| • Imaging | |||||||
Hydrogels exhibit thermo-responsive behavior mainly through the reversible sol–gel state translation at the lower critical solution temperature (LCST) and upper critical solution temperature (UCST). Taking LCST hydrogel as an example, it shows a hydrophilic sol-state at a temperature lower than LCST, and a hydrophobic gel-state at a temperature higher than LCST, which is our familiar morphology in hydrogels. The ideal thermo-response hydrogels undergo this transition near body temperature, providing an accelerated drug release by dissolving or exerting a rapid formation for drug storage by gelation, which enables it to provide local drug delivery and controlled release with hydrogel degradation in vivo.62–64 It has been reported that hydrogels can locally deliver and release colchicine at physiological temperature in human vascular cells at the lesion site, and the cell viability test showed that there was no significant systemic cytotoxicity.65 In addition, thermo-responsive hydrogels can provide long-term storage and sustained release of drugs, reducing drug administration times and improving the comfort and compliance of patients.4
Chemically responsive behavior refers to the response of a polymer chain to one or a class of target molecules. It takes advantage of the activity of the target molecule, such as the peptide-based hydrogel that responds to the degradation of the target enzyme. Alternatively, the target molecular recognition can be transformed into the expansion, collapse or degradation of the polymer chain due to the change of pH, temperature or charge.42 The existing limitations of chemically responsive hydrogels mainly refer to the low recognition of and binding capacity to target molecules. To overcome this challenge, molecularly imprinted polymers (MIPs) are gradually applied to raise the recognition accuracy of hydrogels. MIP-based hydrogels can recognize the binding site of a template molecule (e.g., glucose, enzyme, RGDS peptide) and exert physical adsorption, covalent immobilization or noncovalent immobilization, which is similar to that of antibodies or aptamers. With the ability to bind a particular analyte with high specificity using inexpensive materials that are readily stored long term, MIP-based hydrogels enable many practical applications such as diagnostics, sensors and sorbents.42,66,67
Photoresponsive behavior grants a high level of precise control to hydrogels. Photoresponsive hydrogel polymer chains usually exert structural transformation through the degradation and bonding of polymer chains in response to light including visible light, ultraviolet and near infrared (NIR). Owing to the narrow sensitivity range and high conversion rate, photoresponsive hydrogels achieve precise control of substrates for cell growth in tissue regenerative engineering. The researchers indirectly regulate cell behaviors such as cell proliferation on the substrate by changing the substrate's mechanical properties and chemical biomarkers.42 In recent years, hydrogels have been developed for a novel application approach for a nutritional deprivation strategy to block residual blood vessels around tumors and inhibit angiogenesis. Under the control of NIR, photoresponsive hydrogels release thrombin to the surrounding blood vessels to promote blood coagulation, while reducing the secretion of VEGF to prevent angiogenesis. The photoresponsive hydrogel-based strategy for the disruption of tumor blood supply may be a prospective strategy for the prevention of tumor recurrence or metastasis.68,69
3. Design considerations for hydrogels in oral and maxillofacial environment
The oral and maxillofacial region is a highly dynamic and complex environment. The requirements of motor functions (e.g., chewing, breathing, and speech) as well as facial appearance should be met. Small and irregular anatomic structures such as sinuses, aponeurotic spaces, pulp cavities, TMJ, and periodontal pockets can make drug delivery difficult.70 At the same time, the naturally occurring microbial environment can increase the complexity of treatment.71 Therefore, hydrogels applied in the oral and maxillofacial region usually have these design considerations: (a) high biocompatibility and biodegradability along with low cytotoxicity and carcinogenicity for the hydrogel to integrate itself into the tissue with limited ‘foreign body reactions’; (b) sufficient mechanical strength to meet the requirements of jaw bone, TMJ and muscle movement; (c) excellent rheological properties to adapt to the space of irregular regions; (d) ability to preserve and on-demand deliver different components such as drugs, proteins, and nanoparticles; (e) antibacterial ability, to resist the opportunistic infection of microorganisms inherent in the oral cavity; (f) preferably aesthetically acceptable and easy to use.4. Topical oral anesthesia
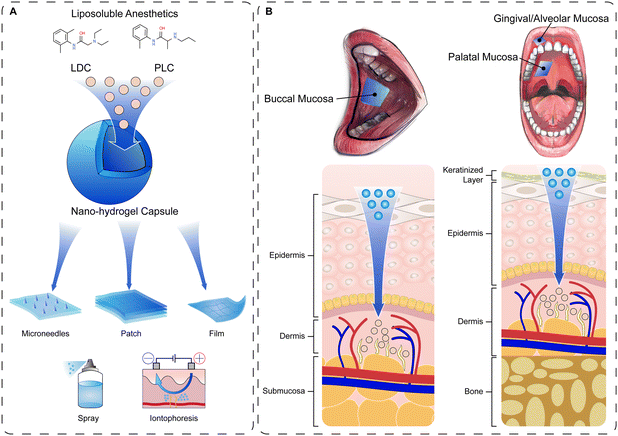
Conventional local injection anesthesia usually causes adverse effects such as pain sensation, infection, needle breakage and cross-infection.72 With the development of anesthetics and drug delivery platforms, needle-free topical anesthesia based on oral mucosa adhesion hydrogels has been tried to replace conventional local injection anesthesia in oral treatments to solve the problems of pain and side effects of injection. One of the major challenges of oral mucoadhesive hydrogels is the moist and highly dynamic oral environment.73 The hydrogels need to exert the ability to firmly adhere to the surface of the moist internal oral environment, resist external forces without separating from the oral mucosa, and be thin and tensile to avoid discomfort of the tongue and oral mucosa. Previously, hydrogels have been applied for mucoadhesion using catechol groups but did not achieve ideal results, probably due to the oxidation of the catechol groups during the synthesis process, which led to a lack of free catechol groups for the oxidative cross-linking to form protein networks with the mucosal surface, resulting in poor adhesion. Therefore, researchers usually modify catechol groups-containing hydrogels (e.g., polydopamine, DPA) by adding ingredients (e.g., polyacrylamide, PAM) via non-covalent bond interaction (e.g., hydrogen bonds and π–π stacking) or covalent bonds (e.g., amino groups) to increase the amounts of catechin groups or protect catechin groups from oxidation.74The latest research has provided an innovative modification strategy that has been developed with the inspiration of light-curing techniques in dentistry. Zhang et al.75 modified HA hydrogel using topical o-nitrobenzyl photocages, enabling the hydrogel to crosslink and anchor to host tissues with imine groups via triggering the S-nitrosylation coupling reaction under 395 nm light curing within 5 seconds. Unlike conventional phototriggered radical polymerization, this hydrogel based on phototriggered mercaptan and nitroso reaction free radical crosslinking avoids the damage to tissues from free radicals, and eliminates the limitation of oxygen blocking in free radical polymerization. This thin, elastic, and degradable mucoadhesive hydrogel could be retained for more than 24 hours with the disturbance of liquid rinsing, tongue movement and food friction in vivo rat models. Light-cured hydrogel provides a rapid, stable and non-invasive preparation strategy for oral mucosal adhesion.
Another challenge for hydrogel containing topical anesthetics is the barrier function of oral mucosa. The epithelial barrier can resist the invasion of microorganisms, while reducing the permeability of local drug delivery on oral mucosa in situ. The permeability is mainly affected by the difference of epithelial thickness, keratinization and lipid composition.76 Anesthetics must penetrate through the paracellular pathway and approach the free nerve endings in the lamina propria in order to exert an anesthetic effect.77 The physical and chemical properties, dosage forms and application approach of anesthetics are closely related to their permeability.76 In order to achieve successful topical anesthesia, people have committed to increasing the permeability and retention of drugs. With small molecular mass and great polarity, which better permeate the epithelial barrier, Lidocaine (LDC) and Prilocaine (PLC) have been applied combined with hydrogels, and have achieved ideal permeation kinetic parameters and amount retained in the epithelium.77 Previously, marketed topical anesthetic creams loaded with LDC and PLC, such as the eutectic mixture of Lidocaine and Prilocaine (EMLA®) and Oraqix®, have been used in oral topical anesthesia, but exhibit the limitations of low drug permeability, bitter taste and a burning sensation during application (Fig. 3).78,79
In order to optimize the anesthetics delivery system, researchers encapsulated 2.5% LDC and PLC into poly(ε-caprolactone) (PCL) nanocapsules, and incorporated these into CARBOPOL hydrogel for mucosal adhesion and long-term release.80 The addition of some biopolymers such as xanthan can further neutralize the inherent toxicity of anesthetics, probably due to the interaction of available Xan-hydroxyl groups with dispersed free topical anesthetics through hydrogen bonds.81 Compared with EMLA®, these nanostructured hydrogels exhibited improved mechanical, rheological, and mucoadhesive properties, providing an effective and long-term superficial anesthetic effect with LDC and PLC. In addition, these anesthetic-loaded hydrogels also diminished the bitterness and burning sensation of existing oral local anesthesia products, making the novel mucoadhesive hydrogels more acceptable for patients.82
Physical methods (e.g., iontophoresis, microneedles) have been applied to improve the anesthetic effect of free diffusion through oral mucosa in anesthetic agent-loaded hydrogels, and to enhance non-invasive drug permeation to the free nerve endings. Iontophoresis is a non-invasive technology which can increase the penetration of ionic compounds into the skin in the presence of an electric field.83 Studies have shown that high concentrations of Prilocaine hydrochloride and Lidocaine hydrochloride in hydrogels along with an external electric field force accelerated ion rapid penetration through the mucosa, and a large amount of retention in the mucosa exerted a long-term anesthetic function.79 Another physical approach, microneedles (usually in the micron range < 1000 μm), can exert a local anesthetic penetration through the stratum corneum of the mucosa and deeply infiltrate the epidermis or dermis layer.84 Hydrogel patches combined with microneedles for local adhesion and anesthesia not only reduce the pain of patients, but also have outstanding properties of viscosity, sealing and formability, preventing the discomfort caused by the infiltration of anesthesia into the oral cavity.85 Inspired by the two auxiliary methods of iontophoresis and microneedles, some researchers combined the two methods with hydrogels to obtain the ideal anesthetic drug penetration and anesthetic effect. Ion-conductive porous microneedles (PMN) significantly enhanced the transdermal molecular penetration or extraction by generating electroosmotic flow (EOF) based on a built-in enzymatic biobattery (fructose/O2 battery). Owing to the penetration through the stratum corneum by microneedles, macromolecular substances including anesthetics were not blocked, and the transdermal resistance to EOF was significantly decreased.85 Generally, topical oral anesthesia can continuously permeate the oral mucosa through the mucoadhesive hydrogel carrier to achieve an excellent and painless local anesthetic effect. This anesthetic effect can be further enhanced by the combination of various physical assistant processes including iontophoresis and microneedle. However, there is not enough evidence to indicate that hydrogels can be applied in a wide range of anesthesia, thus nerve block anesthesia may still be difficult to replace. In addition, current research only puts forward innovative anesthesia strategies or concepts, yet challenges ahead of clinical application still exist, including cost control and complexity of storage and transport.
5. Oral mucosa and skin care
5.1. Treatment of oral mucosal diseases
Oral inflammatory disease (OID) or local lesions including xerostomia, aphthous stomatitis, and oral mucositis still lack effective in situ treatment, thus rely on oral administration. However, acid hydrolysis and primary metabolism of the gastrointestinal tract can result in low drug concentrations at the lesion site and potential systemic side effects.15,26 Oral topical administration such as ointments, sprays, and lozenges has been proposed, but these formulations have been found to be inadequate. In the presence of saliva, existing dosage forms are often unable to adhere stably to the site of the lesion or be dissolved. Swallowing may result in the loss of dissolved or suspended drugs, leading to involuntary removal of dosage forms or even suffocation.86,87 The emergence of hydrogels solves the above shortcomings and makes it possible for local treatment by oral mucosal adhesion through the inherent charge, hydrogen bond, ionic bond or covalent bond on the polymer chain.88 The components are simply diffused in situ, or separated from hydrogels by polymer–drug interaction and stimulus response to achieve controlled local release of the drug at the infected site.86 Hydrogels also protect unstable drugs from degradation by encapsulating drug molecules through different physicochemical interactions.89 Compared with oral administration, mucoadhesive local administration avoids decomposition by the first pass effect and systemic side effects, and provides a higher penetration rate and concentration of components at the lesion site.90 Hydrogels provide convenient application forms for patients, commonly including patch or injection. Recently, some hydrogels have been applied in liquid forms including drops or sprays, sufficiently diffusing into the rough surface of mucosa. The hydrogels of sol-state then exert a rapid gelation in response to body temperature and anchor in situ.91Hydrogels are often used to relieve the discomfort of xerostomia sufferers, using their adhesive properties and ability to retain water. Compared with conventional saliva substitutes, hydrogels have lubricity and viscosity similar to natural saliva92 and can inhibit bacterial and fungal infections to prevent the occurrence of aggressive caries and oral candidiasis.93 Adhesive hydrogel can be used to incorporate antibiotics, glucocorticoids, antimicrobial peptides (AMP)94 and other drugs for the treatment of various oral mucosal diseases in situ (Table 2). Moreover, some types of hydrogel have been applied with iontophoresis, which can help transport them at a greater volume and rate in contrast to passive delivery.95
| Mucosal disease | Hydrogel | Cargo | Characteristics of hydrogel | Therapeutic outcome | Ref. |
|---|---|---|---|---|---|
| Abbreviations: PVA, polyvinyl alcohol; HP-β-CD, hydroxypropyl-beta-cyclodextrin; DMA, dexamethasone acetate; Xan, xanthan; HEC, hydroxyethyl cellulose; BUF, buflomedil hydrochloride; FS-NTF, fluconazole-loaded sesame oil containing nanotransfersomes; HPMC, hydroxypropyl methylcellulose; Hst-5, histatin-5; HA, hyaluronic acid; TA, triamcinolone acetonide; TMC, trimethyl chitosan; EPO, erythropoietin; GP, β-glycerophosphate; DOX, doxycycline. | |||||
| Xerostomia (dry mouth) | Lactoferrin and κ-carrageenan | Oppositely charged hydrogels reinforce surface adsorption and reduce friction | Possessed better lubricity than real saliva | 92 | |
| PVA | Aceclofenac and itraconazole | Applied as spray covering wide range | Mitigate xerostomia and prevent oral candidiasis in HIV patients | 288 | |
| Oral lichen planus | Poloxamer 407 | DMA | Incorporate HP-β-CD and xanthan gum to increase solubility and adhesion | Sustain drug release in situ | 289 |
| Oral submucous fibrosis | 2% HEC hydrogel | BUF | Applied with iontophoresis to enhance BUF transport capacity | Obviously facilitates treatment and compliance | 95 |
| Oral candidiasis | HA | FS-NTF | HA–FS-NTF exhibited significant ex vivo permeation | The synergistic effect of HA–FS-NTF improved antifungal efficacy | 290 |
| HPMC | Hst-5 and its resistant peptide variant | HPMC performed a controlled sustained release of Hst-5 | Clear existing lesions and inflammation, promote wound healing | 291 | |
| Alginate microsphere | Nystatin | Sustained release and intimate contact to mucosa due to high surface-to-volume ratio | Inhibited the C. albicans growth with no systemic absorption or tissue damage | 292 | |
| Recurrent aphthous stomatitis | HA mouth wash/topical gel | — | HA performed barrier function and therapeutic effect in situ | Exhibited reduction of lesion and pain, prevented secondary infection | 293 |
| Oral inflammatory disease | Chitosan/fucoidan | TA | Exhibited enhanced swelling behavior, mechanical strength and adhesion | Exhibited antibacterial property, cyto-compatibility and histocompatibility | 294 |
| Oral mucositis | TMC/GP | EPO | TMC gelled at physiological pH and temperature due to GP | Exhibited antimicrobial and wound-healing property and remained long-term on mucosa | 295 |
| HP-β-CD | DOX | Protected doxycycline from the degradation caused by oxidation and epimerization | Prevented DOX from degradation in an aqueous formulation for up to 5 years | 296 | |
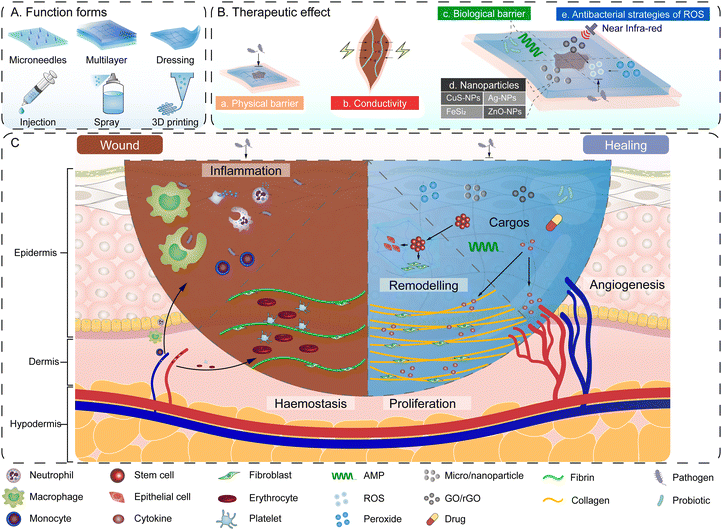
5.2. Wound healing
Wound healing is essential to maintain the barrier function of skin or mucous, but it is often affected by wound infection and scar formation. Wound infection may cause chronic and incurable wounds, leading to adverse effects on the patient's health, feelings of discomfort and pain, and significant medical costs.2 Wounds located in oral and maxillofacial regions also cause hypertrophy or scarring, leading to dysfunction of facial muscle and adversely affecting aesthetics. Scars may cause itching discomfort or even neuropathic pain, which reduces quality of life for patients.96 Therefore, the healing of oral and maxillofacial wounds is very important. Existing wound management strategies include dressings,2 skin substitutes,97 negative pressure wound therapy,98 growth factors and cytokines99 and hyperbaric oxygen,100 but it is difficult to maintain these long term on the oral and maxillofacial skin, or they affect the fundamental functioning of patients in daily life. Therefore, the need to develop a novel anti-infection and self-healing strategy with long-term function in the oral and maxillofacial region is urgent.Hydrogels have been selected as a suitable material to meet the above needs. Hydrogels perform outstanding drug delivery for multiple anti-infection and healing-promoting drugs or other components and adhere to wounds in situ. Based on the 3D structure, mechanical properties and cargo-delivery capacity of hydrogels, researchers have developed various forms of hydrogel carriers loaded with anti-infection drugs or other components for common infection, including injection,21 printed scaffold,101 spray dressing,102 dressing,103 and bio-membrane.104 Compared with conventional drug carriers for wound healing, hydrogels can respond to elevated wound temperatures via sol–gel conversion and changes in pH owing to binding of carboxyl groups to protons on polymer chains and the change of amount of hydrogen bonds.42 The physical cross-linking structure and swelling ratio of hydrogels changes, thus releasing anti-inflammatory drug and antibiotics on demand for better infection control in complicated wounds.105
As well as drugs, other components delivering therapeutic effects, such as silver nanoparticles (AgNPs), hydrogen peroxide (HP) and antimicrobial peptides (AMP), are usually combined with hydrogels for the treatment and healing of complicated wound infection. These components are able to individually exert an anti-infective effect, but lack capacity for long-term preservation at the lesion sites due to oxidation and enzymatic hydrolysis. Hydrogels provide a suitable adhesive carrier. In recent research, multifunctional Ag-Lignin NPs-hydrogels have been reported to significantly speed up the healing of methicillin-resistant Staphylococcus aureus (MRSA)-infected wounds in in vivo experiments. Silver ion has antibacterial and anticancer properties, but it can be easily oxidized which weakens the therapeutic effect. Researchers proposed a novel strategy of using lignin with an antioxidant property to reduce silver ions in situ. Lignin was also used as a crosslinking agent to modified hydroxypropyl cellulose-based hydrogels with phenylboric acid by a dynamic borate bond, endowing the hydrogels with outstanding properties including self-healing, tissue adhesion and electrical conductivity, thus accelerating collagen deposition and promoting wound healing.106 In addition, HP and AMP could deliver an antibacterial/antibiofilm effect, but their short half-life and difficulty remaining at the lesion site has limited their application as wound-healing dressings. Based on cocktail therapy (the combination of several antibacterial agents), researchers co-delivered HP with AMP and chitosan, overcoming the challenges of short half-life and removal of therapeutic ingredients.107
A hydrogel-based antibacterial strategy can also be applied for infection prevention in complex wounds including tumor surface ulcers and diabetic wounds. An in situ-forming nanocomposite hydrogel (NCH) containing PLGA–carboxymethyl CS nanoparticles has been reported for localized pH-responsive skin cancer therapy and wound healing. This hydrogel could respond to the pH at tumor sites and release antimicrobial drugs, an antibacterial function confirmed by observation of significant zones of inhibition in S. aureus and E. coli culture.108 Hydrogels encapsulating bioAgNP-nanocrystals could further demonstrate inhibition of the growth of P. aeruginosa and MRSA, and the proliferation of tumor cells.109 In the case of bacterial inhibition in diabetic wounds, Liu et al.110 prepared a poly(ε-caprolactone)24-block-poly[lysine15-stat-(S-aroylthiooxime)23] polymersome to induce H2S generation and protect AMP from degradation. AMPs provided a membrane disruption effect to inhibit bacterial proliferation, which led to reduced risk of antibiotic resistance. Sustained H2S generated on polymersome membrane further exerted the significant property of bacterial inhibition. These findings have proved that the combination of antimicrobial agents and hydrogels may be a prospective strategy for the treatment of complicated wounds.
