Open Access Article
Open Access ArticleCellular fate and performance of group IV metal organic framework radioenhancers†
Anna Lena
Neuer
 ab,
Alexander
Jessernig
ab,
Lukas R. H.
Gerken
ab,
Alexander
Jessernig
ab,
Lukas R. H.
Gerken
 ab,
Alexander
Gogos
ab,
Alexander
Gogos
 ab,
Fabian H. L.
Starsich
ab,
Fabian H. L.
Starsich
 ab,
Alexandre H. C.
Anthis
ab and
Inge K.
Herrmann
ab,
Alexandre H. C.
Anthis
ab and
Inge K.
Herrmann
 *ab
*ab
aLaboratory for Particles-Biology Interactions, Department of Materials Meet Life, Swiss Federal Laboratories for Materials Science and Technology (Empa), Lerchenfeldstrasse 5, 9014 St. Gallen, Switzerland. E-mail: inge.herrmann@empa.ch; ingeh@ethz.ch; Tel: +41 (0)58 765 7153
bNanoparticle Systems Engineering Laboratory, Institute of Process Engineering, Department of Mechanical and Process Engineering, ETH Zurich, Sonneggstrasse 3, 8092 Zurich, Switzerland
First published on 10th October 2022
Abstract
Nano-sized metal organic frameworks (nanoMOFs) have gained increasing importance in biomedicine due to their tunable properties. In addition to their use as carriers in drug delivery, nanoMOFs containing hafnium have been successfully employed as radio-enhancers augmenting damage caused by X-ray irradiation in tumor tissue. While results are encouraging, there is little mechanistic understanding available, and the biological fate of these radio-enhancer nanoparticles remains largely unexplored. Here, we synthesized a selection of group IV metal-based (Hf, Ti, Ti/Zr) nanoMOFs and investigated their cell compatibility and radio-enhancement performance in direct comparison to the corresponding metal oxides. We report surprising radio-enhancement performance of Ti-containing nanoMOFs reaching dose modifying ratios of 3.84 in human sarcoma cells and no relevant dose modification in healthy human fibroblasts. These Ti-based nanoMOFs even outperformed previously reported Hf-based nanoMOFs as well as equimolar group IV metal oxides in direct benchmarking experiments. While group IV nanoMOFs were well-tolerated by cells in the absence of irradiation, the nanoMOFs partially dissolved in lysosomal buffer conditions showing distinctly different chemical stability compared to widely researched group IV oxides (TiO2, ZrO2, and HfO2). Taken together, this study illustrates the promising potential of Ti-based nanoMOFs for radio-enhancement and provides insight into the intracellular fate and stability of group IV nanoMOFs.
Introduction
Radiotherapy is a key treatment modality in cancer therapy. Despite its wide applicability, X-ray radiation suffers from poor tissue specificity. The radiation penetration depth and the total dose applied to the tumor are limited by the radiation tolerance of the healthy tissue surrounding the tumor.1–3 In order to overcome these limitations and at the same time maximize the dose deposited in the diseased tissue, different strategies have been exploited. These include sophisticated treatment planning, beam modulation, or the use of complex beam geometries. Additionally, the radio-sensitizing effects of chemotherapeutic agents have been exploited with the aim of increasing the radiation susceptibility of diseased cells.4 However, the advancement of the aforementioned approaches have plateaued and new strategies are sought after in order to further augment the effectiveness of radiotherapy. Radio-enhancing metal or metal oxide nanoparticles can serve as promising alternatives to existing approaches.5 Inorganic nanoparticles selectively enhance the X-ray absorption cross-section leading to locally enhanced tissue damage in their vicinity compared to particle-free volumes. A wide variety of metal and metal oxide nanoparticles have been investigated, including gold,6,7 platinum,8,9 and others (see Kuncic et al.10 for a comprehensive review). If these nanoparticles accumulate inside a tumor, they efficiently absorb the energy of the incoming photon beam. The absorbed energy can then lead to the ejection of secondary particles, such as electrons, and depending on the metal oxide and the incident X-ray energy, these secondary electrons can travel from only a couple of μm to a few hundreds of μm.10 On their way through tissue, they produce significant amounts of reactive oxygen species (ROS) through ionization events, which can ultimately lead to cell death.11 As the amount of ROS produced is proportional to the accessible surface area, significant effort has been invested into reducing the nanoparticle sizes to maximize the specific surface area.12 However studies have found that smaller particles can in some cases be more cytotoxic compared to their larger counterparts,13 thus constraining the therapeutic window. Alternatively, nano-sized metal organic frameworks (nanoMOFs) offer a large specific surface area and high compositional modularity, and thus offer a route to unify high specific surface area with biocompatibility. MOFs are three-dimensional coordination polymers composed of secondary building units (SBUs) assembled into a porous lattice structure. The SBUs are generated through the coordinative interaction between metal ion clusters and polydentate organic linker molecules.14,15 This so-called reticular synthesis allows the use of a considerable variety of metals and linkers giving rise to fascinating design possibilities.16,17 Combined with recent advances in nanoparticle synthesis, MOFs have shown great promise in a number of applications ranging from catalysis to biomedicine.18–20 First reports of nanoMOFs as radioenhancers were published in 2018 when the Lin group used hafnium-based SBUs to synthesize high-Z nanoMOFs. By comparing two Hf-MOFs that differ in their SBU, they not only showed that the SBU choice influences the X-ray absorption efficiency but also confirmed that a larger surface area leads to increased ROS production.21 Several of their studies have shown that Hf-based nanoMOFs exhibit radio-enhancement efficiencies surpassing even those of HfO2 nanoparticles, which have most recently been approved as NBTXR3 for clinical use in soft tissue sarcoma and other tumor treatments.22 While various linkers have been probed in nanoMOFs for radiotherapy purposes, there is surprisingly little variation in the choice of the metal centers and studies have focused primarily on hafnium. In addition to poorly understood radio-enhancement mechanisms of metal-based radio-enhancers, the biological fate of nanoMOFs is still largely unexplored. It is well-known that group IV metals, including hafnium, are prone to oxidation in biological environments.23 Compared to metal oxides, the administration of nanoMOFs involves the introduction of a potentially more labile form of group IV non-essential metals into biological systems, with yet poorly understood consequences.In this work, we synthesized Hf-DBA, Hf-TCPP, Ti-MIL-125, and Ti/Zr-PCN-415 group IV nanoMOFs as radioenhancer candidate materials. Following physicochemical characterization, buffer stability and cytocompatibility assessment, we investigated their radioenhancement activity under X-ray irradiation based on ROS detection (in cell free conditions) and based on cell survival. We compared the nanoMOF radioenhancement performance to the corresponding equimolar doses of metal oxide nanoparticles (TiO2, ZrO2 and HfO2) in radio-resistant soft tissue sarcoma cells and healthy human fibroblasts. The assessment of the cellular fate and radioenhancement properties illustrated surprising potential for Ti-based nanoMOFs.
