Current knowledge on the tissue distribution of mRNA nanocarriers for therapeutic protein expression
Abstract
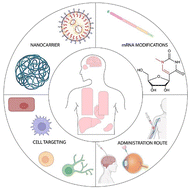
Exogenously delivered mRNA-based drugs are emerging as a new class of therapeutics with the potential to treat several diseases. Over the last decade, advancements in the design of non-viral delivery tools have enabled mRNA to be evaluated for several therapeutic purposes including protein replacement therapies, gene editing, and vaccines. However, in vivo delivery of mRNA to targeted organs and cells remains a critical challenge. Evaluation of the biodistribution of mRNA vehicles is of utmost importance for the development of effective pharmaceutical candidates. In this review, we discuss the recent advances in the design of nanoparticles loaded with mRNA and extrapolate the key factors influencing their biodistribution following administration. Finally, we highlight the latest developments in the preclinical and clinical translation of mRNA therapeutics for protein supplementation therapy.
- This article is part of the themed collection: Biomaterials Science Emerging Investigator Series


 Please wait while we load your content...
Please wait while we load your content...