The treatment strategy of the combination of hydrogel and other physiotherapy can also be found in wound healing. A sprayable β-FeSi2-incorporated sodium alginate (FS/SA) hydrogel combined with photothermal therapy (PTT) and chemodynamic therapy (CDT) was reported to achieve convenient and adequate antibacterial and anti-tumor effects. The hydrogel spray was prepared for instant gelation after spraying in situ, releasing bioactive Fe and Si ions to sterilize and promote the migration and differentiation of endothelial cells and pro-angiogenesis with enhancement of PTT and CDT.111
Recently, antibacterial strategies associated with living bacteria have been proposed. A CuSNPs–HA–Fe3+–EDTA hydrogel (CHFH) demonstrated a bacteria-triggered strategy. The HA skeleton would be decomposed by hyaluronidase secreted by bacteria with Fe3+ released, which would be reduced into Fe2+ in the microenvironment of bacteria to trigger Fenton's reaction. Hydroxyl radicals would be produced and perform a short-distance sterilization based on L-CDT. Combining the photothermal characteristics of CuSNPs with hydrogels, sterilization with low-temperature photothermal therapy (LT-PTT) was realized, improving the antibacterial efficiency and minimizing the damage to normal tissue. CHFH has been applied to prepare a band-aid to effectively adhere to wounds and promote the wound-healing process for Staphylococcus aureus infection in vivo.112 This case indicated the significance of the stimulus-responding properties of hydrogels in providing a better infection control strategy. In another strategy to control chronic infected wounds, a living probiotic, Lactobacillus reuteri, was encapsulated into hydrogel microspheres. Hydrogel microspheres enabled probiotics to adhere to and colonize in situ wounds, exhibiting capabilities against harmful bacteria through microbial competition and secreting antibacterial substances to promote healing of the infected wound and new tissue regeneration.113 Therefore, hydrogel biofilms based on living probiotics may provide a prospective infection control strategy.
With their similarity to the natural extracellular matrix (ECM) and property of adhesion at the moist wound site, hydrogels also perform a promoting effect in hemostasis and wound healing.114 Researchers initially constructed ECM hydrogels from decellularized tissues, but significant batch-to-batch variability limited their applications. Researchers then extracted components from ECM to construct hydrogels such as fibrin and glycoprotein.44 Recently, Javier Navarro et al.115 proposed an innovative hydrogel preparation strategy that extracted keratin from human hair as biological ink for healing dermal burn wounds. The keratin hydrogel accommodated the thickness and contours of wounds by 3D printing and restored the barrier function of skin against water loss or bacterial infection. Loaded with collagen I synthesis inhibitor, halofuginone, keratin hydrogel exhibited a reduction in wound contracture and collagen accumulation in an in vitro collagen gel contracture assay and an in vivo porcine burn model. This novel keratin hydrogel provides a stable and tensile bio-ink for 3D printing in the complex topographical reconstruction of skin tissues.
After various self-healing protein hydrogels had been confirmed, researchers turned to developing stem cell-based wound healing. Researchers combined PLGA and a GelMA–MSC mixture (GMM) to fabricate a detachable hybrid microneedle depot (d-HMND) to deliver and cultivate MSCs. The MSC viability remained above 90% in d-HMND for 24 hours and active VEGF release was found. The d-HMND exhibited elevated wound-closure rates, re-epithelialization, and microvasculature in an in vivo full-thickness skin excisional wound mouse model, which proved that the combination of hydrogels and microneedle is a feasible stem cell delivery strategy for skin regeneration. However, MSC viability demonstrated a sharp decrease to 10% viability at 48 h, indicating that the preservation ability of cell viability of d-HMND needs to be improved before clinical translation.116
Hydrogels also promote wound healing by regulating cell behaviors. A spatiotemporally dynamic therapy (SDT) based on hydrogels has been proposed to provide spatially dynamic responses for regulating cell behaviors for adapting to the wound micro-environment. Dynamic CS–poly(ethylene glycol) Schiff-base linkages were combined with a liquid drug Kangfuxin (KFX) into a gel form. The hydrogels provided space for the regeneration of skin tissues, adapted to irregular wounds by dynamically adjusting the shape, and could regulate biomolecule signals and endogenous cell behaviors, forming an ordered structure similar to normal skin instead of disorderly scar.117 Peroxides such as reduced graphene oxide (rGO) have also been proved to regulate cell behaviors. Hydrogels provide porous scaffolds for rGO, allowing it to enhance cell proliferation and blood vessel formation by increasing the concentration of ROS. The enhanced vascularization by upregulating expression of CD-31 and improved collagen deposition during the wound-healing process reflected the improved promotion of these effects in a full-thickness mouse skin wound model.118 Generally, researchers can modify the micro-environment of stem cell behavior through a hydrogel carrier to regulate skin regeneration and wound healing (Fig. 4).
In recent years, attention has been focused on the effect of the physical environment at wound sites. ROS generated by chronic wounds or bacterial infection have been demonstrated to further impede wound healing. Aimed at scavenging ROS, ceria nanoparticles that exhibit high superoxide dismutase mimetic activity have been incorporated into polymer vesicles to inhibit superoxide free radicals. At a low cerium concentration of 1.25 μg mL−1, the hybrid hydrogel significantly cured infected diabetic wounds within 2 weeks, with a high bacterial inhibition rate of ∼50%.119 Research demonstrated that skin wound healing can be accelerated by appropriate electrical stimulation through physiological mechanisms including immunocyte recruitment, inflammation resolution, and the effect on tissue blood flow and migration and proliferation of cells and so on.120 Based on this theory, the use of conductive materials to promote the activity of electrically responsive cells is an effective means of accelerating wound healing.121 An antibacterial electroactive anti-oxidant injectable hydrogel with self-healing and adhesiveness properties containing quaternized CS-g-polyaniline (QCSP) was reported as an antibacterial, anti-oxidant and electroactive dressing. The hydrogel probably produced electroactivity to transfer bioelectrical signals and promote the wound-healing process.21 Moreover, self-healing conductive hydrogels122 and biodegradable and electroactive regenerated bacterial cellulose/MXene (Ti3C2Tx) composite hydrogels are currently under development. These results suggested that hydrogels combined with external stimulation may be a prospective study direction to promote the healing of oral and maxillofacial skin or mucosal wounds and repair the facial appearance.
6. Treatment of periodontal diseases
Periodontal disease is a destructive inflammatory disease of periodontal tissue caused by bacterial infection, characterized as a degenerative and inflammatory state involving gingiva, cementum, alveolar bone, and periodontal ligament (PDL). Severe periodontitis causes destruction of periodontal support tissues and tooth loss in 10–15% of the global population, mainly including severe aggressive periodontitis (mainly affecting adolescents or young adults) and severe chronic periodontitis (mainly affecting the elderly). Therefore, the prevention and treatment of periodontal disease is an urgent problem. Owing to the colonization form of dental plaque, pathogens such as Porphyromonas gingivalis are difficult to remove and lead to long-term periodontitis. The bacteria themselves with their virulence factors further induce inflammatory responses in the host, leading to irreversible destruction of the alveolar bone and fibrosis.123 Generally, the treatment of periodontal disease faces two main challenges, i.e., control of periodontitis and periodontal tissue reconstruction.Hydrogels provide a prospective therapeutic platform for periodontitis control and periodontal defect repair. Using their transport and controlled release capabilities, hydrogels have been applied as an advanced drug delivery system for the treatment of periodontal infection and inflammation.124 In particular, the periodontal defect lacks self-healing ability, and hydrogels can be used in periodontal tissue regenerative engineering in the form of bio-membrane or regeneration scaffold.125 The current application status of hydrogels for drug/cell delivery and periodontal regeneration in the treatment of periodontitis will be demonstrated in detail below.
6.1. Control of periodontitis
Hydrogels have been proved to be an appropriate drug delivery device to improve the effectiveness of periodontitis treatment, including in complex periodontal pockets. Hydrogels are usually made into an injection to achieve local drug delivery in the periodontal pocket. Through diffusion from hydrogels, the concentration of antibiotics increases and the concentration of periodontal pathogens reduces in the periodontal pocket with no effect in other tissues or systemic reactions.126 Common antibacterial strategies are to incorporate antibiotics (e.g., minocycline,127 doxycycline3), anti-inflammatory drugs (e.g., atorvastatin128) and ions (e.g., silver,129 iodine130) into hydrogel carriers or polymer vesicles for injection into the periodontal pocket.131 These ingredients can slowly diffuse into the periodontal pocket, providing a durable and in situ therapeutic effect due to the adhesion and transport properties of hydrogels without significant side effects. Antibacterial drugs such as chlorhexidine can also be encapsulated in hydrogels and applied for bacterial control in the periodontal pocket or for an auxiliary antibacterial effect after scaling and root planing.132Different from the direct inhibition or sterilization effect of drugs on bacteria, a therapeutic strategy of regulating the microenvironment of bacterial growth in periodontal pockets has been proposed. The novel hydrogel-encapsulated Arenicola marina's hemoglobin (M101) has been reported to inhibit the survival of anaerobic bacteria and promote wound healing through increasing oxygen content in periodontal pockets. With better oxygen-carrying capacity than human hemoglobin and the superoxide dismutase property associated with Cu/Zn, M101-loaded hydrogel significantly increased the oxygen content in the periodontal pocket, avoiding secretion of pro-inflammatory cytokines and periodontal cell death due to hypoxia caused by P. gingivalis lipopolysaccharide.133 Therefore, inhibition of anaerobic bacteria by efficient oxygenation in the periodontal pocket with hemoglobin may be an innovative and prospective treatment strategy for periodontitis.
Compared with simple diffusion administration, controlled administration based on stimulus-response characteristics of hydrogel exhibits a more precise therapeutic effect. Common hydrogel construction strategies typically utilize thermal and pH response characteristics. Aqueous hydrogels initiate gelation to achieve adhesion to the periodontal pocket when responding to body temperature and degrade in acidic environments to accelerate drug release.134 In addition, a novel GelMA–Au NBPs@SiO2 hybrid hydrogel applied with photothermal therapy exhibited better controlled minocycline delivery. 808 nm NIR light irradiation significantly accelerated drug release and performed a photothermal effect for sterilization.135
Gingipains are a family of cysteine proteinases secreted by P. gingivalis and play a role as one of the most important virulence factors in the periodontitis process.136 Aiming at the enzymatic ability of gingipain, Shiyue Liu et al.137 facilitated the hydrogel scaffold to crosslink with a novel designed functional peptide module (FPM) via Michael-type addition reaction, and further incorporated stromal cell-derived factor-1 (SDF-1) into the hydrogel. The FPM demonstrated a structure of anchor peptide–short antimicrobial peptide (SAMP)–anchor peptide that was cleaved by gingipain specifically. SAMP was then released from the hydrogel carrier in response to gingipain to produce an antibacterial effect. The gingipain-responsive hydrogel demonstrated significant inhibition of P. gingivalis reproduction in antibacterial testing and in an in vivo study, indicating that it may be a prospective candidate as an on-demand local drug delivery system for periodontitis. Inspired by the gingipain-responsive hydrogel, we put forward the constructive hypothesis that researchers could focus on drug delivery strategies for periodontitis treatment that specifically recognize virulence factors, including protease or even LPS, fimbriae, and bacterial capsule of the pathogenic bacteria. Specifically, recognition may be a potential strategy to improve the accuracy of drug administration and infection control rate.
Another on-demand drug delivery strategy based on enzymatic hydrolysis was confirmed to be effective for the treatment of stubborn infection caused by Enterococcus faecalis. Researchers covalently linked GO with the peptide linker (Gly–Gly–Leu) to amoxycillin (AMOX) and encapsulated this GO–AMOX complex into an alginate hydrogel capsule. Bromelain (BROM) was then encapsulated into the GO–AMOX capsule to hydrolyze the peptide junction as catalyst and release AMOX, simultaneously producing enzymatic hydrolysis of bacteria. This hydrogel preparation strategy for drug delivery does not exclude the simultaneous attachment of multiple drugs.138 Therefore, the study may provide a more flexible and modifiable sterilization strategy for wide range of bacterial strains, which still needs to be confirmed by further research and pre-clinical investigation.
Although various antibacterial strategies based on hydrogels have been proposed, some researchers have pointed out that bacterial infection is only the initial factor in the progression of periodontitis, while dysregulation of the host immune-inflammatory response mediated by pro-inflammatory macrophages ultimately induces periodontal tissue degeneration. For a sensitive host with severe periodontitis, periodontal system treatments are usually ineffective due to the continuous progress of alveolar bone resorption and periodontium inflammation caused by pro-inflammatory macrophages.139 Therefore, researchers propose that it is necessary to control the immune inflammatory response based on bacterial infection control. Owing to the immunomodulatory and anti-inflammatory properties of DPSCs-derived exosomes, researchers encapsulated DPSC-Exo into CS to constitute a DPSC-Exo/CS injectable hydrogel carrier for modulating the macrophage phenotype. In in vitro and in vivo models, DPSC-Exo/CS facilitated macrophages to convert from a pro-inflammatory phenotype to an anti-inflammatory phenotype through downregulating the chemotaxis pathway, thus alleviating the periodontal inflammatory response and mitigating epithelial lesions and alveolar bone loss.140 Although the authors claimed that DPSCs and PDLSCs could both show the therapeutic effect on periodontitis, they did not explain their reasons for not choosing PDLSCs. Moreover, the authors claimed that the existence of some cell culture supplements in DPSC medium may increase the risk of transmitting infectious bovine pathogens and cause a xenogeneic immune response; thus this anti-inflammatory strategy associated with MSCs still needs to be validated in more studies. Generally, the combination of antibacterial and anti-inflammatory strategies may be more beneficial for the treatment of periodontitis, and both of them can be implemented by hydrogel carriers.