Results & discussion
Synthesis and physicochemical characterization of group IV nanoMOFs
We synthesized four different group IV nanoMOFs containing Hf, Ti, and/or Zr metal clusters as radioenhancer candidate materials (Table 1). Hf-DBA and Hf-TCPP have been used for radiation experiments before and can therefore be considered as radioenhancement benchmark materials.21,24,25 Hf-DBA nanoMOFs, containing Hf12O8(OH)14 clusters linked by 2,5-di(p-benzoato)aniline (DBA), were synthesized based on a procedure adapted and scaled up from Ni et al.21 Similarly, Hf-TCPP nanoMOFs, composed of Hf6O4(OH)4 clusters linked by tetrakis(4-carboxyphenyl)porphyrin (TCPP), were prepared based on scaled up and adapted protocols from Bao et al. and Chen et al.24,25 Additionally, Ti and Ti/Zr based nanoMOFs were synthesized. Ti-MIL-125 has been previously exploited for sensing and catalysis, due to its low bandgap energy, and therefore efficient electron transfer from the linker to the Ti4+ center under UV irradiation. The Ti-MIL-125 was synthesized based on a procedure adapted from Vilela et al.26 They are composed of 8-membered Ti–oxo ring clusters linked by 2-aminoterephtalic acid (NH2–TA). The structure of Ti/Zr-PCN-415 is similar to Ti-MIL-125 with Ti4+ ions partially replaced with Zr4+ ions, and the linker devoid of one amino functional group. Ti/Zr-PCN-415 nanoMOFs are composed of Ti8Zr2O12(MeCOO)16 clusters linked by terephtalic acid (TA) and were previously employed by Yuan et al.27 for water reduction catalysis. Here, the latter nanoMOF was selected with the rationale of unifying strong X-ray absorption and photocatalytic activities.We investigated the morphology and topology of the as-prepared group IV nanoMOFs using scanning transmission electron microscopy (STEM) (Fig. 1A–D). Hf-DBA nanoMOFs exhibited a disc-shape morphology with a diameter of 99 ± 21 nm and a disc thickness of 32 ± 9 nm. STEM imaging revealed a spherical morphology for Hf-TCPP, with a relatively rough surface, and a diameter of 230 ± 45 nm. Morphologically, Ti-MIL-125 had a rice-grain shape with a mean length of 230 ± 41 nm. The cauliflower-like spherical particles of Ti/Zr-PCN-415 had a mean diameter of 192 ± 38 nm. Next to TEM size analysis (Fig. S1†), we also performed dynamic light scattering (DLS) measurements (Table S1†). However, as not all types of nanoMOFs exhibited a spherical morphology (and hence the prerequisites for DLS-based sizing are not fulfilled), these measurements can only be taken as an estimate. DLS sizes in ethanol remained well below 300 nm, thus confirming STEM findings. In cell culture medium, agglomeration was observed, however, hydrodynamic diameters remained in the sub-micron range for all nanoMOFs. It is well known that particle size strongly influences intracellular uptake and distribution,28 with both of them being strong determinants of nanoparticle radio-enhancement performance.7 Interestingly, Orellana-Tavra et al. recently showed that larger nanoMOF particles, such as the ones reported here feature a sub-cellular distribution different from smaller nanoMOF particles (which typically end up in lysosomes), offering potentially new prospects also for drug delivery.29 Fourier transmission infrared spectroscopy (FTIR) of the as-prepared group IV nanoMOFs proved the successful coordination of the linker to the metal clusters through weakening or complete disappearance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch at 1700 cm−1, which is characteristic for carboxylic acid groups (Fig. 1E). Instead, antisymmetric and symmetric COO– stretch modes at 1600 cm−1 and 1300 cm−1 appeared as a result of the deprotonated linker coordinated to metal centers. FTIR reference spectra are available only for Ti-MIL-125 and are in good agreement with our data.30 Also, powder X-ray diffraction (XRD) (Fig. 1F) pattern peaks for all group IV nanoMOFs were found well in line with the respective datasets available in literature, hence once again indicating successful nanoMOF synthesis.21,24,31,32 XRD confirmed crystallinity for Hf-DBA, Hf-TCPP, Ti-MIL-125, and Ti/Zr-PCN-415. Especially for Hf-DBA, high-resolution STEM images (Fig. S2†) showed highly ordered secondary building units with almost atomic-level resolution. The elemental composition of group IV nanoMOFs was measured by carbon hydrogen nitrogen (CHN) elemental analysis and inductively coupled plasma optical emission spectroscopy (ICP-OES). Measured elemental compositions were in excellent agreement with theoretical compositions for all as-prepared group IV nanoMOFs (Tables S2 and S3†) with recoveries of around 100% for Hf-DBA and Ti/Zr-PCN-415 and slightly lower recoveries of around 90% for Hf-TCPP and Ti-MIL-125. Brunauer–Emmett–Teller (BET) surface area measurements yielded specific surface areas (SSA) of 412 ± 13 m2 g−1 for Hf-DBA, 1136 ± 53 m2 g−1 for Hf-TCCP, 835 ± 33 m2 g−1 for Ti-MIL-125, and 587 ± 15 m2 g−1 for PCN-415 nanoMOFs. These physicochemical characterization results confirm the successful synthesis of the four different nanoMOF radioenhancer candidate materials.
O stretch at 1700 cm−1, which is characteristic for carboxylic acid groups (Fig. 1E). Instead, antisymmetric and symmetric COO– stretch modes at 1600 cm−1 and 1300 cm−1 appeared as a result of the deprotonated linker coordinated to metal centers. FTIR reference spectra are available only for Ti-MIL-125 and are in good agreement with our data.30 Also, powder X-ray diffraction (XRD) (Fig. 1F) pattern peaks for all group IV nanoMOFs were found well in line with the respective datasets available in literature, hence once again indicating successful nanoMOF synthesis.21,24,31,32 XRD confirmed crystallinity for Hf-DBA, Hf-TCPP, Ti-MIL-125, and Ti/Zr-PCN-415. Especially for Hf-DBA, high-resolution STEM images (Fig. S2†) showed highly ordered secondary building units with almost atomic-level resolution. The elemental composition of group IV nanoMOFs was measured by carbon hydrogen nitrogen (CHN) elemental analysis and inductively coupled plasma optical emission spectroscopy (ICP-OES). Measured elemental compositions were in excellent agreement with theoretical compositions for all as-prepared group IV nanoMOFs (Tables S2 and S3†) with recoveries of around 100% for Hf-DBA and Ti/Zr-PCN-415 and slightly lower recoveries of around 90% for Hf-TCPP and Ti-MIL-125. Brunauer–Emmett–Teller (BET) surface area measurements yielded specific surface areas (SSA) of 412 ± 13 m2 g−1 for Hf-DBA, 1136 ± 53 m2 g−1 for Hf-TCCP, 835 ± 33 m2 g−1 for Ti-MIL-125, and 587 ± 15 m2 g−1 for PCN-415 nanoMOFs. These physicochemical characterization results confirm the successful synthesis of the four different nanoMOF radioenhancer candidate materials.