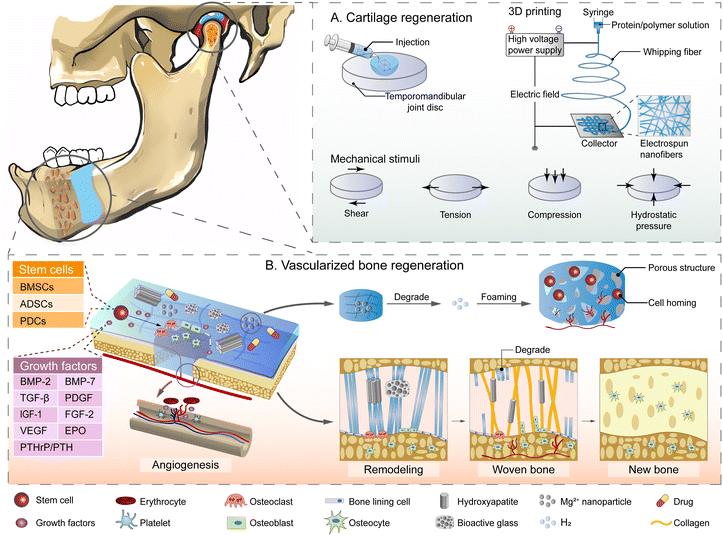
6.2. Periodontal tissue regenerative engineering
Periodontitis can lead to irreversible alveolar bone resorption and attachment loss, resulting in loose or even loss of teeth. After systematic periodontal treatment, the inflammation of periodontal tissue can be controlled, but the damaged periodontal tissue cannot be restored to the original level, thus the problem of tooth loosening and loss is still difficult to solve. Researchers try to restore the periodontal ligament and alveolar bone to the original level through the regeneration of periodontal tissue for stabilizing the loosened teeth.141 At present, the methods of periodontal tissue (including alveolar bone) regeneration mainly refer to guided tissue regeneration (GTR) and guided bone regeneration (GBR),88 which use a barrier membrane to isolate gingival epithelial cells from defects, providing space for periodontal tissue growth. The available barrier membranes can be classified into absorbable films and non-absorbable films, but both have some limitations. The absorbable membranes are often composed of collagen, and have shortcomings of rapid degradation, insufficient mechanical properties, poor antibacterial properties and poor barrier function to prevent the epithelium from growing downward to the defects. Insufficient isolation often leads to the regeneration of long junctional epithelium attached to the root surface through hemidesmosome rather than periodontal ligament, resulting in poorer sealing and antibacterial ability than in periodontal ligament fibers or even the recurrence of periodontitis.88 The non-absorbable membrane requires a secondary surgery to be removed, which leads to unnecessary damage.142 Hydrogels have been proved to be suitable materials for GTR/GBR membranes (Table 3) with significant adhesion and antibacterial properties that precisely match the alveolar bone defects, preventing the invasion of gingival epithelial cells and providing space and a microenvironment for the growth of regenerative periodontal tissue. By adjusting the composition and proportion of polymers, the surface porosity of the hydrogel structure can be adjusted. The adhesion and proliferation of stem cells such as human periodontal ligament fibroblasts (HPDLFs) and human gingival fibroblasts (HGFs) can be regulated on demand through controlling the porous structure of the hydrogel membrane.143 The retention and degradation of hydrogel membranes can also be accurately designed using controllable environmental stimuli.144| Hydrogel | Regulated cell | Function | Main cargo | Crosslinking method | Results | Ref. |
|---|---|---|---|---|---|---|
| Abbreviations: PVA, poly(vinyl alcohol); Col, fish collagen; Si-HPMC, silanized hydroxypropyl methylcellulose; MA-CMCS, methacrylated carboxymethyl chitosan; HAP, hydroxyapatite; β-TCP, β-tricalcium phosphate; EMD, enamel matrix derivatives; GelMA, methacrylated gelatin; PEGDA, poly(ethylene glycol) diacrylate; I2959, 2-hydroxy-1-(4-(hydroxyethoxy)phenyl)-2-methyl-1-propanone; PCEC, polycaprolactone–polyethylene glycol–polycaprolactone; BG, bioactive glass; OPC, oligomeric proanthocyanidin; BC, bacterial cellulose; BD2, beta-defensin 2; MEW, melt electrowriting; PCL, poly(ε-caprolactone); AMP, amorphous magnesium phosphate. | ||||||
| PVA/Col | hPDLFs, hGFs | Physical barrier, cell culture | Physical | Exhibited controllable cell attachment with proper cell morphology in vitro | 143 | |
| Si-HPMC/MA-CMCS | Human primary gingival fibroblasts, human gingival explants | Epithelial barrier | Riboflavin phosphate (photo-crosslinking agent) | Chemical | Performed rapid formation with a dentistry lamp and barrier effect against soft gingival tissue cell invasion | 144 |
| Sodium alginate | BMSCs | Antibacterial, hemolysis, osteogenesis | Cu2O and polydopamine-coated TiO2 (TiO2@PDA) nanoparticles | Chemical | Performed precise match with bone defects and osteogenesis with photothermal effect of NIR, and antibacterial activity with blue light | 297 |
| Gelatin/HA | Fibroblasts | Antibacterial | Hinokitiol (antibacterial agent) | Chemical | Performed significant antibacterial effects with hinokitiol, and prolonged degradation for abundant cell attachment | 298 |
| Gelatin with β-TCP/HAP | MSCs | Physical barrier, osteogenesis | EMD and BMP-2, PDGF, electrospun nanofibers | Physical | Promoted periodontal osteogenesis, showed potential to simultaneously reconstruct alveolar ridge, periodontal ligament and cementum | 299 |
| Alginate | Fibroblasts, osteoblasts | Osteogenesis induction | HAP nanoparticles | Chemical | The bilayered membrane constituted a highly porous fibrous side for fibroblasts growth and a mineral-rich side with higher roughness for osteoblasts growth | 300 |
| GelMA/PEGDA | Mouse osteoblasts MC3T3-E1 culture | Antibacterial, cell culture, rapid formation | I2959 (photocrosslinking agent) | Chemical | Showed high mechanical strength, long degradation time, fast diffusion rate, and high viability, adhesion, and proliferation of osteoblasts | 301 |
| PCEC | DPSC | Physical barrier, osteogenesis | Bismuth-doped BG and GO | Chemical | Showed significant space filling and barrier function, promoted osteogenic effect and mineral deposition property | 302 |
| Oxidized HA/type I collagen | MG-63 human osteoblast-like cells | Antibacterial, drug delivery | β-TCP, tetracycline | Chemical | Showed antibacterial effect by drug release of tetracycline and high mechanical strength with β-TCP | 303 |
| BC/alginate | rBMSC | Antibacterial, osteogenesis, angiogenesis | BD2 (antibacterial peptide) | Physical | Provided suitable space and microenvironment for bone regeneration and showed antibacterial ability of BC | 304 |
| GelMA | Physical barrier, osteogenesis | MEW–PCL, AMP, electrospun nanofibers | Chemical | Enhanced bone formation with bioactive AMP and the reinforcing PCL mesh, reduced the degradability in vivo | 305 | |
The regulation of cell behavior by hydrogel has been a concern of researchers, thus hydrogel containing stem cells has been used in periodontal tissue regenerative engineering. The 3D structure of hydrogels can deliver stem cells such as DPSCs and MSCs to the defect and maintain bioactivity, providing space and a microenvironment to promote their proliferation and osteogenic differentiation, and making hydrogels a suitable carrier for periodontal tissue regenerative engineering.145 In order to further promote the proliferation, migration, and osteogenic differentiation of stem cells, growth factors (GFs) are usually incorporated into hydrogel scaffolds. In a recent study, 6-bromoindirubin-3′-oxime (BIO) was encapsulated into PLGA microspheres and loaded on HA hydrogel. Injected into the gingival sulcus of mice with experimental periodontitis, the PLGA–HA hydrogel system could release BIO via free diffusion and promote the osteogenesis process.146 Other studies demonstrated similar results associated with hydrogels incorporating SDF-1, BMP-2, and NapFFY.147 These results indicated that hydrogels could be an excellent platform for PDLSCs and MSCs to achieve better periodontal bone regeneration under the regulation of active GFs.
Recent reports suggested that PDLSCs’ behavior can be further regulated by modifying the polymer chain of hydrogels. Unlike the strategy of loading regulatory signal molecules, some hydrogels have been proved to regulate cell behavior spontaneously. Previous studies have found that peptides with RGD or laminin cell adhesion motifs promoted the activities of periodontal ligament (PDL) fibroblasts. Some researchers were inspired and used self-assembling peptides (SAPs), series of monomers of short amino acid sequences or repeated amino acid sequences, to develop a hydrogel constitution strategy in recent years.23 In various in vitro model systems, SAP hydrogels showed affinity with the cellular phenotype and cell adhesion, probably due to the adsorption of SAP hydrogel as anchorage point on protein, similar to fibronectin. Furthermore, the stiffness of biomaterials has been proved to influence cellular responses as a crucial parameter in periodontal tissue regenerative engineering. The SAPs can be on-demand designed to yield tailored hydrogel stiffness and elasticities, providing comparatively rigid matrices to induce osteogenic differentiation.148,149 The SAP hydrogel provided a strategy for in vitro cell culture and mineralized deposition for the oriented formation of alveolar bone and periodontal ligament, yet still needs to be confirmed by further in vivo investigation.
Researchers recently noticed that although high-stiffness matrix hydrogel can stimulate osteogenic differentiation of BMSCs, the newly produced macrophages still had a tendency to undergo M1 polarization and compromised cell osteogenesis. Therefore, the strategy of hydrogel incorporating signaling molecules such as IL-4 and stromal cell-derived factor (SDF-1α) to facilitate the M1-to-M2 transition of macrophages was then proposed. BMSCs exhibited an enhanced capability to gather towards SDF-1α in cell migration assays, and macrophages polarized towards an immunomodulatory M2 state in the presence of IL-4, leading to significant attachment recovery (epithelial and connective tissue) and simultaneous regeneration of the entire bone–PDL–cementum complex.150 These results indicated that increasing the capacity for macrophage modulation and cell recruitment in high-stiffness hydrogels represented a practical and effective strategy for promoting in situ periodontal regeneration, simultaneously reminding us of the importance of macrophage polarity in successful alveolar bone reconstruction.
The biggest challenge for researchers is the regeneration of periodontal ligament (PDL). Cementoblasts located at fibroblasts on the tooth root cementum have been proved to be essential for the PDL–root connection.151 Based on this mechanism, researchers have tried to simultaneously constitute cementum–PDL–alveolar bone construction, and initially considered application with laminin. S. Sowmya et al.152 constructed a novel tri-layered nanocomposite hydrogel scaffold which separately loaded cementum protein 1, fibroblast growth factor 2 and platelet-rich plasma to mimic the cementum layer, PDL layer and alveolar bone layer. Microcomputed tomography analysis and histological and immunohistochemical analyses demonstrated new cementum, fibrous PDL, and alveolar bone with well-defined bony trabeculae in comparison. The interposition of a soft hydrogel may trigger a self-arranging alignment response perpendicular to the two hard mineralized surfaces, as new Sharpey's fibers formations have been observed in SEM. There was a significant interface between the relatively dense dentin matrix and the loose collagen structure around the developing root, where cells differentiated into osteogenic phenotypes and had an adjustive ability for fiber mineralization.153 These results indicated that the shaping of new cementum and alveolar bone may provide attachment anchor points for periodontal ligament, which is beneficial for the orderly regeneration of periodontal ligament. Some researchers considered that the key to the regeneration of periodontal ligament is to provide guidance to the orientation of cells with an aligned arrangement of perpendicular collagen fibers. They fabricated functionalized PCL–PEG (PCE) copolymer electrospun nanofibrous mats into porous chitosan (CHI) and applied them in in vivo and in vitro studies. Aligned nanofibers-embedded scaffold could be observed guiding the oriented arrangement and elongation of cells with promoted infiltration, showing a more organized and mature arrangement of regenerated PDL nearly perpendicular against the root surface.154 Therefore, topographic guiding of the hydrogel scaffold may provide an innovative regeneration strategy for aligned arrangement of periodontal ligaments with stabilization function.
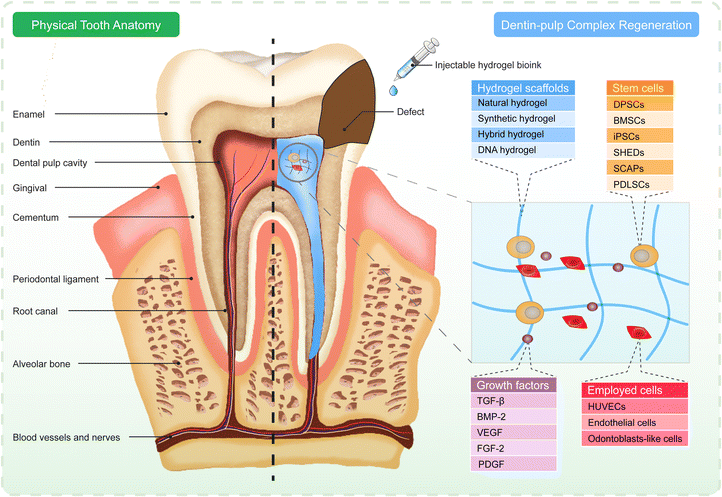
7. Dentin–pulp complex regenerative engineering
Dental pulp contains sufficient fibrous proteins and glycosaminoglycans, which constitute the ECM, to maintain the three-dimensional structure of dental pulp and incorporate vessels, nerves and various cells. Odontoblasts are located along the interface between the dental pulp and dentin and extend cytoplasmic processes into dentinal tubules, making the dentin elastic and full of fluid in order to cushion the bite force. Odontoblasts also regulate the structure of tooth hard tissue by secreting proteoglycans and non-collagen proteins related to nucleation and controlling mineral phase growth. Therefore, the dental pulp is closely related to the structure and function of dentin, forming a closely related complex to protect teeth, namely the dentin–pulp complex.155 In the treatment of pulpitis and necrotic pulp, the dentin with infected pulp and infected microbial membrane needs to be removed by root canal therapy.156 After the removal of dental pulp, the fluid in the dentinal tubules will be rapidly lost and the brittleness of the tooth increased, and the tooth hard tissue structure will become thinner. Both make it difficult for the teeth to resist strong bite forces, thus shortening the lifespan of the teeth. Therefore, researchers try to achieve the preservation of natural teeth through dentin–pulp complex regeneration.The purpose of regenerative endodontics is to reconstruct the dentin–pulp complex to restore the original composition and structure of the teeth. One of the most commonly used methods is pulp revascularization. Successful pulp revascularization case reports show that it is possible to regenerate dental pulp through a cell homing strategy.156 Pulp revascularization activates the proliferation and migration of stem cells from apical papilla (SCAP) to the dental pulp space through apical-induced bleeding, and releases GFs to regulate stem cell behaviors. Pulp revascularization promotes the continuous development of tooth root and prevents the fracture of thin dentin wall, improving the strength and elasticity of tooth resistance to external force.157 Based on the biocompatibility and plasticity of hydrogels, some researchers have applied hydrogels in pulp revascularization to construct an injectable dental pulp vascular regeneration vector. Acellular self-assembled peptide hydrogels have been shown to mimic the structure of ECM and exert neovascularization and tissue deposition in the canine pulp space.158 A GelMA hydrogel loading hDPSC/hUVEC showed the newly formed pulp cells attach to the inner dentin surface with increased matrix deposition of the dentin layer in the root, and partly infiltrate into the dentinal tubules in the root segment of human teeth.159 In addition, the hydrogel loaded with platelet lysate (PL) can not only continuously release chemotactic and angiogenic GFs, but also enhance cell vitality and angiogenic activity, improving the success rate of pulp revascularization.160
However, the current clinical use of pulp revascularization has been proved unable to support dentin–pulp complex regeneration.161 By observing the changes of cementum, bone formation and fibrous tissue after pulp revascularization in 6 purpose-bred mixed-breed canine model dogs, researchers found a lack of organized pulp–dentin complex in the root canal.162 A histological report on the pulp revascularization of the mandibular first molar also confirmed that the root canal was mainly filled with ectopic bone, fibrous tissue and cementum on the inner wall of the root canal dentin, lacking the formation of organized pulp–dentin complex.163 These studies indicated that pulp revascularization can increase the length and thickness of teeth by inducing tissue repair to form osteoid or cementoid tissue to cover the defects, but is unable to support real dentin–pulp complex regeneration. In addition, pulp revascularization has some shortcomings including stagnation of root development, apical insufficiency and pulp space calcification caused by insufficient bleeding.159
Researchers turned to shift their research to hydrogel carriers incorporating stem cells or cytokines. Hydrogels often make use of natural polymers such as HA and decellularized extracellular matrix (dECM) to construct tissue regenerative engineering scaffolds for simulating dental pulp ECM. The structure of hydrogel scaffold is similar to that of collagen fibers in dental pulp, and it has the characteristics of non-toxicity, non-immunogenicity, no significant inflammatory reaction and outstanding biocompatibility.164 Hydrogel scaffolds provide a microenvironment for stem/progenitor cells with odontogenic potential,165 or release GFs to induce peripheral stem cell proliferation.166 Hydrogels usually encapsulate DPSCs, SHED and other regenerative cells (e.g., odontoblast-like cells, HUVEC, SCAP, PDLSCs, endothelial cells and primary dental pulp cells), cell-binding sequences (e.g., RGD), bioactive molecules (e.g., TGF-like 1, BMP-2, VEGF or PDGF) and drugs (e.g., antibiotics).14 In recent years, more attention has been focused on the role played by peptides in stem cell proliferation and differentiation. The ECM-like biomimetic hydrogel multi-functional scaffold composed of self-assembled peptides (SAP) is a novel dental pulp regeneration strategy. The SAP hydrogel scaffold could present RGD and VEGF-mimicking peptide epitopes, providing a microenvironment to promote rapid vascularization for hDPSCs’ attachment and regenerated dental pulp. In in vitro multifunctional groups, hDPSCs showed high survival rate, angiogenesis and odontogenic differentiation in the hydrogel scaffolds. In vivo, hydrogel scaffold can also stimulate pulp recovery and promote dentin regeneration in the histological and functional evaluation of partial pulpectomy rat model.167 We put forward an idea that using peptides to construct a hydrogel skeleton with stem cells or cytokines as dental pulp regeneration scaffolds may achieve a higher success rate of dental pulp–dentin complex regeneration (Fig. 5).
Hydrogel scaffolds are often made into various forms for filling irregular spaces of dental pulp cavities and root canals, the most common of which is injectable hydrogel.168 Injectable hydrogels could ensure a high survival rate of DPSCs of more than 85% and sustained release of VEGF bioactivity for 28 days. After 7 days of hydrogel injection, ECM deposits such as fibronectin (FN) and type I collagen could be found, with the formation of newly formed dental pulp-like tissue and microvessels.169 Recently, 3D bio-printing and bio-ink has enabled hydrogels that accurately match the irregular shape of the dental pulp cavity. One 3D-printed hydroxyapatite-loaded hydrogels scaffold containing peptide and hDPSCs has been reported to be transplanted into immunocompromised mice, showing significant inward growth of blood vessels, differentiation of odontoblast-like cells and cementum deposition.170 Hydrogel bio-ink with excellent viscosity, printability, cytocompatibility and odontogenic capacity may be an outstanding candidate for constituting scaffolds with precise 3D micro-architectures for dentin–pulp complex regeneration.
One of the biggest challenges of regeneration of dentin–pulp complex is the regeneration of dentin. Hydroxypropyl methylcellulose (HPMC) has been reported to be applied as a film and hydrogel carrier for polyaspartic acid-stabilized amorphous calcium phosphate (PAsp–ACP) nanoprecursors to deliver biomimetic mineralization of dentin. The hydrogel induced the early mineralization of demineralized dentin after 24 h, with increasing mineralization of the whole demineralized dentin (3–4 μm) after 72–96 h.171 In another similar study, notoginsenoside R1 (NGR1) was also loaded in Gel–MA as pulp-capping materials to induce dentin formation.172 Thus, hydrogels have the ability to induce dentin or dentin-like layer mineralization and thickening, helping promote reparative dentinogenesis. Moreover, decellularized matrix hydrogels simply incorporating hDPSCs173,174 or loading hDPSCs with platelet-derived growth factor (PDGF)175 have demonstrated that hDPSCs could be found differentiating into odontoblast-like mineralizing cells in the hydrogel scaffold. These results showed that that a dentin-like mineral composition can be produced in the pulp cavity. However, these transformations are unstable, and there is no evidence that odontoblast-like cells can grow into dentin tubules. Therefore, dental pulp regeneration needs to be realized through further research.
8. Bone regenerative engineering
Bone is a dynamic self-healing organ whose structure and function are continuously remodeled to repair small-scale bone defects or facilitate the connection of small displaced fracture segments. However, post-resection of oral and maxillofacial tumors and trauma often results in larger defects of craniofacial bone and jawbone, requiring extensive repair by bone tissue engineering.1 In this procedure, hydrogels are often used to construct bone substitutes or bone grafts.176 Hydrogels can incorporate and on-demand release stem cells and other therapeutic components, with injectable ability to reduce the complexity of surgical resection and degradative property within the bone regeneration process, reducing the postoperative inflammatory response and damage caused by secondary surgical removal.177Hydrogel scaffolds are mainly used to encapsulate growth factors (e.g., TGF, BMP, VEGF, EPO),178 small molecular bioactive factors (e.g. kartogenin,95t-butyl methacrylate179,180), and proteoglycan181 to promote the proliferation and osteogenic differentiation of osteoblasts and osteocytes. Hydrogels have various application forms including injection,181 CAD/CAM,182 3D printing and bioink.183 Adjusted with CAD/CAM and medical imaging systems, hydrogels can be customized or manufactured on multiple scales of length or width of bone by 3D printing. Moreover, the cross-linking density and matrix stiffness of stimulus-responsive hydrogels can be on-demand adjusted by physical stimuli such as ultraviolet, pH, light and temperature, so that the regenerated bone can achieve structure and mechanical strength similar to natural bone fibers.184,185
In novel studies, researchers have focused more attention on enzyme-response hydrogel preparation strategies for better modification of the hydrogel scaffold structure in bone regenerative engineering. Researchers crosslinked chondroitin sulfate–tyramine (TA) and HA–TA by hydrogen peroxide (H2O2) and horseradish peroxidase (HRP) to fabricate an injectable hydrogel system encapsulating BMP-2 and BMSCs as a 3D scaffold for BMSCs culture. In vivo and in vitro studies demonstrated that BMP-2/BMSCs-loaded hydrogel can adhere to bone defect, promoting cell proliferation and differentiation and thus achieving bone regeneration.186 The enzyme-catalyzed crosslinking approach provides an innovative strategy for rapid in situ forming and optimal the scaffold structure. The gelation rate and mechanical strength of the enzyme-responsive hydrogel could be adjusted by the composition of H2O2 and HRP, thus BMP-2 could be controllably released to regulate the growth of BMSCs. In addition, hydrogen and oxygen were produced by H2O2 decomposition, increasing the porous rate and oxygen content of the hydrogel scaffolds and promoting the orderly growth of BMSCs and neovascularization along the scaffolds.