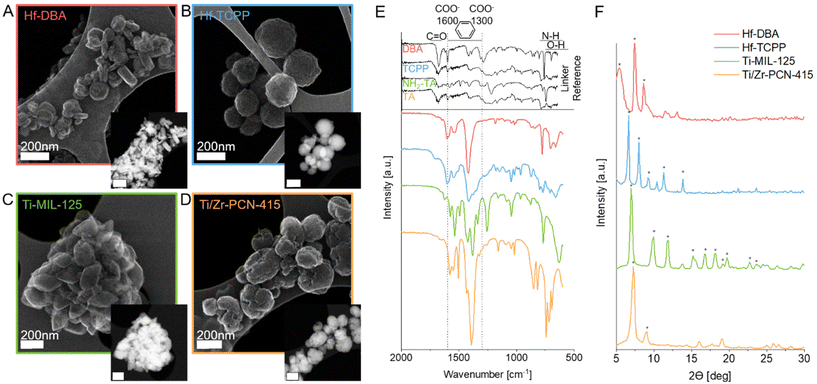 | ||
| Fig. 1 Secondary electron and HAADF (high angle annular dark field, insets) scanning transmission electron micrographs (STEM) of as-prepared group IV nanoMOFs, including Hf-DBA (A), Hf-TCPP (B), Ti-MIL-125 (C), and Ti/Zr-PCN-415 (D) (all scale bars 200 nm). FTIR spectra (E) with organic linker references and XRD patterns (F) of respective group IV nanoMOFs, including reference peak locations from Ni et al.21 for Hf-DBA, Chen et al.25 for Hf-TCPP, Vilela et al.26 for Ti-MIL-125 and Yuan et al.27 for Ti/Zr-PCN-415 indicated by symbols (*). | ||
Stability of nanoMOFs in buffer environments
Prior to cell culture experiments, and due to the expected lower chemical stability of nanoMOFs compared to their metal oxide counterparts, we investigated the buffer stability of the group IV nanoMOFs by assessing the elemental composition of the solid and soluble fractions after 24 h immersion in different media at 37 °C, respectively (Fig. 2, also see Fig. S3†). The solid fraction was defined as the fraction collected after high-speed centrifugation; whereas the respective supernatant is referred to as the soluble fraction. Hf-DBA were perfectly stable in water and PBS with almost 100% of the Hf recovered as part of the solid fraction and less than 1% of Hf found in the supernatant. However, when exposed to the lysosomal buffer instead, again for 24 h, a fraction of 10.7% (lysosomal buffer pH 4.5) and 8.5% (lysosomal buffer pH 5.5) Hf was found in the soluble fraction. Both Hf-containing nanoMOFs showed similar buffer stability with a slightly higher metal release of 3.5% (compared to <1% for Hf-DBA) into the soluble fraction after water incubation for Hf-TCPP. Also, Ti-MIL-125 nanoMOFs were stable in water and PBS (≤0.1% and ≤2.4% in soluble fraction respectively), however, nearly quantitatively dissolved in lysosomal buffer with below 2% metal remaining in the solid fraction after 24 hours nanoMOF immersion. Again, Ti/Zr-PCN-415 nanoMOFs were fully stable in water whereas in PBS and under lysosomal conditions partial metal release was found. For Ti/Zr-PCN-415, more than 85% of both metals remained in the solid fraction after 24 h immersion in PBS. This number decreased to 82.4% and 87.4% for Ti and 75.6% and 81.8% for Zr in lysosomal buffer conditions at pH 4.5 and pH 5.5 respectively.As the lysosomal buffer contains citrate, which might chelate metal ions thus accelerating dissolution, we additionally analyzed the nanoMOF stability in acidified water (containing HNO3) at pH 4.5 and 5.5, respectively. In both Hf containing nanoMOFs, Hf was released into the soluble fraction with 4.0% and 1.4% for Hf-DBA and 7.4% and 7.5% for Hf-TCPP in acidified water at pH 4.5 and 5.5, respectively, confirming a pH-dependent metal release. In contrast, for both Ti containing nanoMOFs below 1% metal was found in the soluble fraction independent of nanoMOF and pH. (Amino-)terephtalic acid linker nanoMOFs seem thus to be less prone to pH-induced dissolution than DBA and TCPP, however, Ti and Zr ions are chelated by citrate more than Hf ions. With regard to simulating the nanoMOF degradation in acidic compartments, these experiments showed that the nanoMOF stability was higher in acidic water compared to lysosomal buffer at the respective pH for all materials, indicating that in lysosomal buffer not only pH plays a role in dissolution but also citrate as a chelator.
Taken together, these data show that all group IV nanoMOFs are stable with above 96% metal content found in the solid fraction when exposed to water and PBS, except for Ti/Zr-PCN-415 with slightly diminished stability in PBS. Lysosomal conditions simulated by citrate buffer (pH 4.5 and 5.5) led to the highest metal release with either partial (Hf-DBA, Hf-TCPP, and Ti/Zr-PCN-415) or quantitative (Ti-MIL-125) nanoMOF dissolution. The higher metal release in lysosomal buffer compared to PBS, water and acidified water may not only be caused by the acidic environment, but also by the chelation of metal ions by citrate.33 Taken together, these buffer stability data indicate that all group IV nanoMOFs were stable in physiological buffers, however, are prone to (partial) degradation in acidic environments.
NanoMOF-catalyzed reactive oxygen species generation under irradiation
Due to the importance of reactive oxygen species (ROS) in radio-enhancement, the ability of the nanoMOFs to generate ROS was quantified in a cell-free assay using a DCF (di-chloro-di-hydro-fluorescein) fluorescent assay (Fig. S4†), with high sensitivity for hydroxyl radicals (HO˙). Based on the DCF signal change, dose enhancement factors (DEF) relative to nanoparticle-free controls were highest for Hf-TCPP nanoMOFs. As expected, all nanoMOFs showed a dose-dependent DEF increase, but interestingly, in comparison to respective equi-molar oxide nanoparticles (HfO2, ZrO2, and TiO2, see Fig. S5† for characterization data), only Hf-TCPP outperformed HfO2. All other oxide nanoparticles showed even higher DEFs at equimolar concentrations. This is surprising as it is expected that in an equimolar dosed experiment due to the large surface area in nanoMOFs the number of accessible metal atoms would be higher than in respective metal oxide nanoparticles. As the Hf-TCPP nanoMOFs with the largest linker and therefore the expectedly biggest pore size performed best, this might indicate a potential limitation in diffusion time of the fluorescent dye into the framework structure and therefore the assay may not account for all ROS effectively generated. Also, the chemical environment, including the buffer system and the cellular system, strongly influences ROS production. Moreover, many of the available fluorescent dye-based ROS quantification assays including the ones available for other H2O2 and superoxide anions, are prone to assay interferences34 (that cannot easily be overcome by nanomaterial removal by centrifugation or precipitation).35 Thus, the development of alternative methods for ROS quantification is warranted in order to be able to gain additional mechanistic insights.Cytocompatibility and cellular uptake
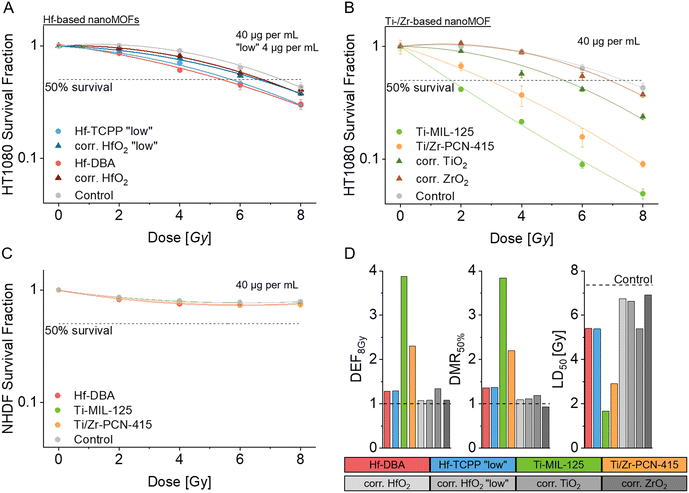
Following physicochemical characterization, buffer stability, and ROS generation capability assessment of the as-synthesized group IV nanoMOFs, we investigated mammalian cell compatibility and cellular uptake, as high cell-compatibility is imperative for further radiation enhancement experiments and safe prospective clinical application. We performed short- (Fig. 3A) and long-term (Fig. 3B and C) cytotoxicity studies to assess acute toxicity after 24 h and potential delayed toxicity due to the limited nanoMOF stability (after 5 days) based on a metabolic activity assay (ATP quantification assay). Short-term cytotoxicity in human sarcoma cells (HT1080) (Fig. 3A) showed the lowest impact on viability for Hf-DBA even for high concentrations (250 μg mL−1). In contrast, Hf-TCPP nanoMOFs showed up to 80% viability reduction for the highest dose with a clear dose-dependent viability reduction. The high toxicity for Hf-TCPP nanoMOFs is likely caused by the porphyrin linker, as porphyrins are known for their toxicity in photodynamic therapy applications.36 The two Ti-based nanoMOFs, Ti-MIL-125 and Ti/Zr-PCN-415, showed comparable cytotoxicity effects on HT1080 cells with a dose-dependent viability reduction of around 40% for the highest concentration (250 μg mL−1). Due to their similarity in composition with the linker molecule only differing in one amino group, the similar toxicity profile is not unexpected. Toxicity might be caused by free metal ions after intracellular nanoMOF degradation.37To further evaluate long-term toxicity, which becomes relevant for the radio-enhancement investigation with an overall observation period of 5 days, we investigated the long-term biocompatibility of two sub-toxic group IV nanoMOFs concentrations (4 and 40 μg mL−1) after 5 days in HT1080 cells (Fig. 3B) and normal human dermal fibroblasts (NHDF) (Fig. 3C). The latter served as a non-cancerous control in radio-enhancement studies. For both cell types, no drastic group IV nanoMOF long-term cytotoxicity was observed, except for the Hf-TCPP nanoMOFs which showed a viability reduction of almost 100% for the higher dose (40 μg mL−1). Notably, this dose showed no significant viability reduction in the short-term experiment, however, led to almost complete cell death after 5 days, thus highlighting the importance of assessing long-term effects. In contrast, the slightly diminished viability observed for the higher concentration of the Ti/Zr-PCN-415 nanoMOFs during short-term exposure recovered to values comparable to the untreated controls at 5 days, indicating a transient nature of this effect. The distinct differences in long-term toxicity between Hf-TCPP and Ti/Zr-PCN-415, leading in one case to complete cell death, and in the other to full recovery may be explained by the fact that Ti/Zr-PCN-415 were significantly better tolerated than Hf-TCPP at the highest concentrations, which might be reached over time due to sedimentation and continuous uptake. Additionally, the cell response to an initial trigger and the proliferation rate is a highly dynamic and multifactorial process.38,39
To be able to contextualize radiation enhancement data, we assessed cellular uptake with ICP-OES (Fig. 4A and B) for metal content quantification and transmission electron microscopy (TEM) imaging (Fig. 4C) for intracellular distribution of the group IV nanoMOFs. We assessed uptake in metal mass per cell in both HT1080 (Fig. 4A) and NHDF (Fig. 4B) cells indicating a comparable nanoparticle uptake (see also Fig. S6† for particle mass per cell). A higher intracellular metal content was found in group IV nanoMOF treated cells compared to oxide nanoparticles (HfO2, ZrO2, and TiO2, see Fig. S5† for characterization data) administered at equimolar metal doses, which can likely be attributed to the smaller size and correspondingly lower sedimentation40 rate of the metal oxide nanoparticles. While the uptake was relatively comparable for the different types of nanoMOFs at equivalent doses, uptake in all particle-treated samples was cell type- and particle-dose dependent. The overall uptake in NHDF cells for group IV nanoMOFs was slightly lower compared to the uptake in HT1080 cells. Uptake for Hf-DBA into cancerous cells has previously been quantified by the Lin group21 and is in close agreement with our data. To analyze the nanoMOF intracellular fate, we assessed the uptake of group IV nanoMOFs in HT1080 cells based on TEM imaging (Fig. 4C). As expected, after 24 h incubation, group IV nanoMOFs were internalized by the cells and localized mainly in vesicular compartments, such as endosomes and lysosomes.41 NanoMOFs were found partially agglomerated inside these vesicular compartments. After 24 h nanoMOF incubation with HT1080 sarcoma cells, Hf-DBA appeared stable, which was well in line with the buffer stability results (Fig. 2), where over 80% of Hf was found in the solid phase after lysosomal buffer incubation. The stability is also in line with data by Ni et al., who found that Hf-DBA nanoMOFs were stable in cell culture medium over at least 72 h.21 For Hf-TCCP and Ti/Zr-PCN-415 some pronounced intracellular nanoMOF aggregation and compaction was observed. Ti-MIL-125 appeared morphologically largely unchanged. These results are in line with the buffer stability investigations.