The phenomenon of oxygen produced by the decomposition of H2O2 inspired researchers to focus on the function of gas in the process of increasing the porosity of osteogenic scaffolds. Researchers have proved that porosity is necessary to drive angiogenesis and osteogenesis behaviors through enhancing oxygen and nutrient diffusion and promoting integration between the hydrogel scaffold and host tissue after homing of BMSCs.187 In order to increase the porosity rate, researchers have constituted interconnected porous structure by 3D-printing,188 electrospinning,189 and magnetic control.190 The enzyme-catalyzed crosslinking hydrogel inspired researchers and provided an innovative strategy for biologically friendly regulation of hydrogel porosity. A novel approach of foaming in hydrogels through generating gas by metal degradation has been proposed. Magnesium (Mg) particles were added into the cell-loaded hydrogels and then generated H2 gas in its degrading process, which indirectly regulated the porosity at a mean size of 20 μm of newly formed bone. In vivo, viability and proliferation of BMSCs in the Mg particles group were significantly improved, probably due to the enhancement of nutrients and oxygen diffusion and infiltration of surrounding tissue and vessels.187
The porous structure of hydrogel scaffolds not only increases the oxygen content and promotes the growth of cells into the hydrogel scaffold, but also promotes the growth of neovascularization, which is also essential for bone regenerative engineering. The plasticity of hydrogels enables the construction of vascular structure by 3D biological printing or biological ink, or promotion of vascularization by releasing biological factors.191 With cells and hydrogels, 3D bioprinting can create vascular/nutrient channels with a diameter of more than 150 μm. The microvascular system supports the self-assembly of the body into a capillary network structure by introducing bioactive biological ink based on hydrogel.192 Hydrogel bioinks are usually composed of a combination of ECM proteins and dECM to provide tissue-specific biological cues and improve cell function and tissue.193 Due to the batch-to-batch variation of ECM, researchers began to use purified peptides and GFs as stable functional fragments. Peptide-driven self-assembling hydrogel has been proposed as an improved biological ink for angiogenesis in vivo.194,195 Shuai Yuan et al.196 encapsulated platelet-rich fibrin (PRF) into gelatin nanoparticles (GNPs) to construct a composite injectable hydrogel. Histological results demonstrated that the GNPs + PRF hydrogel significantly produced an enrichment area of vessels and woven bone with low osteoclast activity in bone defect at 2 weeks, and generated corticalization on the new bone at 8 weeks. These results support the characteristics of blood clotting and favorable capacity of promoting angiogenesis and osteogenesis of hydrogels.
Based on the porous structure and delivery characteristics, hydrogel has been widely used in various types of bone tissue regeneration research projects in repairing oral and maxillofacial defects (Table 4). However, several key challenges have not been overcome, including: (1) the mechanical properties of hydrogels need to be enhanced at the stress site; (2) in the process of printing and injecting hydrogels, the activity of cells and cytokines should be maintained; (3) when designing the printed hydrogels, the time dimension needs to be considered. Therefore, improving the mechanical strength of the structure while maintaining the bioactivity of the content may still be the future development direction of hydrogels in bone tissue engineering.
| Hydrogel | Cargo | Mechanism | Application | Form | Ref. |
|---|---|---|---|---|---|
| Abbreviations: WHMP, whitlockite microparticle; MRONJ, medication-related osteonecrosis of the jaw; MAPK pathway, mitogen-activated protein kinase pathway; MBGN, mesoporous bioglass nanoparticles; BSA, bovine serum albumin; BrdU, 5-bromo-2′-deoxyuridine; GMSC, gingival mesenchymal stem cells. | |||||
| Gelatin–HA | VEGF | Pro-angiogenic and immunomodulatory mechanism | Prevention and treatment for MRONJ | Injection | 306 |
| Alginate | WHMP | Upregulate the MAPK pathway | Repair craniofacial bone | Injection | 307 |
| GelMA | BMP-2, MBGN | Promote cell adhesion and osteogenic differentiation | Repair craniofacial bone | Artificial periosteum | 308 |
| PVP | Ti–6Al–7Nb powder encapsulated PLA microspheres | Osteoconduction | Reconstruct jawbone after tumor resection | Coating | 309 |
| PEG–PLGA–PNIPAM | MicroRNA-222 and aspirin | Promote BMSC differentiation into neural-like cells via Wnt/β-catenin/nemo-like kinase signaling | Repair mandibular bone defect | Injection | 310 |
| Calcium alginate–PLA | BSA | Osteoinduction | Repair mandibular bone defect | Injection | 311 |
| Gelatin | BMP-2, RANKL-binding peptide (OP3–4) | Increase BMP-2-induced BrdU-positive cells | Repair alveolar ridge | Injection | 312 |
| Alginate | GMSCs, HAP microparticles | Osteoconduction | Repair peri-implant bone loss | Injection | 16 |
9. Cartilage regenerative engineering
The temporomandibular joint (TMJ) is the only joint in the oral and maxillofacial region, and the TMJ disc is a fundamental fibrocartilage structure between the mandible and the temporal bone and functions in mandibular movement.197 Aging changes and trauma often lead to TMJ dysfunction or structural abnormalities, resulting in TMJ disorders. A specific subset of TMJ disorders involve discal pathologies including internal derangement (ID), disc perforation and osteoarthritis (OA), and can cause substantial damage to the TMJ disc.197 Therefore, the purpose of cartilage tissue engineering based on hydrogel is to repair damaged articular cartilage. The key components of cartilage regenerative engineering are signal molecules, stem cells and scaffolds. With outstanding biocompatibility, adhesion and water absorption ability, hydrogels are applied as scaffold in cartilage tissue engineering.198 In previous studies, the researchers treated porcine TMJ discs with detergents and enzymes to prepare dECM hydrogels containing large amounts of collagen, which has been proved to have rheological characterization and mechanical properties, rapid gelation at physiological temperature (37 °C) and a multiaperture fiber ultrastructure observed under SEM.199 However, foreign implants are often absorbed by the body, thus researchers turned their attention to stem cell-mediated cartilage regenerative engineering. Hydrogel scaffolds commonly incorporate MSCs, pre-differentiated mesenchymal stem cells (PBMCs), embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs).199 Hongzhe Wang et al.18 used a self-crosslinking thiolated hyaluronic acid (HA-SH)/type I collagen (Col I) blend hydrogel and biphasic calcium phosphate (BCP) ceramics to incorporate BMSCs/chondrocytes for fabricating a new bi-layer scaffold, achieving simulation of the specific structure of rabbit condylar osteochondral defects. Compared with acellular implants, the rBMSCs/chondrocytes bi-layer hydrogel scaffolds effectively promoted cell-mediated fibrocartilage regeneration, indicating that a cellular hydrogel regeneration scaffold may achieve better cartilage regeneration (Fig. 6).Hydrogel is mainly used in cartilage regenerative engineering in injectable form and 3D printing owing to its excellent plasticity.200 Injectable hydrogels can be implanted into the deep closed site to fully fill the irregularly shaped defect space and repair it. In recent years, 3D bio-printing has become a more promising strategy for cartilage regeneration, accurately designing and fabricating hydrogel scaffolds. Bioink for cartilage engineering has become the key to the development of 3D scaffolds for repairing cartilage defects to meet the requirements of maintaining cell viability, sustained release of drugs or cytokines and structural strength.200 Some stimulus-responsive (e.g., light, temperature, enzyme) hydrogels can accurately induce morphological or structural transformation of hydrogels.201 However, the mechanical strength of hydrogel bioink may still be difficult to adapt to the frequent extrusion and stretching of the TMJ disc. Therefore, researchers have proposed some strategies to improve the mechanical strength of hydrogels, including the optimization of cross-linking structure and polymer composition. Compared with the conventional single cross-linking structure, the structural strength and toughness of hydrogels with a double cross-linking structure have been further improved. Simultaneously, different polymer chains can usually respond to different external stimuli to achieve more dimensional regulation of the structure of hydrogels.202 In another strategy, researchers have combined 3D electrospun nanofibers with higher toughness and porosity into hydrogel to produce composite 3D scaffolds for better cell infiltration, mechanical properties and adjustable degradability to meet the needs of tissue engineering scaffolds.203
Considering that the friction generated during the TMJ motion process can cause abrasion to the joint disc, the regenerated joint disc must have a stable supporting structure and excellent lubrication function. A shear-responsive hydrogel combined with a supramolecular N-fluorenylmethoxycarbonyl-L-tryptophan network and PAAm/PVA double network (FT-PAAm/PVA) provides a novel hydrogel constitution strategy. The PAAm and PVA double network exhibited a stable supporting structure. In the friction test, gel-state FT disassembled when the shear force proximately exceeded 0.76 mN and transformed into sol-state, forming a lubricating layer between the hydrogel surface and the moving object. Moreover, the loose and porous structures became dense, which further improved the mechanical strength of the hydrogel.204 The addition of FT network in response to shear stress may provide a hydrogel preparation strategy to reduce friction and improve strength for movable temporomandibular joint disc regeneration, which still needs to be further verified by in vivo models.
During motion of the mandible the shape of the TMJ disc changes continuously, so keeping its original structure without deformation is a major challenge. The requirement for shape recovery reminds us that shape memory polymers (SMPs) may be combined with 3D printing to fabricate shape memory hydrogels. As a class of stimulus-response polymers, SMPs can be restored to their original shape from their programmed temporary shape under external stimuli including light, heat, shear stress, magnetism and electricity.205 Here we put forward an idea that the hydrogel scaffold may be designed and manufactured on demand in vitro and the original shape can be restored after input into the defect, which may be more suitable for repairing a complex defect.
10. Salivary gland regeneration
Radiotherapy for head and neck cancer206 and Sjögren's syndrome207 usually leads to reduction of salivary secretory acinar cells and loss of salivary gland function, thus causing xerostomia. Xerostomia has a negative effect on diet, speech, and oral hygiene.208 Currently, there are few treatments for xerostomia, limited to medications of the parasympathetic agonists or saliva substitutes.209 Therefore, researchers are exploring a biomaterial-based approach for cell transplantation to regenerate acinar cells in salivary glands.207The construction of salivary gland tissue in vitro based on hydrogels has been an alternative approach.210 Salivary gland tissue is a complex physiological system and requires the coordination of multiple cell types, including acinar cells and myoepithelial cells that initiate salivary secretion, and ductal cells that modify the ionic components of secretions.208,211 Thus, salivary gland tissue engineering requires essential components, including primary cells, extracellular matrix proteins which orchestrate the differentiation of primary cells into functional structures, and a scaffold that can orderly integrate these components and reconstruct the microenvironment mimicking natural ECM.212 Primary cells commonly used in salivary gland tissue engineering include human salivary stem/progenitor cells (hS/PCs),213 salivary gland-resident stem cells (SGSCs),214 primary submandibular gland (SMG) cells215 and primary human salivary myoepithelial cells (hSMECs).216 Extracellular matrix proteins including basement membrane proteins (e.g., laminin, collagen type I/IV perlecan, nidogen), tight junction proteins (e.g., occludins and claudins) and aquaporins enable acinar cells to exhibit apicobasal polarity and ensure that saliva flows in the right direction.217 Depending on the biocompatibility and biodegradability, researchers use hydrogels to create cell culture systems or scaffolds.
In previous studies of SMG cells’ reconstruction, a commercial ECM, Matrigel (MG), has been proved to apply in 3D culture system in vitro. Matrix proteins initially deposited and formed organizing de novo basement membrane. Salivary stem cell-derived epithelial microstructures then attached to the basement membrane to integrate with connective tissue or support cells, and subsequently exhibited polarity. As the epithelial microstructure surrounded by organized basement membrane matured, it performed spontaneous rotation and formed lumens.218 These cells have increased expression of progenitor markers (e.g., K5, K14, MYC, ETV4, ETV5),219 salivary stem cell markers (e.g., LGR5 and THY1), pluripotency markers (e.g., OU5F1 and NANOG)214 and released intracellular calcium [Ca2+i],216 which indicated that the salivary gland tissue cultured in vitro can perform the function of saliva secretion.
Although the human salivary gland (HSG) cell line differentiated into the acinar phenotype accompanied by acinar cell proliferation and myoepithelial cells, yet programmed cell death ensued which might due to the downregulation of cell markers in vitro.220 Moreover, MG originated from rat sarcoma which is rich in extracellular matrix protein, thus shows significant variability between different batches, and tumorigenicity, limiting its application for salivary gland reconstruction.206,215 Aiming at developing safer hydrogel scaffolds, researchers fabricated hydrogels composed of different polymer molecules, including natural hydrogels (e.g., Matrigel®, decellularized ECM, laminin I/IV) and synthetic hydrogels (e.g., PEG, PLGA).206,217,221,222 Moreover, the addition of regulators in hydrogels such as laminin-1 peptides (e.g., YIGSR, A99), human growth factors (e.g., VEGF, FGF9), matrix metalloproteinase (MMP) and integrin resulted in synergistic effects on salivary gland proliferation and differentiation.223,224
Recent evidence suggests that the growth of salivary gland tissue can be regulated in vitro through modifying hydrogel with peptide. Fibrin hydrogels (FHs) modified with laminin 1 peptide (L1p-FH) have been reported that encapsulate salivary gland stem cells for improving cell migration, proliferation and adhesion, and promoting organized collagen formation. The mechanism of gland regeneration is still not completely understood. One possible explanation is that L1p-FH could have activated resident hS/PCs that contributed to gland homeostasis through interactions with specific integrins. Likewise, a fibronectin (RGDSP)-modified HA hydrogel also improved cell viability, accelerated formation of epithelial spheroids and promoted the progenitor cell population expansion.225 Peptide-modified hydrogels probably promote the proliferation and differentiation of salivary gland stem cells by regulating the growth microenvironment of salivary gland stem cells. For example, neurotrophic factor e.g., neurturin (NRTN) and neurite have been proved essential for morphogenesis of salivary gland cells. Therefore, KP24 peptide was used to modify alginate hydrogel substrate, and SMG tissue growth was found to be promoted by increasing neuronal growth and enhancing neural innervation.210 Collectively, culture of salivary gland stem cells through a modified hydrogel structure may be a more effective strategy to promote stem cell proliferation and differentiation.
Furthermore, some researchers have developed engineered ECM with microbubble (MB) array technology to develop salivary gland tissue chip arrays for functional mouse culture and screening. They demonstrated that mouse and human salivary tissues encapsulated in degradable MMP–PEG hydrogels could be formed into MB arrays, expressing key salivary gland markers and showing polarization localization of functional proteins. The salivary gland simulator (SGm) secreted salivary proteins in response to calcium signal agonists, which could be applied in a high-throughput manner for surveying salivary gland radioprotective drugs.226 After successfully cultivating the salivary gland model in vitro, the researchers tried to implant hydrogels into in vivo models to evaluate cell viability and receptor expression in vivo. Some researchers encapsulated salivary cells into HA hydrogels spheroids in vitro and further implanted them into athymic rats with partial parotidectomy. The salivary cell/hydrogel scaffolds exhibited stable adhesion to the residual parotid gland with no significant inflammatory signs, staining high-expression progenitor cell markers including CD168/RHAMM and CD44.212 Moreover, a study of hS/PC-HA hydrogel scaffold in vivo demonstrated that hydrogel-based cell implants could replace lost or damaged salivary tissues. Immunosuppressed miniswine were developed as large animal models for implant testing, having a similar size and anatomy of gland and similar tissue response as human. The hS/PC-HA hydrogel was inserted under the renal capsule of immunosuppressed miniswine and exposed to a single radical fraction of 15 Gy. Viability studies demonstrated that hS/PCs were still viable at least 8 weeks post-implant, producing α-amylase and expressing significant initiation of bio-integration without obvious signs of rejection or host immune response.227 Hydrogel scaffolds can also promote the secretory function of irradiated salivary glands in vivo. A laminin-1 peptides (A99, YIGSR)-modified fibrin hydrogel was injected into irradiated salivary glands, and exhibited promotion of formation of functional salivary tissue.221 Therefore, the in vitro culture and drug testing of hydrogel in salivary gland tissue, and transplantation in vivo to promote the regeneration of salivary gland tissue may provide a novel and prospective approach for the treatment of irradiation damage of the salivary glands.
11. Treatment of head and neck squamous cell carcinoma
Head and neck squamous cell carcinoma (HNSCC) has become the seventh most common cancer type worldwide. Chemotherapy is a well-established noninvasive therapy, but presents potentially serious risks of patient harm such as systemic side effects. Localized chemotherapy has been put forward for diminishing the toxicity of systemic chemotherapy while providing a sustained release of the chemotherapeutics at the target tumor site.228 In recent years, immunotherapy has become a novel effective therapeutic strategy for cancer treatment, such as checkpoint inhibitors, lymphocyte-promoting cytokines, and cancer vaccines. The implementation of immunotherapy in cancer also faces challenges from the controlled regulation of the immune system due to significant side effects including autoimmunity and nonspecific inflammation. The key to improving the curative effect and controlling adverse reactions is to improve the response rate of immunotherapy.229 Collectively, in order to improve the response rate, it is necessary to undertake further investigation of the epigenetic characteristics of various tumor cells and the ability of drug penetration to develop drugs for localized chemotherapy and immunotherapy for targeted cancer therapy. In addition, these drugs need to be equipped with an appropriate and efficient drug delivery system to precisely deliver to the target for local administration. With a stable 3D structure, outstanding fluidity, plasticity and characteristics similar to ECM, hydrogels have been applied to meet both requirements, and have been applied as prospective bio-materials to facilitate a tumor cell culture system imitating the natural internal microenvironment and novel delivery technology. At present, hydrogel has been used in the development of a 3D culture system of tumor cells230 and a novel drug delivery system.23111.1. 3D culture system
One challenge in cancer treatment is to develop better methods to predict, detect and eradicate the spread of tumor cells to distant tissues. After infiltrating surrounding tissue, dynamic interactions between tumor cells and their surrounding micro-environment, including loss of immune checkpoints, ECM deposition and remodeling and angiogenesis, usually lead to serious consequences such as structural destruction and chronic inflammation around the infiltration site.232 When becoming over-mutated and genomically unstable, the tumor cells break away from the primary site and metastasize, leading to colonization and dissemination of distant tumor tissue.233 In the oral and maxillofacial region, tumor cells often lead to long-lasting ulcers of the skin or mucosa, or distal metastasis to the lungs and other organs through blood and lymphatic pathways. Therefore, appropriate tumor culture models are essential for studying the biological behaviors and mechanism of tumor cell growth, surrounding ECM remodeling and even distal metastasis, which enable researchers to explore a wide range of variables that affect tumor growth, invasion and metastasis and develop effective tumor-targeting drugs.Tumor cell growth occurs in a complex microenvironment containing immune cells, cancer-associated fibroblasts, cytokines, growth factors, extracellular vesicles and ECM. Conventional 2D in vitro culture models can provide a high-throughput drug-screening ability that cannot be achieved by animal models or clinical samples in vivo, but it is difficult to simulate the natural tumor cell microenvironment with them. Tumor cells can only grow and expand in a single layer on the plate with flat and slender shape, cannot withstand gravity, and their growth rate is extremely fast. In addition, there are few interactions between tumor cells and extracellular matrix.234 To overcome these shortcomings, researchers developed a 3D culture system based on hydrogel scaffold, namely multicellular cancer spheroid (MCS). Hydrogel-based MCSs are commonly composed of dECM hydrogels, with a filamentous structure comprising fibrils of collagen, fibrin, or fibronectin that provides structural support and a specific distribution of cell adhesion receptors.235 Similar to the ECM microenvironment in vivo, dECM hydrogels can be used for 3D tumor-like tissue culture to effectively study the physiological pathways and mechanisms of tumor cells, and test the therapeutic effects of anticancer drugs.236 However, dECM hydrogel scaffolds have some shortcomings, such as diversity of component sources, batch-to-batch variation, and uncontrolled degradation, which usually lead to the difficulty of separating single components in tumor cells and affect the repeatable growth in comparative tumor cell studies. Researchers have turned to synthetic hydrogel (e.g., PEG, PLA, PVA, AMP) scaffolds with appropriate cell adhesion ligands and biodegradable crosslinkers. However, these synthetic hydrogels can not simulate the filamentous characteristics and mechanical signal transduction of natural ECM, leading to not readily remodeled by tumor cells. Therefore, researchers have combined the advantages of both natural and synthetic ingredients to create hybrid hydrogels.237
Different from previous hydrogels synthesized using natural or synthetic polymers, hydrogels with a DNA framework show great potential in 3D cell culture systems. It has been reported that a novel hydrogel composed of dendritic DNA with four branches can inoculate tumor cells and somatic cells in situ, achieving high proliferation and vitality. In addition, the dual entity of the branch of the tree process enables the specific loading of biological activity cues to regulate cell behaviors.238 Therefore, the hydrogel assembled using dendritic DNA can be used as a hydrogel platform with high biocompatibility, efficient functionalization and casting for 3D cell culture (Fig. 7).