Radio-enhancement performance of group IV nanoMOFs compared to metal oxides
After confirming and quantifying group IV nanoMOF uptake and identifying sub-toxic concentrations (4 μg mL−1 Hf-TCPP and 4 and 40 μg mL−1 for all other nanoMOFs), we assessed their radio-enhancement efficacies for the aforementioned concentrations in HT1080 and NHDF cells. The survival fraction was assessed five days after irradiation with X-rays (150 keV, Fig. 5) for the nanoMOFs and the corresponding metal oxide nanoparticles at equimolar doses. Survival fractions showed radio-enhancement effects in human sarcoma cells (HT1080), which were strongly dependent on nanoMOF type and dose (Fig. 5A and B for high and Fig. S7† for low nanoMOF concentrations). In sarcoma cells, Hf-based nanoMOFs (Fig. 5A and D) showed dose modifying ratios (DMR50%) of 1.36, which was higher than the HfO2 nanoparticles with a DMR50% of around 1.1. These findings indicate the same trend found by Ni et al.,21 however with a lower overall magnitude. Ni et al. showed more pronounced effects with DMR50% for Hf12-DBA of 1.8–3.5 and for equimolar HfO2 of 1.1–2.4 for different cell lines (none of the cell lines studied by Ni et al. were HT1080 cells).21 Interestingly, in our study, we found that a 10-fold lower concentration of Hf-TCPP (4 μg mL−1) performed almost identical to Hf-DBA (40 μg mL−1) with DMR50% of 1.36 and 1.37 respectively (Fig. 5D). This can only partially be explained by the larger specific surface area of Hf-TCPP (3× higher compared to Hf-DBA) and indicates a significant role of other parameters, including the particle size, surface roughness, linker molecule, etc. In contrast to Hf-MOFs, Ti-based nanoMOFs (Fig. 5B and D) strongly outperformed the corresponding equidosed oxide nanoparticles in vitro with DMR50% values of 3.8 in Ti-MIL-125 and 2.2 in Ti/Zr-PCN-415 compared to 0.9 for ZrO2 and 1.2 for TiO2. Again, for the low nanoMOF doses (Fig. S6†), no significant enhancement effects were found. Table S4† summarizes DEF8Gy, DMR50%, and LD50 values for all nanoMOFs, oxide nanoparticles, and concentrations investigated.Generally, all Ti-containing particles presented higher DMR50% compared to the Hf-based materials. This illustrates that high atomic number alone is not the only determinant for a high radio-enhancement capability (in line with DCF findings, see Fig. S4†),42 but other factors, such as catalytic activity, may also play an important role.43 Indeed, the material with the highest atomic number (Hf-DBA) investigated here, presented the lowest DMR50% values at uptake levels comparable to the other nanoMOFs. In contrast, Ti-MIL-125 with a lower atomic number (compared to Hf-based materials), presented the highest DMR50% in this study, strengthening the hypothesis of catalytic activity through electron transfer from the linker to the metal might impact the radiation enhancement.30,44 The higher ROS generation in Ti-based materials could partially be explained by a radical generation through a pseudo-Fenton reaction, which is known for titanium active sites.45,46 A linker excitation followed by a linker-mediated metal reduction of Ti4+ to Ti3+, might lead to an overall better performance of Ti-based nanoMOFs compared to Hf-based materials as seen in this study. A possible catalytic cycle includes a pseudo-Fenton reaction mechanism for switching from Ti4+ to Ti3+ as proposed in parts by Liu et al.45 for TiO2 and electron transfer from the linker to the metal (Fig. S8†). While in this work, we have employed as-synthesized Ti-MIL-125, interestingly, recent research in photocatalysis has demonstrated that the activity of Ti-MIL-125 may be boosted even further by oxygen vacancy engineering, e.g. using propionic acid as an etching agent.47,48 It has also been shown that the MOF catalytic activity is crystal facet-dependent; facets containing a higher proportion of the metal clusters on the surface possess a higher activity and surface energy. However, compared to the well-established concept in hard materials, such as metal oxides, crystal facet characterization, and engineering in MOFs requires additional efforts due to the complexity of these soft materials and the coexistence of various types of chemical bonds.49
Interestingly, in the non-cancerous NHDF cells, no radio-enhancement effect was detectable, neither for high (Fig. 5C) nor low (Fig. S7†) nanoMOF concentrations, at uptake values comparable between cancerous and non-cancerous cell lines. These findings indicate a higher therapeutic ratio and are in line with previous studies on metal oxide nanoparticles, where considerably lower dose-enhancement efficacies have been observed in non-cancerous fibroblasts compared to cancerous cells.42 The better performance of nanoMOFs compared to the corresponding oxides in equimolar conditions can in part be explained by the higher cell internalization of nanoMOFs as well as the higher accessible surface area of nanoMOFs.
Conclusion
With this work, we introduce group IV transition metal nanoMOFs beyond Hf-MOFs as promising radio-enhancers with high therapeutic ratios. Greatest in vitro radio-enhancement effects were found for Ti-MIL-125, followed by Ti/Zr-PCN-415 nanoMOFs, confirming that not only high atomic number metals but also catalytic43 and radioluminescence50 processes should be considered in the molecular design of high-performance radio-enhancers. While radio-enhancement efficiencies in human sarcoma cells were high, especially for Ti-containing nanoMOFs, they were attenuated to untreated control values in healthy fibroblasts at comparable nanoMOF uptake values, offering new prospects for targeted (tissue-specific) radiotherapy. However, in addition to high radio-enhancement efficiencies, a detailed understanding of stability and intracellular fate is imperative with regard to prospective clinical applications. We show high buffer stability for all nanoMOFs in water and PBS with less than 20% metal release from the as-synthesized framework whereas in acidic, lysosome-mimicking conditions, nanoMOFs showed partial dissolution. Nonetheless, three out of four nanoMOFs (Hf-DBA, Ti-MIL-125, Ti/Zr-PCN-415) showed high short and long-term cytocompatibility, indicating that gradual stability reduction might even offer new prospects for radio-enhancer clearance. Taken together, the here presented group IV nanoMOFs, especially the Ti-based nanoMOFs, outperformed corresponding equimolar metal oxide nanoparticles in in vitro X-ray radio-enhancement and therefore offer promising prospects for nanoMOF-based high-performance radio-enhancement.Experimental section
NanoMOF synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 45 minutes. The precipitate was then washed once with DMF, then once with 1% trimethylamine/ethanol, and then twice with ethanol and finally dried overnight at 40 °C under vacuum.