The hydrogel-based MCSs need to replicate several of the characteristics of the natural tumor microenvironment in vivo. The physical properties of the hydrogels, such as hardness, structure, stiffness and permeability, usually influence the proliferation and spread of MCSs. Hydrogels must have network degradability in order to create space for tumor cells to diffuse, migrate, generate cell–cell interactions and deposit matrix components.235 It has been reported that hydrogel-based MCSs can be degraded by hydrolysis, cell command and photodegradation.239,240 Matrix mechanics and mechanical loading are also required for MCSs, making them different from the traditional 2D medium, and can resist gravity and maintain a 3D structure. In addition, cell-to-cell and cell–extracellular matrix interactions in hydrogels depend on multiple signals such as mechanical signals, topography, and the presentation of growth factors. Some researchers bind charged polysaccharides such as heparan sulfate to chelating growth factors, or bind and retain the sequence of peptides secreting ECM protein to indicate the behavior of encapsulated tumor cells.241,242 Most crucially, hydrogel-based MCSs provide anchor sites for cell adhesion. Specific cell–matrix adhesion is required for cell nutrition, diffusion, migration and mechanical sensing. A class of heterodimeric receptors called integrins binds intracellular cytoskeleton to specific cell adhesion ligands on ECM proteins encapsulated in hydrogels, such as RGD peptides.243 Hydrogels containing RGD peptides can adhere to various tumor cells, and regulating the concentration of RGD peptides can lead to increased adhesion, diffusion and migration of tumor cells.244
Hydrogel-based 3D culture systems exhibit many characteristics of tumor cells formed in vivo, thus could be a promising in vitro model for basic research on tumor biology. Integrating patient-derived tumor cells allows for the precise control of microenvironmental elements of each tumor cell through experimental design, thus providing high-throughput screening of drugs and supporting the development of personalized tumor treatment regimens.235,245 At present, the 3D culture system has been used in the basic research of HNSCC. Researchers encapsulated HNSCC in collagen hydrogel for 3D culture, and observed the differences in anti-cancer drug responses in different culture environments.246 The cisplatin and docetaxel IC50 calculated from 3D HNSCC culture systems by drug sensitivity assays in vitro were also found to be similar to responses to drug treatment in vivo.247 The 3D culture system can also be used to simulate tumor angiogenesis and interaction with stromal cells. Some researchers modified the alginate hydrogel of oral squamous cell carcinoma (OSCC) with RGD peptides and found that the secretion of IL-8 (a chemokine ligand which functions in tumor angiogenesis) was up-regulated.248 The results obtained by these in vitro models need further investigation to be verified for their biological correlation.
By modifying the composition and structure of the 3D culture system, researchers were able to modify the composition and content of polymers to achieve adjustable mechanical and permeable properties, mainly regarding spheroid dimension and density. They were often influenced by the semi-IPN stiffness and permeability.249 A novel microfluidic chip was demonstrated to simultaneously facilitate MCS formation, culture and analysis at a similar size.250 Moreover, researchers can also control the distribution and geometric shape of the MCS by implanting magnetic beads or magnetic nanoparticles.251 Generally, researchers can ensure that the tumor structures of the same cell types cultured under the same external conditions are basically similar in morphology, structure, microenvironment and cell physiology.
Hydrogel-based MCSs can also achieve high-throughput drug screening. The MCS allows direct and rapid comparison of drug efficacy in monolayer and oval cultures and identification of drug candidates that fail in 3D tissue structures. Compared with less complex analytical systems and in vivo models, MCS testing of many drugs used in clinical use can be used to clearly demonstrate the clinical predictive significance of the testing strategy.235 Studies have shown that MCSs cultured in collagen hydrogel exhibit stronger resistance to chemotherapeutic drugs than MCSs cultured in the absence of hydrogel, in which hydrogel may act as a barrier, slowing down drug diffusion and reducing the number of drug molecules reaching cells.252 For example, a collagen-based MCS was used to evaluate the therapeutic efficacy of different drug delivery methods. Compared with the MCS treated with free paclitaxel, the MCS cultured from osteosarcoma and breast adenocarcinoma showed a more significant reduction in size after treatment with paclitaxel-loaded polymer nanoparticles (NPs).253
Generally, hydrogel-based MCSs have become a prospective cancer screening tool in anticancer drug development projects. However, most engineered models of MCSs are only morphologically similar to real tumor tissues, but they are still unable to simulate the complex tumor microenvironment with an authenticity that falls between 2D culture systems and solid tumor.254 Moreover, the synthesis of hydrogels and characterization of MCSs embedded in hydrogels are labor-intensive processes.
11.2. Localized cancer therapy
Chemotherapeutic and immunotherapeutic drugs are expected to be a more effective and less systemically toxic therapeutic strategy for HNSCC. Their anticancer mechanisms mainly include strengthening T-cell immune response, promoting tumor cell apoptosis or autophagy and inhibiting tumor cell mitosis or energy metabolism.255,256 Using traditional drug administration methods it is difficult to concentrate drugs on the tumor tissues in situ, resulting in toxicity and side effects from the remaining circulating drugs that are distributed throughout the body and cause damage to normal tissues. Therefore, novel targeted drug delivery systems are required to provide precise treatment to improve the efficacy of chemotherapy drugs and reduce toxic side effects.257 Hydrogel-based local drug delivery systems are being developed to centralize the concentration of chemotherapeutics at the tumor in situ and reduce systemic toxic side effects. Stimuli-responsive hydrogels can further respond to endogenous or exogenous triggers, enabling on-demand drug delivery controlled by the researcher.4Hydrogels can carry multiple components including anticancer metallodrugs,256 immune checkpoint inhibitors,20 cancer vaccines,258 micro/nanomaterials259 and STING (Stimulator of Interferon Genes) agonists.260 Recently, self-assembled peptides (SAPs) have attracted attention from researchers. SAPs can have selected amino acid sequences to control the physical and chemical properties of conjugates23 and actively target specific receptors on the surface of tumor cells. They can be integrated with hydrogels to prepare supramolecular hydrogels to form peptide–drug conjugates (PDCs) for tumor treatment.261
Hydrogels are often applied in the form of injectable agents to achieve stable adhesion to the surface of the tumor.262 Since OSCC often occurs in superficial sites of the oral cavity such as lingual, buccal and palate areas, oral mucosal adhesive hydrogels can be applied for in situ tumor therapy in the forms of patch or spray. A novel nanocarriers-in-ion-triggered mucoadhesive hydrogel has been prepared to enhance site-specific delivery of clinically relevant radiosensitizers i.e., cisplatin and paclitaxel. The nanoparticles released from the hydrogel enhanced the tumor bioaccumulation of chemotherapeutic drugs with reduced systemic absorption, resulting in improvement of efficacy and safety confirmed by PET-CT imaging.263
Some hydrogels can respond to external stimuli such as heat, light, pH, and enzymes to achieve on-demand drug delivery at the tumor site.262 Recently, photothermal therapy (PTT) based on photoresponsive hydrogels has become a novel method of in situ tumor therapy. An injectable hydrogel network was formed by negatively charged proteins and chitosan as a nanocarrier of Ag3AuS2 nanoparticles (NPs) to perform photothermal therapy (PTT). The Ag3AuS2 NPs with high photothermal conversion efficiency absorbed NIR energy and converted it into heat to eradicate tumor tissues in an in situ lingual tumor model. PTT provided an effective non-surgical therapeutic strategy for tumor eradication and inhibition of potential tumor recurrence with no significant side effects on the surrounding normal tissue.264
Accordingly, a synergistic anti-tumor effect through the combination of chemotherapy and phototherapy has been developed. Wu et al.265 develop an injectable and NIR photoresponsive hydrogel which incorporates mesoporous silica nanoparticles (MSNs) as doxorubicin (DOX) carriers and a green cyanine dye (IR820) as a photosensitizer. MSNs achieved on-demand DOX release through self-degradation via the ROS-induced cleavage of diselenide bonds, while IR820 induced a photothermal effect against tumor tissues without interference. In other similar research, a nano doxorubicin–indocyanine green matrix metalloproteinase (MMP)-responsive hydrogel (NDIMH) further proved the anti-tumor efficacy in synergistic therapy of phototherapy and chemotherapy. NDIMH inhibited viability, invasion, and metastasis of tumor cells under 808 nm NIR illumination with no significant interference.266 The hydrogel-based chemophotothermal combination therapy exhibited a significant synergistic effect in anti-tumor therapy, which could be a promising alternative for HNSCC.
12. Discussion
Taking advantage of the various properties of hydrogels, researchers have developed multiple application strategies based on hydrogels in recent years. In the treatment of periodontitis and wound infection, researchers have proposed bacteriostatic strategies based on hydrogel to regulate the microenvironment of pathogens. Compared with the traditional drug delivery methods that can create an imbalance in the inherent microbial ecosystem in the oral cavity, the anti-infective strategy of using probiotics to build biological barriers against the colonization and proliferation of pathogens may be more effective and less damaging.113 In studies on the control of periodontitis and deep wound infection, researchers incorporated peroxides106 and proteins133 into hydrogels to produce oxygen to inhibit anaerobic bacteria. These findings indicated the feasibility of antibacterial hydrogels by regulating the microenvironment in periodontal tissue, skin and mucosa. However, whether complications such as subcutaneous emphysema will occur still needs further investigation. Researchers have also emphasized the importance of macrophages in the treatment of periodontitis and proposed a hydrogel preparation strategy to induce macrophages polarized towards an immunomodulatory M2 state.140 These findings indicated that host immune status should also be taken into account in the coming antibacterial hydrogel preparation strategies.In the tissue engineering field, hydrogels not only provide carriers for stem cells, drugs and therapeutic components, but also regulate cell behavior in the process of regeneration to a certain extent. For example, researchers can adjust the porosity and stiffness of the hydrogel by adding Mg nanoparticles that can produce H2 for foaming within its degradation process in cellular hydrogels, thereby providing a proliferation and crawling scaffold for cells and new blood vessels.187 However, challenges still exist in developing commercial products when considering the safety, efficacy, and difficulty of synthesis for regulatory approval, which is significant in the field of cellular tissue regenerative engineering. Although the application of synthetic hydrogels in tissue regenerative engineering has gradually increased due to their mechanical properties and controllable components, the biocompatibility and biosafety of synthetic hydrogels are still not comparable to natural hydrogels such as dECM.194,195 The uncertainty of the degradation rate in vivo and the interaction between vehicles and bioactive molecules that interfere with the release behavior of drugs are still affecting the ultimate therapeutic effect. Moreover, many types of hydrogels have been studied in animal models, yet very few human model-based hydrogel products have been put into practice. Further investigations in human models are urgently needed in clinical trials.
We also found that hydrogels have been studied in nerve regenerative engineering, but there are few reports associated with the oral and maxillofacial region. Oral and maxillofacial surgeries such as tooth extraction, salivary gland removal, and orthognathic surgery often cause reversible or irreversible damage to the alveolar and facial nerve, which requires repair by nerve regeneration. Hydrogels have been reported to co-deliver basic fibroblast growth factor (bFGF) and nerve growth factor (NGF) to enhance axonal regeneration and remyelination of Schwann cells, with an increased expression of nerve-associated proteins including PI3K/Akt, JAK/STAT3, and MAPK/ERK.267 Some evidence indicates that it is possible for hydrogels to reconstruct peripheral nerves in the oral and maxillofacial region. However, several key difficulties of hydrogels in alveolar and facial nerve regenerative engineering have not been resolved, including: (1) surrounded by vital anatomical structures such as the jawbones, vessels and salivary glands; (2) whether the regenerated nerve can innervate the frequent movement of mastication, facial expression and articulation of related muscles; (3) restoration of sensation in the craniofacial area. Segmental facial nerve defect models in rats have been reported as significantly improved, with functional recovery and axonal regeneration through GMSC-derived NCSC/SCP-like cells incorporated into 3D-collagen hydrogels.268 However, few cases of hydrogels for inferior alveolar nerve repair have been reported, although it is the most frequently injured facial nerve seen in the clinic owing to the narrow mandibular cavity that the inferior alveolar nerve is located in. Hydrogels with injectability and structural strength are helpful for entering the defect and promoting good recovery of the injured nerve; thus, hydrogels are expected to be a strong candidate for the treatment of oral and maxillofacial nerve injury in the future.
In addition, the complexity of synthesis and the safety of non-biodegradable residuals are obstacles to regulatory research of synthetic hydrogels. The storage of hydrogels also limits the development of commercial products for tissue regenerative engineering. Aqueous hydrogels are more susceptible to damage during storage and transportation, which can eventually lead to the failure of commercial products.269 With the development of polymer chemistry and the emerging bio-inspired synthesis strategy, the boundary between synthetic and natural polymer is blurring; we believe that innovative biomimetic hydrogels can overcome the limitations of biocompatibility soon.
13. Conclusion
In this review, we briefly summarize the structure and properties of hydrogels, and introduce the great progress in their research and application in the treatment of various oral and maxillofacial diseases in detail. Hydrogels are insoluble 3D crosslinked networks of hydrophilic copolymers which retain large amounts of water in their swollen states. Hydrogels do not, on their own, have the function of treatment or repair, but act as flexible and multi-functional carriers which can carry various components. In addition, their unique stimulus response characteristics enable hydrogels to exert on-demand drug delivery and sustained release to achieve better therapeutic effects. Furthermore, adjustive physical and chemical methods have been applied to promote cargo delivery or simultaneously achieve therapeutic effects. The combination of various methods with hydrogels has broadened their further development for research and application in the treatment of oral and maxillofacial diseases. Therefore, stimulus-responsive hydrogels combined with multifunctional adjustment approaches will be beneficial for extending their applications in the future.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the Science and Technology Project of Guangzhou City (201802020018) and the Guangdong Science and Technology Program (2019A1515010408).References
- X. Feng and J. M. McDonald, Annu. Rev. Pathol.: Mech. Dis., 2011, 6, 121–145 CrossRef CAS PubMed.
- M. A. Fonder, G. S. Lazarus, D. A. Cowan, B. Aronson-Cook, A. R. Kohli and A. J. Mamelak, J. Am. Acad. Dermatol., 2008, 58, 185–206 CrossRef PubMed.
- B. Wang, H. E. Booij-Vrieling, E. M. Bronkhorst, J. Shao, P. H. J. Kouwer, J. A. Jansen, X. F. Walboomers and F. Yang, Acta Biomater., 2020, 116, 259–267 CrossRef CAS PubMed.
- N. Oliva, J. Conde, K. Wang and N. Artzi, Acc. Chem. Res., 2017, 50, 669–679 CrossRef CAS PubMed.
- Q. Huang, Y. Zou, M. C. Arno, S. Chen, T. Wang, J. Gao, A. P. Dove and J. Du, Chem. Soc. Rev., 2017, 46, 6255–6275 RSC.
- M. S. Jhon and J. D. Andrade, J. Biomed. Mater. Res., 1973, 7, 509–522 CrossRef CAS PubMed.
- Q. Chai, Y. Jiao and X. Yu, Gels, 2017, 3, 6 CrossRef PubMed.
- S. J. Buwalda, K. W. M. Boere, P. J. Dijkstra, J. Feijen, T. Vermonden and W. E. Hennink, J. Controlled Release, 2014, 190, 254–273 CrossRef CAS PubMed.
- S. Li, S. Dong, W. Xu, S. Tu, L. Yan, C. Zhao, J. Ding and X. Chen, Adv. Sci., 2018, 5, 1700527 CrossRef PubMed.
- J. Yang, Y. S. Zhang, K. Yue and A. Khademhosseini, Acta Biomater., 2017, 57, 1–25 CrossRef CAS PubMed.
- M. Franz-Montan, L. N. d. M. Ribeiro, M. C. Volpato, C. M. S. Cereda, F. C. Groppo, G. R. Tofoli, D. R. de Araújo, P. Santi, C. Padula and E. de Paula, Expert Opin. Drug Delivery, 2017, 14, 673–684 CrossRef CAS PubMed.
- X. Chen, G. Wu, Z. Feng, Y. Dong, W. Zhou, B. Li, S. Bai and Y. Zhao, Crit. Rev. Biotechnol., 2016, 36, 760–775 CrossRef CAS PubMed.
- Z. Xu, S. Han, Z. Gu and J. Wu, Adv. Healthc. Mater., 2020, 9, e1901502 CrossRef.
- M. M. S. Abbass, A. A. El-Rashidy, K. M. Sadek, S. E. Moshy, I. A. Radwan, D. Rady, C. E. Dörfer and K. M. Fawzy El-Sayed, Polymers, 2020, 12, 2935 CrossRef CAS.
- T. de Jong, A. D. Bakker, V. Everts and T. H. Smit, J. Periodontal Res., 2017, 52, 965–974 CrossRef CAS PubMed.
- M. M. Hasani-Sadrabadi, P. Sarrion, S. Pouraghaei, Y. Chau, S. Ansari, S. Li, T. Aghaloo and A. Moshaverinia, Sci. Transl. Med., 2020, 12, eaay6853 CrossRef CAS PubMed.
- R. M. Gruber, S. Krohn, C. Mauth, M. Dard, A. Molenberg, K. Lange, C. Perske and H. Schliephake, J. Clin. Periodontol., 2014, 41, 820–826 CrossRef CAS PubMed.
- H. Wang, Y. Xu, P. Wang, J. Ma, P. Wang, X. Han, Y. Fan, D. Bai, Y. Sun and X. Zhang, Acta Biomater., 2021, 123, 364–378 CrossRef CAS.
- L. Gu and D. J. Mooney, Nat. Rev. Cancer, 2016, 16, 56–66 CrossRef CAS PubMed.
- D.-W. Zheng, W.-W. Deng, W.-F. Song, C.-C. Wu, J. Liu, S. Hong, Z.-N. Zhuang, H. Cheng, Z.-J. Sun and X.-Z. Zhang, Nat. Biomed. Eng., 2022, 6, 32–43 CrossRef CAS PubMed.
- X. Zhao, H. Wu, B. Guo, R. Dong, Y. Qiu and P. X. Ma, Biomaterials, 2017, 122, 34–47 CrossRef CAS.
- A. Francesko, P. Petkova and T. Tzanov, Curr. Med. Chem., 2018, 25, 5782–5797 CrossRef CAS PubMed.
- F. Gelain, Z. Luo and S. Zhang, Chem. Rev., 2020, 120, 13434–13460 CrossRef CAS PubMed.
- C. Mandrycky, Z. Wang, K. Kim and D.-H. Kim, Biotechnol. Adv., 2016, 34, 422–434 CrossRef CAS PubMed.
- K. d. S. Amorim, M. Franz-Montan, F. C. Groppo, B. V. Muniz, J. S. M. d. Araújo, J. V. F. Santana, A. C. G. C. Dantas, E. de Paula and L. M. d. A. Souza, J. Craniomaxillofac. Surg., 2020, 48, 815–819 CrossRef.
- J. Zhang, Q. Huang and J. Du, Polym. Int., 2016, 65, 1365–1372 CrossRef CAS.
- Y. Samchenko, Z. Ulberg and O. Korotych, Adv. Colloid Interface Sci., 2011, 168, 247–262 CrossRef CAS.
- W. E. Roorda, H. E. Boddé, A. G. De Boer, J. A. Bouwstra and H. E. Junginer, Pharm. Weekbl., Sci. Ed., 1986, 8, 165–189 CAS.
- K. S. Anseth, C. N. Bowman and L. Brannon-Peppas, Biomaterials, 1996, 17, 1647–1657 CrossRef CAS.
- L. Francis, K. V. Greco, A. R. Boccaccini, J. J. Roether, N. R. English, H. Huang, R. Ploeg and T. Ansari, J. Biomater. Appl., 2018, 33, 447–465 CrossRef CAS PubMed.
- M. C. Catoira, L. Fusaro, D. Di Francesco, M. Ramella and F. Boccafoschi, J. Mater. Sci. Mater. Med., 2019, 30, 115 CrossRef PubMed.
- E. M. Ahmed, J. Adv. Res., 2015, 6, 105–121 CrossRef CAS PubMed.
- M. T. Spang and K. L. Christman, Acta Biomater., 2018, 68, 1–14 CrossRef CAS.
- Z. Bao, C. Xian, Q. Yuan, G. Liu and J. Wu, Adv. Healthc. Mater., 2019, 8, e1900670 CrossRef PubMed.
- R. Dimatteo, N. J. Darling and T. Segura, Adv. Drug Delivery Rev., 2018, 127, 167–184 CrossRef CAS PubMed.
- Z. Zhu, Z. Guan, S. Jia, Z. Lei, S. Lin, H. Zhang, Y. Ma, Z.-Q. Tian and C. J. Yang, Angew. Chem., Int. Ed., 2014, 53, 12503–12507 CAS.
- G. Pan, Q. Guo, Y. Ma, H. Yang and B. Li, Angew. Chem., Int. Ed., 2013, 52, 6907–6911 CrossRef CAS PubMed.
- A. GhavamiNejad, N. Ashammakhi, X. Y. Wu and A. Khademhosseini, Small, 2020, 16, e2002931 CrossRef PubMed.
- Y. Jiang, J. Chen, C. Deng, E. J. Suuronen and Z. Zhong, Biomaterials, 2014, 35, 4969–4985 CrossRef CAS PubMed.
- S. Sharma and S. Tiwari, Int. J. Biol. Macromol., 2020, 162, 737–747 CrossRef CAS.
- S. Van Vlierberghe, P. Dubruel and E. Schacht, Biomacromolecules, 2011, 12, 1387–1408 CrossRef CAS PubMed.
- M. C. Koetting, J. T. Peters, S. D. Steichen and N. A. Peppas, Mater. Sci. Eng., R, 2015, 93, 1–49 CrossRef PubMed.
- M. Vázquez-González and I. Willner, Angew. Chem., Int. Ed., 2020, 59, 15342–15377 CrossRef.
- L. T. Saldin, M. C. Cramer, S. S. Velankar, L. J. White and S. F. Badylak, Acta Biomater., 2017, 49, 1–15 CrossRef CAS PubMed.
- A. Fallacara, E. Baldini, S. Manfredini and S. Vertuani, Polymers, 2018, 10, 701 CrossRef PubMed.
- J. Shi, L. Yu and J. Ding, Acta Biomater., 2021, 128, 42–59 CrossRef CAS PubMed.
- J. E. Scott and F. Heatley, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 4850–4855 CrossRef CAS.
- J. R. Pappenheimer, Physiol. Rev., 1953, 33, 387–423 CrossRef CAS PubMed.
- N. Salamat-Miller, M. Chittchang and T. P. Johnston, Adv. Drug Delivery Rev., 2005, 57, 1666–1691 CrossRef CAS PubMed.
- T. Chen, Y. Chen, H. U. Rehman, Z. Chen, Z. Yang, M. Wang, H. Li and H. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 33523–33531 CrossRef CAS PubMed.
- H. Lee, N. F. Scherer and P. B. Messersmith, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12999–13003 CrossRef CAS PubMed.
- J. Li, A. D. Celiz, J. Yang, Q. Yang, I. Wamala, W. Whyte, B. R. Seo, N. V. Vasilyev, J. J. Vlassak, Z. Suo and D. J. Mooney, Science, 2017, 357, 378–381 CrossRef CAS.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- Y. Yi, C. Xie, J. Liu, Y. Zheng, J. Wang and X. Lu, J. Mater. Chem. B, 2021, 9, 8739–8767 RSC.
- D. Gan, W. Xing, L. Jiang, J. Fang, C. Zhao, F. Ren, L. Fang, K. Wang and X. Lu, Nat. Commun., 2019, 10, 1487 CrossRef PubMed.
- M. F. P. Graça, S. P. Miguel, C. S. D. Cabral and I. J. Correia, Carbohydr. Polym., 2020, 241, 116364 CrossRef.
- Y. Wang, Y. Wu, L. Long, L. Yang, D. Fu, C. Hu, Q. Kong and Y. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 33584–33599 CrossRef CAS PubMed.
- X. Xu, Z. Gu, X. Chen, C. Shi, C. Liu, M. Liu, L. Wang, M. Sun, K. Zhang, Q. Liu, Y. Shen, C. Lin, B. Yang and H. Sun, Acta Biomater., 2019, 86, 235–246 CrossRef CAS PubMed.
- Y. Samchenko, Z. Ulberg and O. Korotych, Adv. Colloid Interface Sci., 2011, 168, 247–262 CrossRef CAS.
- L. A. Sharpe, A. M. Daily, S. D. Horava and N. A. Peppas, Expert Opin. Drug Delivery, 2014, 11, 901–915 CrossRef CAS.
- I. Adroher-Benítez, A. Moncho-Jordá and J. Dzubiella, Langmuir, 2017, 33, 4567–4577 CrossRef.
- K. Zhang, K. Xue and X. J. Loh, Gels, 2021, 7, 77 CrossRef CAS PubMed.
- M. J. Taylor, P. Tomlins and T. S. Sahota, Gels, 2017, 3, 4 CrossRef.
- L. Klouda and A. G. Mikos, Eur. J. Pharm. Biopharm., 2008, 68, 34–45 CrossRef CAS PubMed.
- S. J. Wilson, A. V. Gorelov, Y. A. Rochev, F. McGillicuddy, K. A. Dawson, W. M. Gallagher and A. K. Keenan, J. Biomed. Mater. Res., Part A, 2003, 67, 667–673 CrossRef CAS.
- Z. Zeng, Y. Hoshino, A. Rodriguez, H. Yoo and K. J. Shea, ACS Nano, 2010, 4, 199–204 CrossRef CAS PubMed.
- G. Pan, Q. Guo, Y. Ma, H. Yang and B. Li, Angew. Chem., Int. Ed., 2013, 52, 6907–6911 CrossRef CAS PubMed.
- H. Wang, Y. Jin, Y. Tan, H. Zhu, W. Huo, P. Niu, Z. Li, J. Zhang, X.-J. Liang and X. Yang, Biomaterials, 2021, 275, 120992 CrossRef CAS PubMed.
- J. Jiao, H. Lu and S. Wang, Acta Biomater., 2021, 126, 421–432 CrossRef CAS PubMed.
- M. Sadrameli and M. Mupparapu, Radiol. Clin. N. Am., 2018, 56, 13–29 CrossRef PubMed.
- L. Sedghi, V. DiMassa, A. Harrington, S. V. Lynch and Y. L. Kapila, Periodontol 2000, 2021, 87, 107–131 CrossRef PubMed.
- F. Zhang, W. Bao, R. Li, S. Zhao, Y. Liu, Y. Xu, L. Liao and X. Wang, Biomater. Sci., 2019, 7, 4503–4507 RSC.
- W. Zhang, B. Bao, F. Jiang, Y. Zhang, R. Zhou, Y. Lu, S. Lin, Q. Lin, X. Jiang and L. Zhu, Adv. Mater., 2021, e2105667, DOI:10.1002/adma.202105667.
- L. Han, L. Yan, K. Wang, L. Fang, H. Zhang, Y. Tang, Y. Ding, L.-T. Weng, J. Xu, J. Weng, Y. Liu, F. Ren and X. Lu, NPG Asia Mater., 2017, 9, e372 CrossRef CAS.
- W. Zhang, B. Bao, F. Jiang, Y. Zhang, R. Zhou, Y. Lu, S. Lin, Q. Lin, X. Jiang and L. Zhu, Adv. Mater., 2021, 33, e2105667 CrossRef PubMed.
- V. F. Patel, F. Liu and M. B. Brown, J. Controlled Release, 2011, 153, 106–116 CrossRef CAS PubMed.
- R. O. do Couto, C. Cubayachi, P. L. Calefi, R. F. V. Lopez, V. Pedrazzi, C. M. De Gaitani and O. de Freitas, J. Controlled Release, 2017, 266, 205–215 CrossRef CAS PubMed.
- A. Z. Petrikas, E. B. Ol'khovskaia, D. V. Medvedev and M. V. Diubaĭlo, Stomatologiia, 2013, 92, 71–76 Search PubMed.
- C. Cubayachi, R. O. do Couto, C. M. de Gaitani, V. Pedrazzi, O. de Freitas and R. F. V. Lopez, Colloids Surf., B, 2015, 136, 1193–1201 CrossRef CAS.
- B. V. Muniz, D. Baratelli, S. Di Carla, L. Serpe, C. B. da Silva, V. A. Guilherme, L. N. d. M. Ribeiro, C. M. S. Cereda, E. de Paula, M. C. Volpato, F. C. Groppo, L. F. Fraceto and M. Franz-Montan, Sci. Rep., 2018, 8, 17972 CrossRef CAS PubMed.
- L. N. M. Ribeiro, M. Franz-Montan, M. C. Breitkreitz, G. H. Rodrigues da Silva, S. R. de Castro, V. A. Guilherme, D. R. de Araujo and E. de Paula, Int. J. Nanomed., 2018, 13, 6453–6463 CrossRef PubMed.
- J. G. Meechan, Periodontol 2000, 2008, 46, 56–79 CrossRef.
- M. Bok, Z.-J. Zhao, S. Jeon, J.-H. Jeong and E. Lim, Sci. Rep., 2020, 10, 2027 CrossRef CAS PubMed.
- F. Zhang, W. Bao, R. Li, S. Zhao, Y. Liu, Y. Xu, L. Liao and X. Wang, Biomater. Sci., 2019, 7, 4503–4507 RSC.
- S. Kusama, K. Sato, Y. Matsui, N. Kimura, H. Abe, S. Yoshida and M. Nishizawa, Nat. Commun., 2021, 12, 658 CrossRef CAS PubMed.
- N. Salamat-Miller, M. Chittchang and T. P. Johnston, Adv. Drug Delivery Rev., 2005, 57, 1666–1691 CrossRef CAS PubMed.
- V. G. R. Patlolla, W. Peter Holbrook, S. Gizurarson and T. Kristmundsdottir, Gels, 2019, 5, 47 CrossRef CAS.
- H. N. Woo, Y. J. Cho, S. Tarafder and C. H. Lee, Bioact. Mater., 2021, 6, 3328–3342 CrossRef CAS PubMed.
- J. Li and D. J. Mooney, Nat. Rev. Mater., 2016, 1, 16071 CrossRef CAS PubMed.
- V. Sankar, V. Hearnden, K. Hull, D. V. Juras, M. S. Greenberg, A. R. Kerr, P. B. Lockhart, L. L. Patton, S. Porter and M. Thornhill, Oral Dis., 2011, 17(Suppl. 1), 73–84 CrossRef PubMed.
- N. A. Peppas and J. J. Sahlin, Biomaterials, 1996, 17, 1553–1561 CrossRef CAS PubMed.
- J. Hu, E. Andablo-Reyes, S. Soltanahmadi and A. Sarkar, ACS Macro Lett., 2020, 9, 1726–1731 CrossRef CAS.
- J. Tsibouklis, A. M. Middleton, N. Patel and J. Pratten, J. Biomed. Mater. Res., Part A, 2013, 101, 3327–3338 Search PubMed.
- Y.-J. Zou, S.-S. He and J.-Z. Du, Chin. J. Polym. Sci., 2018, 36, 1239–1250 CrossRef CAS.
- V. Tyagi, S. Del Río-Sancho, M. Lapteva and Y. N. Kalia, Int. J. Pharm., 2019, 569, 118610 CrossRef CAS PubMed.
- C. J. Hoogewerf, M. J. Hop, M. K. Nieuwenhuis, I. M. Oen, E. Middelkoop and M. E. Van Baar, Cochrane Database Syst. Rev., 2020, 7, CD008058 Search PubMed.
- H. S. Kim, X. Sun, J.-H. Lee, H.-W. Kim, X. Fu and K. W. Leong, Adv. Drug Delivery Rev., 2019, 146, 209–239 CrossRef CAS PubMed.
- Z. Iheozor-Ejiofor, K. Newton, J. C. Dumville, M. L. Costa, G. Norman and J. Bruce, Cochrane Database Syst. Rev., 2018, 7, CD012522 Search PubMed.
- S. Werner and R. Grose, Physiol. Rev., 2003, 83, 835–870 CrossRef CAS PubMed.
- S. Schreml, R. M. Szeimies, L. Prantl, S. Karrer, M. Landthaler and P. Babilas, Br. J. Dermatol., 2010, 163, 257–268 CrossRef CAS PubMed.
- K. Nuutila, M. Samandari, Y. Endo, Y. Zhang, J. Quint, T. A. Schmidt, A. Tamayol and I. Sinha, Bioact. Mater., 2022, 8, 296–308 CrossRef CAS PubMed.
- A. Kumar and H. Kaur, Int. J. Biol. Macromol., 2020, 145, 950–964 CrossRef CAS PubMed.
- Y. Yang, Y. Liang, J. Chen, X. Duan and B. Guo, Bioact. Mater., 2022, 8, 341–354 CrossRef CAS PubMed.
- P. Sacco, A. Travan, M. Borgogna, S. Paoletti and E. Marsich, J. Mater. Sci.: Mater. Med., 2015, 26, 128 CrossRef PubMed.
- A. S. Montaser, M. Rehan and M. E. El-Naggar, J. Mater. Sci.: Mater. Med., 2019, 124, 1016–1024 CAS.
- P. Deng, F. Chen, H. Zhang, Y. Chen and J. Zhou, ACS Appl. Mater. Interfaces, 2021, 13, 52333–52345 CrossRef CAS PubMed.
- V. O. Fasiku, C. A. Omolo, N. Devnarain, U. H. Ibrahim, S. Rambharose, M. Faya, C. Mocktar, S. D. Singh and T. Govender, ACS Omega, 2021, 6, 21994–22010 CrossRef CAS PubMed.
- A. Gonsalves, P. Tambe, D. Le, D. Thakore, A. S. Wadajkar, J. Yang, K. T. Nguyen and J. U. Menon, J. Mater. Chem. B, 2021, 9, 9533–9546 RSC.
- H. Murugaiah, C. L. Teh, K. C. Loh, A. R. Mohamad Yahya, N. A. Md Noh, N. H. H. Abu Bakar, D. Kernain, R. Hashim and Y. Bustami, Molecules, 2021, 26, 6414 CrossRef CAS PubMed.
- D. Liu, Y. Liao, E. J. Cornel, M. Lv, T. Wu, X. Zhang, L. Fan, M. Sun, Y. Zhu, Z. Fan and J. Du, Chem. Mater., 2021, 33, 7972–7985 CrossRef CAS.
- W. Ma, H. Ma, P. Qiu, H. Zhang, Z. Yang, B. Ma, J. Chang, X. Shi and C. Wu, Biomaterials, 2021, 279, 121225 CrossRef CAS PubMed.
- X. Lin, Y. Fang, Z. Hao, H. Wu, M. Zhao, S. Wang and Y. Liu, Small, 2021, 17, e2103303 CrossRef.
- Z. Ming, L. Han, M. Bao, H. Zhu, S. Qiang, S. Xue and W. Liu, Adv. Sci., 2021, 8, e2102545 CrossRef PubMed.
- L. Huang, Z. Zhu, D. Wu, W. Gan, S. Zhu, W. Li, J. Tian, L. Li, C. Zhou and L. Lu, Carbohydr. Polym., 2019, 225, 115110 CrossRef CAS PubMed.
- J. Navarro, R. M. Clohessy, R. C. Holder, A. R. Gabard, G. J. Herendeen, R. J. Christy, L. R. Burnett and J. P. Fisher, Tissue Eng., Part A, 2020, 26, 265–278 CrossRef CAS.
- K. Lee, Y. Xue, J. Lee, H.-J. Kim, Y. Liu, P. Tebon, E. Sarikhani, W. Sun, S. Zhang, R. Haghniaz, B. Çelebi-Saltik, X. Zhou, S. Ostrovidov, S. Ahadian, N. Ashammakhi, M. R. Dokmeci and A. Khademhosseini, Adv. Funct. Mater., 2020, 30, 2000086 CrossRef CAS PubMed.
- Y.-N. Fu, Y. Li, B. Deng, Y. Yu, F. Liu, L. Wang, G. Chen, L. Tao, Y. Wei and X. Wang, Bioact. Mater., 2022, 8, 165–176 CrossRef CAS PubMed.
- Y. Liang, X. Zhao, T. Hu, B. Chen, Z. Yin, P. X. Ma and B. Guo, Small, 2019, 15, e1900046 CrossRef PubMed.
- T. Wang, Y. Li, E. J. Cornel, C. Li and J. Du, ACS Nano, 2021, 15, 9027–9038 CrossRef CAS PubMed.
- R. Luo, J. Dai, J. Zhang and Z. Li, Adv. Healthcare Mater., 2021, 10, e2100557 CrossRef PubMed.
- C. Korupalli, H. Li, N. Nguyen, F.-L. Mi, Y. Chang, Y.-J. Lin and H.-W. Sung, Adv. Healthcare Mater., 2021, 10, e2001384 CrossRef.
- Z. Deng, H. Wang, P. X. Ma and B. Guo, Nanoscale, 2020, 12, 1224–1246 RSC.
- D. F. Kinane, P. G. Stathopoulou and P. N. Papapanou, Nat. Rev. Dis. Primers, 2017, 3, 17038 CrossRef.
- S. Ivanovski, C. Vaquette, S. Gronthos, D. W. Hutmacher and P. M. Bartold, J. Dent. Res., 2014, 93, 1212–1221 CrossRef CAS PubMed.
- G. Goyal, T. Garg, G. Rath and A. K. Goyal, Crit. Rev. Ther. Drug Carrier Syst., 2014, 31, 89–119 CrossRef CAS PubMed.
- M. Zieba, P. Chaber, K. Duale, M. Martinka Maksymiak, M. Basczok, M. Kowalczuk and G. Adamus, Polymers, 2020, 12, 1574 CrossRef CAS PubMed.
- J. Mou, Z. Liu, J. Liu, J. Lu, W. Zhu and D. Pei, Drug Delivery, 2019, 26, 179–187 CrossRef CAS PubMed.
- N. Baranov, M. Popa, L. I. Atanase and D. L. Ichim, Molecules, 2021, 26, 2735 CrossRef CAS PubMed.
- E. V. Lengert, A. A. Savkina, A. V. Ermakov, M. S. Saveleva, D. D. Lagutina, T. V. Stepanova and A. N. Ivanov, Mater. Sci. Eng., C, 2021, 126, 112144 CrossRef CAS PubMed.