000g for 45 minutes. The precipitate was then washed once with DMF, then once with 1% trimethylamine/ethanol, and then twice with ethanol and finally dried overnight at 40 °C under vacuum.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 45 minutes. The precipitate was washed twice with DMF, then once with 1% triethylamine/ethanol, and then twice with ethanol and finally dried overnight at 40 °C under vacuum.
000g for 45 minutes. The precipitate was washed twice with DMF, then once with 1% triethylamine/ethanol, and then twice with ethanol and finally dried overnight at 40 °C under vacuum.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g at 4 °C for 45 min) and finally dried overnight at 40 °C under vacuum.
000g at 4 °C for 45 min) and finally dried overnight at 40 °C under vacuum.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 45 minutes) and finally dried overnight at 40 °C under vacuum.
000g for 45 minutes) and finally dried overnight at 40 °C under vacuum.
Corresponding oxide nanoparticles HfO2, TiO2 and ZrO2 were synthesized via flame spray pyrolysis based on procedures reported elsewhere.42
NanoMOF characterization
Scanning transmission electron micrographs (STEM) images of as prepared nanoparticles were recorded on a Talos F200X TEM (Super-X EDS, 4 detector configuration, FEI, USA) at an acceleration voltage of 200 kV. NP samples were prepared via a drop through grid method of 1 mg ml−1 nanoMOF suspension in ethanol on nickel (Hf-DBA; Hf-TCPP)/copper (Ti-MIL-125; Ti/Zr-PCN-415) grids (EMR, Holey Carbon Film 200 Mesh Nickel/Copper). NanoMOF suspension was placed on the respective grid for 2 min and pulled through the grid with a filter paper, the grid was then washed three times with water.Fourier transform infrared spectroscopy (FTIR) measurements were performed using a Varian 640 IR FT-IR spectrometer. Transmission of samples were measured in solid form and previously measure background subtracted.
X-ray diffraction (XRD) patterns were obtained using a Bruker D2 Phaser (30 kV, mA). Dry MOF sample were measured at the diffraction angle 2θ from 5° to 50° in 0.15° per step.
Dynamic light scattering (DLS) hydrodynamic size measurements were obtained using a Zetasizer Nano ZS90 (Malvern Instruments Ltd, Worcestershire, United Kingdom) with a 90°scattering angle. To assess dispersant effects on hydrodynamic size, particles were dispersed in water, ethanol and cell culture medium at a concentration of 1 mg mL−1. The 1 mg ml−1 stock solutions were diluted dependent on the particle from 0.1–0.9 mg mL−1 to ensure a count rate between 200–400 kcps at an attenuator level of 7–9.
Inductively coupled plasma optical emission spectrometry (ICP-OES) measurements of 1 mg ml−1 nanoMOFs dispersion in water. 500 μl of sample were mixed with 1500 μl sulfuric acid (H2SO4) and 1500 μl ultrapure water in a quartz digestion tube. For digestion, a pressurized microwave (turboWAVE I500 MWS GmbH, Germany) at 1000 W, 200 °C and 150 bar pressure for 40 minutes was used. After digestion in the microwave, 1000 μl hydrogen peroxide (H2O2) was added to each sample, carefully mixed and allowed to react for 20 minutes. The tube contents were then transferred to 50 ml falcon tubes and filled to the 25 ml mark with 2% nitric acid (HNO3). To verify the adequacy of this digestion method, each nanoMOF was also digested once using the gold standard method, where 500 μl of sample were mixed with 1 mL 40% hydrofluoric acid (HF) in 3 ml HNO3 and digested in a pressurized microwave as above. Recoveries of the sulfuric acid method were at 95–98% of the content determined using the HF method, which were considered 100%. The respective metal content was quantified using an Agilent 5110 ICP-OES (Agilent, Switzerland) with external calibration from 0 to 5 ppm.
Brunauer–Emmett–Teller (BET) method was used for specific surface area analysis by a five-point N2 adsorption isotherm measurement at 77 K after degassing for 2 h at 180 °C using a Micromeritics Tristar II PLUS.
Cell-free experiments
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 45 min the supernatant was collected as soluble fraction. After washing twice with water the residual solid was dispersed in 0.9 ml water as solid fraction and considered as a concentration of 10 mg ml−1 (as 1 ml was removed for total fraction). For total and soluble fraction 250 μl and 50 μl for solid fraction were digested with 750 μl H2SO4 and 750 μl water in pressurized microwave. After microwave digestion 500 μl H2O2 were added and allowed to react for 20 min before transfer to 50 ml tube and filled to 25 ml mark with 2% HNO3. The respective metal content was then quantified using an Agilent 5110 ICP-OES (Agilent, Switzerland) with external calibration from 0 to 5 ppm.
000g for 45 min the supernatant was collected as soluble fraction. After washing twice with water the residual solid was dispersed in 0.9 ml water as solid fraction and considered as a concentration of 10 mg ml−1 (as 1 ml was removed for total fraction). For total and soluble fraction 250 μl and 50 μl for solid fraction were digested with 750 μl H2SO4 and 750 μl water in pressurized microwave. After microwave digestion 500 μl H2O2 were added and allowed to react for 20 min before transfer to 50 ml tube and filled to 25 ml mark with 2% HNO3. The respective metal content was then quantified using an Agilent 5110 ICP-OES (Agilent, Switzerland) with external calibration from 0 to 5 ppm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g at 4 °C for 5 min. Then, 500 μl supernatant was transferred to a new tube without nanoMOF contamination and stored on ice for transportation. Technical triplicates of 150 μl each sample were transferred to a clear bottom 96 well plate and fluorescence was measured at an excitation wavelength of 485 nm and an emission wavelength of 528 nm at 40% lamp energy using a Mithras LB 943 Multimode plate reader. The dose enhancement factor DEF was calculated as:
000g at 4 °C for 5 min. Then, 500 μl supernatant was transferred to a new tube without nanoMOF contamination and stored on ice for transportation. Technical triplicates of 150 μl each sample were transferred to a clear bottom 96 well plate and fluorescence was measured at an excitation wavelength of 485 nm and an emission wavelength of 528 nm at 40% lamp energy using a Mithras LB 943 Multimode plate reader. The dose enhancement factor DEF was calculated as:where f12S stands for the mean fluorescence intensity of the 12 Gy irradiated nanoMOF containing sample and f12C for the respective 12 Gy irradiated particle free control. Similarly, f0S and f0C indicates the respective non-irradiated sample and control.