- S. Lu, X. Ren, T. Guo, Z. Cao, H. Sun, C. Wang, F. Wang, Z. Shu, J. Hao, S. Gui, C. Lei and J. Zhang, Carbohydr. Polym., 2021, 267, 118187 CrossRef CAS PubMed.
- Y. Xi, Y. Wang, J. Gao, Y. Xiao and J. Du, ACS Nano, 2019, 13, 13645–13657 CrossRef CAS PubMed.
- H. Zhao, J. Hu and L. Zhao, BMC Oral Health, 2020, 20, 34 CrossRef CAS PubMed.
- H. Özçelik, F. Batool, M. Corre, A. Garlaschelli, G. Conzatti, C. Stutz, C. Petit, E. Delpy, F. Zal, E. Leize-Zal and O. Huck, Int. J. Pharm., 2021, 605, 120810 CrossRef.
- P.-C. Chang, Y.-C. Chao, M.-H. Hsiao, H.-S. Chou, Y.-H. Jheng, X.-H. Yu, N. Lee, C. Yang and D.-M. Liu, J. Periodontol., 2017, 88, 190–196 CrossRef CAS PubMed.
- J. Lin, Z. He, F. Liu, J. Feng, C. Huang, X. Sun and H. Deng, Int. J. Nanomed., 2020, 15, 5377–5387 CrossRef CAS.
- W. Xu, W. Zhou, H. Wang and S. Liang, Adv. Protein Chem. Struct. Biol., 2020, 120, 45–84 Search PubMed.
- S. Liu, Y.-N. Wang, B. Ma, J. Shao, H. Liu and S. Ge, ACS Appl. Mater. Interfaces, 2021, 13, 36880–36893 CrossRef CAS PubMed.
- A. Trusek and E. Kijak, Materials, 2021, 14, 3182 CrossRef CAS PubMed.
- H. R. Preus, P. Gjermo and V. Baelum, J. Periodontol., 2017, 88, 144–152 CrossRef PubMed.
- Z. Shen, S. Kuang, Y. Zhang, M. Yang, W. Qin, X. Shi and Z. Lin, Bioact. Mater., 2020, 5, 1113–1126 CrossRef PubMed.
- D. F. Kinane, P. G. Stathopoulou and P. N. Papapanou, Nat. Rev. Dis. Primers, 2017, 3, 17038 CrossRef PubMed.
- M. M. Hasani-Sadrabadi, P. Sarrion, N. Nakatsuka, T. D. Young, N. Taghdiri, S. Ansari, T. Aghaloo, S. Li, A. Khademhosseini, P. S. Weiss and A. Moshaverinia, ACS Nano, 2019, 13, 3830–3838 CrossRef CAS.
- T. Zhou, K. Zheng, B. Sui, A. R. Boccaccini and J. Sun, J. Mater. Sci.: Mater. Med., 2020, 163, 1938–1946 CAS.
- P. M. Chichiricco, R. Riva, J.-M. Thomassin, J. Lesoeur, X. Struillou, C. Le Visage, C. Jérôme and P. Weiss, Dent. Mater., 2018, 34, 1769–1782 CrossRef CAS PubMed.
- Y. Song, C. Zhang, P. Wang, L. Wang, C. Bao, M. D. Weir, M. A. Reynolds, K. Ren, L. Zhao and H. H. K. Xu, Mater. Sci. Eng., C, 2017, 75, 895–905 CrossRef CAS PubMed.
- S. Shen, Y. Zhang, S. Zhang, B. Wang, L. Shang, J. Shao, M. Lin, Y. Cui, S. Sun and S. Ge, ACS Biomater. Sci. Eng., 2021, 7, 232–241 CrossRef CAS PubMed.
- J. Tan, M. Zhang, Z. Hai, C. Wu, J. Lin, W. Kuang, H. Tang, Y. Huang, X. Chen and G. Liang, ACS Nano, 2019, 13, 5616–5622 CrossRef CAS PubMed.
- F. Koch, K. Ekat, D. Kilian, T. Hettich, O. Germershaus, H. Lang, K. Peters and B. Kreikemeyer, Adv. Healthcare Mater., 2019, 8, e1900167 CrossRef.
- F. Koch, A. Wolff, S. Mathes, U. Pieles, S. S. Saxer, B. Kreikemeyer and K. Peters, Int. J. Nanomed., 2018, 13, 6717–6733 CrossRef CAS.
- P. Qiu, M. Li, K. Chen, B. Fang, P. Chen, Z. Tang, X. Lin and S. Fan, Biomaterials, 2020, 227, 119552 CrossRef CAS PubMed.
- M. Tsukasaki and H. Takayanagi, Nat. Rev. Immunol., 2019, 19, 626–642 CrossRef CAS PubMed.
- S. Sowmya, U. Mony, P. Jayachandran, S. Reshma, R. A. Kumar, H. Arzate, S. V. Nair and R. Jayakumar, Adv. Healthcare Mater., 2017, 6, 1601251 CrossRef PubMed.
- T. de Jong, A. D. Bakker, V. Everts and T. H. Smit, J. Periodontal Res., 2017, 52, 965–974 CrossRef CAS PubMed.
- W. Jiang, L. Li, D. Zhang, S. Huang, Z. Jing, Y. Wu, Z. Zhao, L. Zhao and S. Zhou, Acta Biomater., 2015, 25, 240–252 CrossRef CAS PubMed.
- F. Bleicher, Exp. Cell Res., 2014, 325, 65–71 CrossRef CAS PubMed.
- J. Yang, G. Yuan and Z. Chen, Front. Physiol., 2016, 7, 58 Search PubMed.
- R. Bansal, A. Jain, S. Mittal, T. Kumar and D. Kaur, J. Clin. Diagn. Res., 2014, 8, ZE20–ZE24 Search PubMed.
- Z. Siddiqui, B. Sarkar, K.-K. Kim, N. Kadincesme, R. Paul, A. Kumar, Y. Kobayashi, A. Roy, M. Choudhury, J. Yang, E. Shimizu and V. A. Kumar, Acta Biomater., 2021, 126, 109–118 CrossRef CAS PubMed.
- A. Khayat, N. Monteiro, E. E. Smith, S. Pagni, W. Zhang, A. Khademhosseini and P. C. Yelick, J. Dent. Res., 2017, 96, 192–199 CrossRef CAS.
- C. R. Silva, P. S. Babo, M. Gulino, L. Costa, J. M. Oliveira, J. Silva-Correia, R. M. A. Domingues, R. L. Reis and M. E. Gomes, Acta Biomater., 2018, 77, 155–171 CrossRef CAS PubMed.
- Y. Cao, M. Song, E. Kim, W. Shon, N. Chugal, G. Bogen, L. Lin, R. H. Kim, N. H. Park and M. K. Kang, J. Dent. Res., 2015, 94, 1544–1551 CrossRef CAS.
- X. Wang, B. Thibodeau, M. Trope, L. M. Lin and G. T. J. Huang, J. Endod., 2010, 36, 56–63 CrossRef CAS PubMed.
- G. Martin, D. Ricucci, J. L. Gibbs and L. M. Lin, J. Endod., 2013, 39, 138–144 CrossRef.
- X. Zhang, H. Li, J. Sun, X. Luo, H. Yang, L. Xie, B. Yang, W. Guo and W. Tian, Cell Proliferation, 2017, 50, e12361 CrossRef PubMed.
- J. L. Drury and D. J. Mooney, Biomaterials, 2003, 24, 4337–4351 CrossRef CAS PubMed.
- Y.-T. Lin, T.-T. Hsu, Y.-W. Liu, C.-T. Kao and T.-H. Huang, Biomedicines, 2021, 9, 929 CrossRef CAS PubMed.
- K. Xia, Z. Chen, J. Chen, H. Xu, Y. Xu, T. Yang and Q. Zhang, Int. J. Nanomed., 2020, 15, 6631–6647 CrossRef CAS.
- P. Zarrintaj, M. Khodadadi Yazdi, M. Youssefi Azarfam, M. Zare, J. D. Ramsey, F. Seidi, M. Reza Saeb, S. Ramakrishna and M. Mozafari, Tissue Eng., Part A, 2021, 27, 821–843 CrossRef CAS.
- R. Zhang, L. Xie, H. Wu, T. Yang, Q. Zhang, Y. Tian, Y. Liu, X. Han, W. Guo, M. He, S. Liu and W. Tian, Acta Biomater., 2020, 113, 305–316 CrossRef CAS.
- I. Lambrichts, R. B. Driesen, Y. Dillen, P. Gervois, J. Ratajczak, T. Vangansewinkel, E. Wolfs, A. Bronckaers and P. Hilkens, J. Endod., 2017, 43, S12–S16 CrossRef.
- Z. Wang, Z. Zhou, J. Fan, L. Zhang, Z. Zhang, Z. Wu, Y. Shi, H. Zheng, Z. Zhang, R. Tang and B. Fu, J. Nanobiotechnol., 2021, 19, 385 CrossRef CAS PubMed.
- L. Wang, H. Fu, W. Wang, Y. Liu, X. Li, J. Yang, L. Li, G. Wu and Y. Pan, Acta Biomater., 2021, 122, 160–171 CrossRef CAS.
- J. Li, Z. Rao, Y. Zhao, Y. Xu, L. Chen, Z. Shen, Y. Bai, Z. Lin and Q. Huang, J. Endod., 2020, 46, 1438–1447 CrossRef PubMed .e5.
- Y. Itoh, J. I. Sasaki, M. Hashimoto, C. Katata, M. Hayashi and S. Imazato, J. Dent. Res., 2018, 97, 1137–1143 CrossRef.
- M. Zhang, F. Jiang, X. Zhang, S. Wang, Y. Jin, W. Zhang and X. Jiang, Stem Cells Transl. Med., 2017, 6, 2126–2134 CrossRef CAS PubMed.
- V. Gradinaru, J. Treweek, K. Overton and K. Deisseroth, Annu. Rev. Biophys., 2018, 47, 355–376 CrossRef CAS PubMed.
- Q. Ding, X. Xu, Y. Yue, C. Mei, C. Huang, S. Jiang, Q. Wu and J. Han, ACS Appl. Mater. Interfaces, 2018, 10, 27987–28002 CrossRef CAS.
- J. Yang, Y. S. Zhang, K. Yue and A. Khademhosseini, Acta Biomater., 2017, 57, 1–25 CrossRef CAS.
- K. Johnson, S. Zhu, M. S. Tremblay, J. N. Payette, J. Wang, L. C. Bouchez, S. Meeusen, A. Althage, C. Y. Cho, X. Wu and P. G. Schultz, Science, 2012, 336, 717–721 CrossRef CAS PubMed.
- D. S. Benoit, M. P. Schwartz, A. R. Durney and K. S. Anseth, Nat. Mater., 2008, 7, 816–823 CrossRef CAS PubMed.
- K. Y. Wang, X. Y. Jin, Y. H. Ma, W. J. Cai, W. Y. Xiao, Z. W. Li, X. Qi and J. Ding, J. Nanobiotechnol., 2021, 19, 214 CrossRef CAS PubMed.
- L. Roseti, V. Parisi, M. Petretta, C. Cavallo, G. Desando, I. Bartolotti and B. Grigolo, Mater. Sci. Eng., C, 2017, 78, 1246–1262 CrossRef CAS.
- L. Dong, Z. Bu, Y. Xiong, H. Zhang, J. Fang, H. Hu, Z. Liu and X. Li, J. Mater. Sci.: Mater. Med., 2021, 188, 72–81 CAS.
- S. V. Murphy and A. Atala, Nat. Biotechnol., 2014, 32, 773–785 CrossRef CAS.
- Q. Mei, J. Rao, H. P. Bei, Y. Liu and X. Zhao, Int. J. Bioprint., 2021, 7, 367 CrossRef PubMed.
- Y. Zhang, H. Chen, T. Zhang, Y. Zan, T. Ni, Y. Cao, J. Wang, M. Liu and R. Pei, Mater. Sci. Eng., C, 2019, 96, 841–849 CrossRef CAS PubMed.
- Y. Tang, S. Lin, S. Yin, F. Jiang, M. Zhou, G. Yang, N. Sun, W. Zhang and X. Jiang, Biomaterials, 2020, 232, 119727 CrossRef CAS PubMed.
- A. Iglesias-Mejuto and C. A. García-González, Mater. Sci. Eng., C, 2021, 131, 112525 CrossRef CAS PubMed.
- A. De Mori, M. Peña Fernández, G. Blunn, G. Tozzi and M. Roldo, Polymers, 2018, 10, 285 CrossRef PubMed.
- S. Panseri, A. Russo, M. Sartori, G. Giavaresi, M. Sandri, M. Fini, M. C. Maltarello, T. Shelyakova, A. Ortolani, A. Visani, V. Dediu, A. Tampieri and M. Marcacci, Bone, 2013, 56, 432–439 CrossRef CAS.
- Z. Jing, Z. Liang, L. Yang, W. Du, T. Yu, H. Tang, C. Li and L. Guo, J. Controlled Release, 2021, 338, 571–582 CrossRef PubMed.
- D. Richards, J. Jia, M. Yost, R. Markwald and Y. Mei, Ann. Biomed. Eng., 2017, 45, 132–147 CrossRef PubMed.
- J. Visser, P. A. Levett, N. C. te Moller, J. Besems, K. W. Boere, M. H. van Rijen, J. C. de Grauw, W. J. Dhert, P. R. van Weeren and J. Malda, Tissue Eng., Part A, 2015, 21, 1195–1206 CrossRef CAS PubMed.
- C. Y. Lin, Y. R. Wang, C. W. Lin, S. W. Wang, H. W. Chien, N. C. Cheng, W. B. Tsai and J. Yu, BioRes. Open Access, 2014, 3, 297–310 CrossRef CAS PubMed.
- L. Wang, M. Zhao, S. Li, U. J. Erasquin, H. Wang, L. Ren, C. Chen, Y. Wang and C. Cai, ACS Appl. Mater. Interfaces, 2014, 6, 8401–8406 CrossRef CAS PubMed.
- S. Yuan, Q. Li, K. Chen, Z. Mu, T. Chen, H. Wang and P. Ji, J. Biol. Eng., 2021, 15, 19 CrossRef CAS PubMed.
- R. P. Donahue, J. C. Hu and K. A. Athanasiou, Trends Mol. Med., 2019, 25, 241–256 CrossRef CAS PubMed.
- A. H. Reddi, J. Becerra and J. A. Andrades, Tissue Eng., Part B, 2011, 17, 301–305 CrossRef CAS PubMed.
- J. Liang, P. Yi, X. Wang, F. Huang, X. Luan, Z. Zhao and C. Liu, J. Biomed. Mater. Res., Part B, 2020, 108, 2995–3007 CrossRef CAS PubMed.
- W. Zhang, Y. Zhang, Y. Zhang, Y. Dai, F. Xia and X. Zhang, J. Mater. Chem. B, 2021, 9, 5954–5966 RSC.
- Q. Mei, J. Rao, H. P. Bei, Y. Liu and X. Zhao, Int. J. Bioprint., 2021, 7, 367 CrossRef PubMed.
- W. Chu, M. Nie, X. Ke, J. Luo and J. Li, Macromol. Biosci., 2021, 21, e2100109 CrossRef.
- S. Han, K. Nie, J. Li, Q. Sun, X. Wang, X. Li and Q. Li, Stem Cells Int., 2021, 2021, 8790143 Search PubMed.
- X. Zhang, J. Wang, H. Jin, S. Wang and W. Song, J. Am. Chem. Soc., 2018, 140, 3186–3189 CrossRef CAS PubMed.
- Y. Xia, Y. He, F. Zhang, Y. Liu and J. Leng, Adv. Mater., 2021, 33, e2000713 CrossRef PubMed.
- K. Shin, K. H. Koo, J. Jeong, S. J. Park, D. J. Choi, Y. G. Ko and H. Kwon, Tissue Eng., Part A, 2019, 25, 1396–1403 CrossRef CAS.
- A. D. Shubin, A. Sharipol, T. J. Felong, P. L. Weng, B. E. Schutrum, D. S. Joe, M. H. Aure, D. S. W. Benoit and C. E. Ovitt, Cell Tissue Res., 2020, 380, 487–497 CrossRef PubMed.
- A. D. Shubin, T. J. Felong, D. Graunke, C. E. Ovitt and D. S. Benoit, Tissue Eng., Part A, 2015, 21, 1733–1751 CrossRef CAS PubMed.
- K. Nam, C. S. Wang, C. L. M. Maruyama, P. Lei, S. T. Andreadis and O. J. Baker, J. Dent. Res., 2017, 96, 798–806 CrossRef CAS.
- A. Ikeda, H. Taketa, G. A. Sathi, Y. Hirano, S. Iida and T. Matsumoto, Regener. Ther., 2016, 3, 108–113 CrossRef PubMed.
- T. Ozdemir, E. W. Fowler, S. Liu, D. A. Harrington, R. L. Witt, M. C. Farach-Carson, S. Pradhan-Bhatt and X. Jia, ACS Biomater. Sci. Eng., 2016, 2, 2217–2230 CrossRef CAS.
- S. Pradhan-Bhatt, D. A. Harrington, R. L. Duncan, M. C. Farach-Carson, X. Jia and R. L. Witt, Laryngoscope, 2014, 124, 456–461 CrossRef CAS.
- D. Wu, P. Chapela and M. C. Farach-Carson, Methods Mol. Biol., 2018, 1817, 19–32 CrossRef CAS PubMed.
- H. S. Shin, S. Lee, H. J. Hong, Y. C. Lim, W. G. Koh and J. Y. Lim, Stem Cell Res. Ther., 2018, 9, 74 CrossRef CAS PubMed.
- A. D. Shubin, T. J. Felong, B. E. Schutrum, D. S. L. Joe, C. E. Ovitt and D. S. W. Benoit, Acta Biomater., 2017, 50, 437–449 CrossRef CAS PubMed.
- T. Ozdemir, P. P. Srinivasan, D. R. Zakheim, D. A. Harrington, R. L. Witt, M. C. Farach-Carson, X. Jia and S. Pradhan-Bhatt, Biomaterials, 2017, 142, 124–135 CrossRef CAS PubMed.
- C. M. L. Barrows, D. Wu, M. C. Farach-Carson and S. Young, Tissue Eng., Part A, 2020, 26, 1332–1348 CrossRef CAS PubMed.
- D. Wu, R. L. Witt, D. A. Harrington and M. C. Farach-Carson, Front. Cell Dev. Biol., 2019, 7, 224 CrossRef.
- P. P. Srinivasan, V. N. Patel, S. Liu, D. A. Harrington, M. P. Hoffman, X. Jia, R. L. Witt, M. C. Farach-Carson and S. Pradhan-Bhatt, Stem Cells Transl. Med., 2017, 6, 110–120 CrossRef CAS PubMed.
- V. Szlávik, J. Vág, K. Markó, K. Demeter, E. Madarász, I. Oláh, T. Zelles, B. C. O'Connell and G. Varga, J. Cell. Biochem., 2008, 103, 284–295 CrossRef PubMed.
- K. Nam, H. T. Dos Santos, F. Maslow, B. G. Trump, P. Lei, S. T. Andreadis and O. J. Baker, Front. Bioeng. Biotechnol., 2021, 9, 729180 CrossRef.
- S. V. Patil and L. S. Y. Nanduri, Int. J. Biol. Macromol., 2017, 104, 1398–1406 CrossRef CAS PubMed.
- A. D. Shubin, T. J. Felong, B. E. Schutrum, D. S. L. Joe, C. E. Ovitt and D. S. W. Benoit, Acta Biomater., 2017, 50, 437–449 CrossRef CAS PubMed.