In vitro experiments
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 HT1080 or NHDF cells were seeded in 12-well plates, allowed to attach overnight and treated with 4 or 40 μg mL−1 (total volume 1 mL) nanoMOF and respective metalmass equi-dosed oxide particle in cellculture medium. After 24 h incubation remaining nanoMOFs/particle were removed and cells washed two times with PBS for extracellular nanoMOF removal. Cells were detached with trypsin, counted and 10
000 HT1080 or NHDF cells were seeded in 12-well plates, allowed to attach overnight and treated with 4 or 40 μg mL−1 (total volume 1 mL) nanoMOF and respective metalmass equi-dosed oxide particle in cellculture medium. After 24 h incubation remaining nanoMOFs/particle were removed and cells washed two times with PBS for extracellular nanoMOF removal. Cells were detached with trypsin, counted and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per sample were pelleted by 5 min centrifugation at 200g. Matrix free cells were digested and further procedure as described above in the ICP-OES section. The respective metal content was then quantified using an Agilent 5110 ICP-OES (Agilent, Switzerland) with external calibration from 0 to 5 ppm.
000 cells per sample were pelleted by 5 min centrifugation at 200g. Matrix free cells were digested and further procedure as described above in the ICP-OES section. The respective metal content was then quantified using an Agilent 5110 ICP-OES (Agilent, Switzerland) with external calibration from 0 to 5 ppm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Epon 1
Epon 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 RT, overnight 100% Epon with open lid RT) and finally polymerized in fresh 100% Epon at 60 °C for 48 h. Sections of 60 nm thickness were cut and STEM images recorded on a Talos F200X TEM (Super-X EDS, 4 detector configuration, FEI, USA) at an acceleration voltage of 200 kV.
1 RT, overnight 100% Epon with open lid RT) and finally polymerized in fresh 100% Epon at 60 °C for 48 h. Sections of 60 nm thickness were cut and STEM images recorded on a Talos F200X TEM (Super-X EDS, 4 detector configuration, FEI, USA) at an acceleration voltage of 200 kV.
The dose enhancement factor54 (DEFxGy) was calculated as following:
The dose modifying ratio54 (DMR) was calculated as following:
| LD50 = xGy50% SF. |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was in parts supported by the Gebauer Foundation, the OPO Foundation, the Swiss National Science Foundation (Eccellenza grant number 181290), the Swiss Cancer Research Foundation (KFS-4868-08-2019) and an ETH Grant (ETH-07 21-2). We acknowledge the Microscopy Center of the University of Zurich and ScopeM (ETHZ) for microscopy sample preparation and access to their microscopes, and the Empa X-ray center for access to their irradiation facilities. We thank Ralf Kägi and Andreas Voegelin for access and Brian Sinnet for assistance at the HF digestion facilities of the Swiss Federal Institute of Aquatic Science and Technology (EAWAG). The graphical abstract was created with Biorender.com.References
- H. Krieger and W. Petzold, Strahlenphysik, Dosimetrie und Strahlenschutz, Vieweg + Teubner Verlag, Wiesbaden, 1992 Search PubMed.
- C. R. Lewanski and W. J. Gullick, Lancet Oncol., 2001, 2, 366 CrossRef CAS PubMed.
- G. C. Barnett, C. M. L. West, A. M. Dunning, R. M. Elliott, C. E. Coles, P. D. P. Pharoah and N. G. Burnet, Nat. Rev. Cancer, 2009, 9, 134 CrossRef CAS PubMed.
- Y. Matsuno, M. Hyodo, H. Fujimori, A. Shimizu and K. Yoshioka, Cancers, 2018, 10, 364 CrossRef CAS PubMed.
- A. Guerreiro, N. Chatterton, E. M. Crabb and J. P. Golding, Cancer Nanotechnol., 2019, 10, 10 CrossRef CAS.
- K. T. Butterworth, S. J. McMahon, F. J. Currell and K. M. Prise, Nanoscale, 2012, 4, 4830 RSC.
- A. L. Neuer, L. R. H. Gerken, K. Keevend, A. Gogos and I. K. Herrmann, Nanoscale Adv., 2020, 2, 2992 RSC.
- A. Pottier, E. Borghi and L. Levy, Anticancer Res., 2014, 34, 443 CAS.
- L. Maggiorella, G. Barouch, C. Devaux, A. Pottier, E. Deutsch, J. Bourhis, E. Borghi and L. Levy, Future Oncol., 2012, 8, 1167 CrossRef CAS PubMed.
- Z. Kuncic, Phys. Med. Biol., 2018, 28(63), 02TR01 CrossRef PubMed.
- D. Howard, S. Sebastian, Q. V.-C. Le, B. Thierry and I. Kempson, Int. J. Mol. Sci., 2020, 21, 579 CrossRef CAS PubMed.
- A. Abdal Dayem, M. K. Hossain, S. B. Lee, K. Kim, S. K. Saha, G.-M. Yang, H. Y. Choi and S.-G. Cho, Int. J. Mol. Sci., 2017, 18, 120 CrossRef PubMed.
- Y.-W. Huang, M. Cambre and H.-J. Lee, Int. J. Mol. Sci., 2017, 18, 2702 CrossRef PubMed.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276 CrossRef CAS.
- J. L. C. Rowsell and O. M. Yaghi, Microporous Mesoporous Mater., 2004, 73, 3 CrossRef CAS.
- O. M. Yaghi, G. Li and H. Li, Nature, 1995, 378, 703 CrossRef CAS.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705 CrossRef CAS PubMed.
- Q. Wang and D. Astruc, Chem. Rev., 2020, 120, 1438 CrossRef CAS PubMed.
- X. Ma, M. Lepoitevin and C. Serre, Mater. Chem. Front., 2021, 5, 5573 RSC.
- S. Wang, C. M. McGuirk, A. d'Aquino, J. A. Mason and C. A. Mirkin, Adv. Mater., 2018, 30, 1800202 CrossRef PubMed.
- K. Ni, G. Lan, C. Chan, B. Quigley, K. Lu, T. Aung, N. Guo, P. La Riviere, R. R. Weichselbaum and W. Lin, Nat. Commun., 2018, 9, 2351 CrossRef PubMed.
- S. Bonvalot, P. L. Rutkowski, J. Thariat, S. Carrère, A. Ducassou, M.-P. Sunyach, P. Agoston, A. Hong, A. Mervoyer and M. Rastrelli, et al. , Lancet Oncol., 2019, 20, 1148 CrossRef CAS PubMed.
- M. Kleczkowski and M. Garncarz, Pol. J. Vet. Sci., 2012, 15, 165 CAS.
- J. Bao, X. Zu, X. Wang, J. Li, D. Fan, Y. Shi, Q. Xia and J. Cheng, Int. J. Nanomed., 2020, 15, 7687 CrossRef CAS PubMed.
- Y. Chen, H. Zhong, J. Wang, X. Wan, Y. Li, W. Pan, N. Li and B. Tang, Chem. Sci., 2019, 10, 5773 RSC.
- S. Vilela, P. Salcedo-Abraira, I. Colinet, F. Salles, M. de Koning, M. Joosen, C. Serre and P. Horcajada, Nanomaterials, 2017, 7, 321 CrossRef PubMed.
- S. Yuan, J.-S. Qin, H.-Q. Xu, J. Su, D. Rossi, Y. Chen, L. Zhang, C. Lollar, Q. Wang and H.-L. Jiang, et al. , ACS Cent. Sci., 2018, 4, 105 CrossRef CAS PubMed.
- P. Foroozandeh and A. A. Aziz, Nanoscale Res. Lett., 2018, 13, 339 CrossRef PubMed.
- C. Orellana-Tavra, S. A. Mercado and D. Fairen-Jimenez, Adv. Healthcare Mater., 2016, 5, 2261 CrossRef CAS PubMed.
- Y. Fu, D. Sun, Y. Chen, R. Huang, Z. Ding, X. Fu and Z. Li, Angew. Chem., Int. Ed., 2012, 51, 3364 CrossRef CAS PubMed.
- S. Vilela, P. Salcedo-Abraira, I. Colinet, F. Salles, M. de Koning, M. Joosen, C. Serre and P. Horcajada, Nanomaterials, 2017, 7, 321 CrossRef PubMed.
- S. Yuan, J.-S. Qin, H.-Q. Xu, J. Su, D. Rossi, Y. Chen, L. Zhang, C. Lollar, Q. Wang and H.-L. Jiang, et al. , ACS Cent. Sci., 2018, 4, 105 CrossRef CAS PubMed.
- R. Uppal, C. D. Incarvito, K. V. Lakshmi and A. M. Valentine, Inorg. Chem., 2006, 45, 1795 CrossRef CAS PubMed.
- J. Zhao and M. Riediker, J. Nanopart. Res., 2014, 16, 2493 CrossRef PubMed.
- S. Zwiehoff, J. Johny, C. Behrends, A. Landmann, F. Mentzel, C. Bäumer, K. Kröninger, C. Rehbock, B. Timmermann and S. Barcikowski, Small, 2022, 18, 2106383 CrossRef CAS PubMed.
- J. Kou, D. Dou and L. Yang, Oncotarget, 2017, 8, 81591 CrossRef PubMed.
- À. Ruyra, A. Yazdi, J. Espín, A. Carné-Sánchez, N. Roher, J. Lorenzo, I. Imaz and D. Maspoch, Chem. – Eur. J., 2015, 21, 2508 CrossRef PubMed.
- J. Liu, Y. Yang, W. Zhu, X. Yi, Z. Dong, X. Xu, M. Chen, K. Yang, G. Lu and L. Jiang, et al. , Biomaterials, 2016, 97, 1 CrossRef CAS PubMed.
- J. Wang, D. Chen, B. Li, J. He, D. Duan, D. Shao and M. Nie, Sci. Rep., 2016, 6, 26126 CrossRef CAS PubMed.
- A. Spyrogianni, I. K. Herrmann, M. S. Lucas, J.-C. Leroux and G. A. Sotiriou, Nanomedicine, 2016, 11, 2483 CrossRef CAS PubMed.
- S. S. Teske and C. S. Detweiler, Int. J. Environ. Res. Public Health, 2015, 12, 1112 CrossRef PubMed.
- L. Gerken, A. Neuer, P. Gschwend, K. Keevend, A. Gogos, A. Anthis, L. Aengenheister, S. Pratsinis, L. Plasswilm and I. Herrmann, Chem. Mater., 2021, 33, 3098 CrossRef CAS.
- L. R. H. Gerken, A. Gogos, F. H. L. Starsich, H. David, M. E. Gerdes, H. Schiefer, S. Psoroulas, D. Meer, L. Plasswilm and D. C. Weber, et al. , Nat. Commun., 2022, 13, 3248 CrossRef CAS PubMed.
- H. L. Nguyen, New J. Chem., 2017, 41, 14030 RSC.
- Z. Liu, T. Wang, X. Yu, Z. Geng, Y. Sang and H. Liu, Mater. Chem. Front., 2017, 1, 1989 RSC.
- G. Lan, K. Ni, S. S. Veroneau, X. Feng, G. T. Nash, T. Luo, Z. Xu and W. Lin, J. Am. Chem. Soc., 2019, 141, 4204 CrossRef CAS PubMed.
- Y. Chang, H. Huang, T. Yang, L. Wang, H. Zhu and C. Zhong, J. Colloid Interface Sci., 2021, 599, 785 CrossRef CAS PubMed.
- L. Wang, S. Wang, M. Li, X. Yang, F. Li, L. Xu and Y. Zou, J. Alloys Compd., 2022, 909, 164751 CrossRef CAS.
- J. Kolosnjaj-Tabi, L. Lartigue, Y. Javed, N. Luciani, T. Pellegrino, C. Wilhelm, D. Alloyeau and F. Gazeau, Nano Today, 2016, 11, 280 CrossRef CAS.
- C. Wang, O. Volotskova, K. Lu, M. Ahmad, C. Sun, L. Xing and W. Lin, J. Am. Chem. Soc., 2014, 136, 6171 CrossRef CAS PubMed.
- Y. Zhao, Q. Zhang, Y. Li, R. Zhang and G. Lu, ACS Appl. Mater. Interfaces, 2017, 9, 15079 CrossRef CAS PubMed.
- R. Zhou, H. Wang, Y. Yang, C. Zhang, X. Dong, J. Du, L. Yan, G. Zhang, Z. Gu and Y. Zhao, Biomaterials, 2019, 189, 11 CrossRef CAS PubMed.
- Promega Corporation·2800 Woods Hollow Road·Madison, WI 53711-5399 USA, n.d.
- A. Subiel, R. Ashmore and G. Schettino, Theranostics, 2016, 6, 1651 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2bm00973k |
| This journal is © The Royal Society of Chemistry 2022 |