- K. Nam, S. M. Dean, C. T. Brown, R. J. Smith, P. Lei, S. T. Andreadis and O. J. Baker, Acta Biomater., 2019, 91, 186–194 CrossRef CAS PubMed.
- E. W. Fowler, A. Ravikrishnan, R. L. Witt, S. Pradhan-Bhatt and X. Jia, ACS Biomater. Sci. Eng., 2021, 13, 5749–5761 CrossRef.
- Y. Song, H. Uchida, A. Sharipol, L. Piraino, J. A. Mereness, M. H. Ingalls, J. Rebhahn, S. D. Newlands, L. A. DeLouise, C. E. Ovitt and D. S. W. Benoit, Commun. Biol., 2021, 4, 361 CrossRef CAS PubMed.
- D. Wu, I. M. A. Lombaert, M. DeLeon, S. Pradhan-Bhatt, R. L. Witt, D. A. Harrington, M. G. Trombetta, M. J. Passineau and M. C. Farach-Carson, Front. Mol. Biosci., 2021, 8, 711602 CrossRef CAS PubMed.
- M. D. Mody, J. W. Rocco, S. S. Yom, R. I. Haddad and N. F. Saba, Lancet, 2021, 398, 2289–2299 CrossRef.
- R. S. Riley, C. H. June, R. Langer and M. J. Mitchell, Nat. Rev. Drug Discovery, 2019, 18, 175–196 CrossRef CAS PubMed.
- S. Nath and G. R. Devi, Pharmacol. Ther., 2016, 163, 94–108 CrossRef CAS PubMed.
- Y. Xiao, Y. Gu, L. Qin, L. Chen, X. Chen, W. Cui, F. Li, N. Xiang and X. He, Colloids Surf., B, 2021, 200, 111581 CrossRef CAS PubMed.
- H. F. Dvorak, Cancer Immunol. Res., 2015, 3, 1–11 CrossRef CAS PubMed.
- V. Papalazarou, M. Salmeron-Sanchez and L. M. Machesky, Biophys. Rev., 2018, 10, 1695–1711 CrossRef PubMed.
- E. C. Costa, A. F. Moreira, D. de Melo-Diogo, V. M. Gaspar, M. P. Carvalho and I. J. Correia, Biotechnol. Adv., 2016, 34, 1427–1441 CrossRef PubMed.
- Y. Li and E. Kumacheva, Sci. Adv., 2018, 4, eaas8998 CrossRef PubMed.
- G. G. Giobbe, C. Crowley, C. Luni, S. Campinoti, M. Khedr, K. Kretzschmar, M. M. De Santis, E. Zambaiti, F. Michielin, L. Meran, Q. Hu, G. van Son, L. Urbani, A. Manfredi, M. Giomo, S. Eaton, D. Cacchiarelli, V. S. W. Li, H. Clevers, P. Bonfanti, N. Elvassore and P. De Coppi, Nat. Commun., 2019, 10, 5658 CrossRef.
- Y. Li, N. Khuu, A. Gevorkian, S. Sarjinsky, H. Therien-Aubin, Y. Wang, S. Cho and E. Kumacheva, Angew. Chem., Int. Ed., 2017, 56, 6083–6087 CrossRef CAS PubMed.
- J. Wu, B. R. Liyarita, H. Zhu, M. Liu, X. Hu and F. Shao, ACS Appl. Mater. Interfaces, 2021, 13, 49705–49712 CrossRef CAS.
- K. M. Galler, L. Aulisa, K. R. Regan, R. N. D'Souza and J. D. Hartgerink, J. Am. Chem. Soc., 2010, 132, 3217–3223 CrossRef CAS PubMed.
- A. M. Kloxin, A. M. Kasko, C. N. Salinas and K. S. Anseth, Science, 2009, 324, 59–63 CrossRef CAS.
- M. Guvendiren and J. A. Burdick, Curr. Opin. Biotechnol, 2013, 24, 841–846 CrossRef CAS PubMed.
- C. M. Madl and S. C. Heilshorn, Annu. Rev. Biomed. Eng., 2018, 20, 21–47 CrossRef CAS.
- M. Barczyk, S. Carracedo and D. Gullberg, Cell Tissue Res., 2010, 339, 269–280 CrossRef CAS PubMed.
- U. Hersel, C. Dahmen and H. Kessler, Biomaterials, 2003, 24, 4385–4415 CrossRef CAS.
- N. Brassard-Jollive, C. Monnot, L. Muller and S. Germain, Front. Cell Dev. Biol., 2020, 8, 594903 CrossRef.
- J. M. Ayuso, R. Vitek, A. D. Swick, M. C. Skala, K. B. Wisinski, R. J. Kimple, P. F. Lambert and D. J. Beebe, Sci. Rep., 2019, 9, 12480 CrossRef PubMed.
- N. Tanaka, A. A. Osman, Y. Takahashi, A. Lindemann, A. A. Patel, M. Zhao, H. Takahashi and J. N. Myers, Oral Oncol., 2018, 87, 49–57 CrossRef CAS PubMed.
- C. Fischbach, H. J. Kong, S. X. Hsiong, M. B. Evangelista, W. Yuen and D. J. Mooney, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 399–404 CrossRef CAS.
- G. Morello, A. Quarta, A. Gaballo, L. Moroni, G. Gigli, A. Polini and F. Gervaso, Carbohydr. Polym., 2021, 274, 118633 CrossRef CAS PubMed.
- C.-Y. Fu, S.-Y. Tseng, S.-M. Yang, L. Hsu, C.-H. Liu and H.-Y. Chang, Biofabrication, 2014, 6, 015009 CrossRef CAS PubMed.
- S. Libring, Á. Enríquez, H. Lee and L. Solorio, Cancers, 2021, 13, 4440 CrossRef CAS PubMed.
- W. Zhang, C. Li, B. C. Baguley, F. Zhou, W. Zhou, J. P. Shaw, Z. Wang, Z. Wu and J. Liu, Anal. Biochem., 2016, 515, 47–54 CrossRef CAS PubMed.
- K. M. Charoen, B. Fallica, Y. L. Colson, M. H. Zaman and M. W. Grinstaff, Biomaterials, 2014, 35, 2264–2271 CrossRef CAS PubMed.
- X. Xu, M. C. Farach-Carson and X. Jia, Biotechnol. Adv., 2014, 32, 1256–1268 CrossRef CAS PubMed.
- M. Sun, C. Wang, M. Lv, Z. Fan and J. Du, Biomaterials, 2021, 278, 121168 CrossRef CAS PubMed.
- M. Sun, L. He, Z. Fan, R. Tang and J. Du, Biomaterials, 2020, 257, 120252 CrossRef CAS PubMed.
- J. Conde, J. T. Dias, V. Grazú, M. Moros, P. V. Baptista and J. M. de la Fuente, Front. Chem., 2014, 2, 48 Search PubMed.
- S. Polla Ravi, Y. Shamiya, A. Chakraborty, C. Elias and A. Paul, Trends Pharmacol. Sci., 2021, 42, 813–828 CrossRef CAS PubMed.
- N. Taneja, A. Alam, R. S. Patnaik, T. Taneja, S. Gupta and S. M. K, Crit. Rev. Ther. Drug Carrier Syst., 2021, 38, 1–48 CrossRef PubMed.
- D. G. Leach, N. Dharmaraj, S. L. Piotrowski, T. L. Lopez-Silva, Y. L. Lei, A. G. Sikora, S. Young and J. D. Hartgerink, Biomaterials, 2018, 163, 67–75 CrossRef CAS PubMed.
- Y. Wang, A. G. Cheetham, G. Angacian, H. Su, L. Xie and H. Cui, Adv. Drug Delivery Rev., 2017, 110–111, 112–126 CrossRef CAS PubMed.
- N. Oliva, M. Carcole, M. Beckerman, S. Seliktar, A. Hayward, J. Stanley, N. M. A. Parry, E. R. Edelman and N. Artzi, Sci. Transl. Med., 2015, 7, 272ra211 Search PubMed.
- P. Bhardwaj, V. Gota, K. Vishwakarma, V. Pai, P. Chaudhari, B. Mohanty, R. Thorat, S. Yadav, M. Gurjar, J. S. Goda and R. Banerjee, J. Controlled Release, 2022, 343, 288–302 CrossRef CAS PubMed.
- J. Su, S. Lu, S. Jiang, B. Li, B. Liu, Q. Sun, J. Li, F. Wang and Y. Wei, Adv. Mater., 2021, 33, e2100619 CrossRef PubMed.
- Y. Wu, F. Chen, N. Huang, J. Li, C. Wu, B. Tan, Y. Liu, L. Li, C. Yang, D. Shao and J. Liao, Nanoscale, 2021, 13, 17168–17182 RSC.
- H.-H. Wang, Z.-G. Fu, W. Li, Y.-X. Li, L.-S. Zhao, L. Wen, J.-J. Zhang and N. Wen, Int. J. Nanomed., 2019, 14, 623–638 CrossRef CAS.
- R. Li, Y. Li, Y. Wu, Y. Zhao, H. Chen, Y. Yuan, K. Xu, H. Zhang, Y. Lu, J. Wang, X. Li, X. Jia and J. Xiao, Biomaterials, 2018, 168, 24–37 CrossRef CAS.
- Q. Zhang, P. Nguyen, J. C. Burrell, J. Zeng, S. Shi, R. M. Shanti, G. Kulischak, D. K. Cullen and A. D. Le, npj Regener. Med., 2021, 6, 59 CrossRef CAS PubMed.
- Y. Gao, K. Peng and S. Mitragotri, Adv. Mater., 2021, 33, e2006362 CrossRef.
- B. Wang, J. Wang, J. Shao, P. H. J. Kouwer, E. M. Bronkhorst, J. A. Jansen, X. F. Walboomers and F. Yang, J. Controlled Release, 2020, 324, 134–145 CrossRef CAS PubMed.
- H. An, Y. Yang, Z. Zhou, Y. Bo, Y. Wang, Y. He, D. Wang and J. Qin, Acta Biomater., 2021, 131, 149–161 CrossRef CAS.
- Q. U. A. Malik, S. Iftikhar, S. Zahid, S. Z. Safi, A. F. Khan, M. Nawshad, S. Ghafoor, A. S. Khan and A. Tufail Shah, Mater. Sci. Eng., C, 2020, 113, 110956 CrossRef CAS.
- P. He, H. Zhang, Y. Li, M. Ren, J. Xiang, Z. Zhang, P. Ji and S. Yang, Mater. Sci. Eng., C, 2020, 109, 110551 CrossRef CAS.
- S. Ge, J. Li, J. Geng, S. Liu, H. Xu and Z. Gu, Mater. Horiz., 2021, 8, 1189–1198 RSC.
- W. Lu, X. Li, Y. Yang, J. Yi, L. Xie, Z. Zhao and Y. Li, J. Periodontal Res., 2021, 56, 885–896 CrossRef CAS PubMed.
- R. C. Op’t Veld, O. I. van den Boomen, D. M. S. Lundvig, E. M. Bronkhorst, P. H. J. Kouwer, J. A. Jansen, E. Middelkoop, J. W. Von den Hoff, A. E. Rowan and FADTG. Wagener, Biomaterials, 2018, 181, 392–401 CrossRef.
- I. M. A. Diniz, A. C. O. Carreira, C. R. Sipert, C. M. Uehara, M. S. N. Moreira, L. Freire, C. Pelissari, P. M. Kossugue, D. R. de Araújo, M. C. Sogayar and M. M. Marques, J. Cell Physiol., 2018, 233, 4907–4918 CrossRef CAS PubMed.
- L. Han, X. Lu, M. Wang, D. Gan, W. Deng, K. Wang, L. Fang, K. Liu, C. W. Chan, Y. Tang, L.-T. Weng and H. Yuan, Small, 2017, 13, 1601916 CrossRef.
- F.-Z. Yuan, H.-F. Wang, J. Guan, J.-N. Fu, M. Yang, J.-Y. Zhang, Y.-R. Chen, X. Wang and J.-K. Yu, Pharmaceutics, 2021, 13, 1487 CrossRef CAS PubMed.
- M. L. Fabiilli, C. G. Wilson, F. Padilla, F. M. Martín-Saavedra, J. B. Fowlkes and R. T. Franceschi, Acta Biomater., 2013, 9, 7399–7409 CrossRef CAS PubMed.
- W. Shi, F. Fang, Y. Kong, S. E. Greer, M. Kuss, B. Liu, W. Xue, X. Jiang, P. Lovell, A. M. Mohs, A. T. Dudley, T. Li and B. Duan, Biofabrication, 2021, 14, 014107 CrossRef PubMed.
- O. Guaresti, C. García-Astrain, T. Palomares, A. Alonso-Varona, A. Eceiza and N. Gabilondo, J. Mater. Sci.: Mater. Med., 2017, 102, 1–9 CAS.
- M. C. Mañas-Torres, C. Gila-Vilchez, F. J. Vazquez-Perez, P. Kuzhir, D. Momier, J.-C. Scimeca, A. Borderie, M. Goracci, F. Burel-Vandenbos, C. Blanco-Elices, I. A. Rodriguez, M. Alaminos, L. Á. de Cienfuegos and M. T. Lopez-Lopez, ACS Appl. Mater. Interfaces, 2021, 13, 49692–49704 CrossRef PubMed.
- M. Wang, C. Wang, Y. Zhang and Y. Lin, Mater. Sci. Eng., C, 2021, 129, 112376 CrossRef CAS.
- M. Mackiewicz, J. Romanski, K. Drabczyk, E. Waleka, Z. Stojek and M. Karbarz, Int. J. Pharm., 2019, 569, 118589 CrossRef CAS PubMed.
- Y. Ma, Y. Yin, L. Ni, H. Miao, Y. Wang, C. Pan, X. Tian, J. Pan, T. You, B. Li and G. Pan, Bioact. Mater., 2021, 6, 1308–1317 CrossRef CAS PubMed.
- C. Xin, D. Jin, Y. Hu, L. Yang, R. Li, L. Wang, Z. Ren, D. Wang, S. Ji, K. Hu, D. Pan, H. Wu, W. Zhu, Z. Shen, Y. Wang, J. Li, L. Zhang, D. Wu and J. Chu, ACS Nano, 2021, 15, 18048–18059 CrossRef CAS.
- R. D. Durai, J. Nallakkannu, K. Rajaraman and V. H. Bodethala Narayanan, Assay Drug Dev. Technol., 2021, 19, 139–152 CrossRef CAS PubMed.
- R. Diaz-Salmeron, B. Toussaint, N. Huang, E. Bourgeois Ducournau, G. Alviset, S. Goulay Dufaÿ, H. Hillaireau, A. Dufaÿ Wojcicki and V. Boudy, Pharmaceutics, 2021, 13, 117 CrossRef PubMed.
- H. M. Alkhalidi, K. M. Hosny and W. Y. Rizg, Pharmaceutics, 2020, 13, 27 CrossRef.
- E. F. Kong, C. Tsui, H. Boyce, A. Ibrahim, S. W. Hoag, A. J. Karlsson, T. F. Meiller and M. A. Jabra-Rizk, Antimicrob. Agents Chemother., 2016, 60, 881–889 CrossRef CAS PubMed.
- M. J. Martín, A. C. Calpena, F. Fernández, M. Mallandrich, P. Gálvez and B. Clares, Carbohydr. Polym., 2015, 117, 140–149 CrossRef.
- D. Dalessandri, F. Zotti, L. Laffranchi, M. Migliorati, G. Isola, S. Bonetti and L. Visconti, BMC Oral Health, 2019, 19, 153 CrossRef PubMed.
- W. Zheng, Y. Hao, D. Wang, H. Huang, F. Guo, Z. Sun, P. Shen, K. Sui, C. Yuan and Q. Zhou, Carbohydr. Polym., 2021, 272, 118493 CrossRef CAS PubMed.
- M. Rezazadeh, N. Jafari, V. Akbari, M. Amirian, M. Tabbakhian, M. Minaiyan and M. Rostami, Drug Delivery Transl. Res., 2018, 8, 1226–1237 CrossRef CAS PubMed.
- V. G. R. Patlolla, W. P. Holbrook, S. Gizurarson and T. Kristmundsdottir, Curr. Drug Discovery Technol., 2020, 17, 376–386 CrossRef PubMed.
- Y. Xu, S. Zhao, Z. Weng, W. Zhang, X. Wan, T. Cui, J. Ye, L. Liao and X. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 54497–54506 CrossRef CAS PubMed.
- K.-C. Chang, W.-C. Chen, C.-H. Chen, C.-L. Ko, S.-M. Liu and J.-C. Chen, Mater. Sci. Eng., C, 2021, 119, 111576 CrossRef CAS PubMed.
- R.-Y. Huang, W.-C. Tai, M.-H. Ho and P.-C. Chang, J. Periodontal Res., 2020, 55, 529–538 CrossRef CAS PubMed.
- N. L. D'Elía, R. Rial Silva, J. Sartuqui, D. Ercoli, J. Ruso, P. Messina and G. Mestres, J. Colloid Interface Sci., 2020, 572, 408–420 CrossRef.
- Y. Wang, M. Ma, J. Wang, W. Zhang, W. Lu, Y. Gao, B. Zhang and Y. Guo, Materials, 2018, 11, 1345 CrossRef PubMed.
- A. E. Pazarçeviren, Z. Evis, D. Keskin and A. Tezcaner, Biomed. Mater., 2019, 14, 035018 CrossRef.
- Y. Wei, Y.-H. Chang, C.-J. Liu and R.-J. Chung, Pharmaceutics, 2018, 10, 37 CrossRef.
- Q. Yuan, L. Li, Y. Peng, A. Zhuang, W. Wei, D. Zhang, Y. Pang and X. Bi, Biomater. Sci., 2021, 9, 1256–1271 RSC.
- N. Dubey, J. A. Ferreira, A. Daghrery, Z. Aytac, J. Malda, S. B. Bhaduri and M. C. Bottino, Acta Biomater., 2020, 113, 164–176 CrossRef CAS PubMed.
- D. Sharma, S. Hamlet, C. Vaquette, E. B. Petcu, P. Ramamurthy and S. Ivanovski, Sci. Rep., 2021, 11, 23371 CrossRef CAS.
- S. Pouraghaei Sevari, J. K. Kim, C. Chen, A. Nasajpour, C.-Y. Wang, P. H. Krebsbach, A. Khademhosseini, S. Ansari, P. S. Weiss and A. Moshaverinia, ACS Appl. Mater. Interfaces, 2021, 13, 35342–35355 CrossRef CAS.
- T. Xin, J. Mao, L. Liu, J. Tang, L. Wu, X. Yu, Y. Gu, W. Cui and L. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 6840–6851 CrossRef CAS.
- R. Major, P. Kowalczyk, M. Surmiak, I. Łojszczyk, R. Podgórski, P. Trzaskowska, T. Ciach, G. Russmueller, K. Kasperkiewicz, Ł. Major, R. Jabłoński, J. Kropiwnicki and J. M. Lackner, Colloids Surf., B, 2020, 193, 111056 CrossRef CAS PubMed.
- L. Lei, Z. Liu, P. Yuan, R. Jin, X. Wang, T. Jiang and X. Chen, J. Mater. Chem. B, 2019, 7, 2722–2735 RSC.
- L. Chen, R. Shen, S. Komasa, Y. Xue, B. Jin, Y. Hou, J. Okazaki and J. Gao, Int. J. Mol. Sci., 2017, 18, 989 CrossRef PubMed.
- T. Uehara, S. Mise-Omata, M. Matsui, Y. Tabata, R. Murali, M. Miyashin and K. Aoki, J. Dent. Res., 2016, 95, 665–672 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |