Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Evaluation of injectable nucleus augmentation materials for the treatment of intervertebral disc degeneration
Matthew P.
Culbert
 *a,
James P.
Warren
*a,
James P.
Warren
 a,
Andrew R.
Dixon
a,
Andrew R.
Dixon
 a,
Hazel L.
Fermor
a,
Hazel L.
Fermor
 a,
Paul A.
Beales
a,
Paul A.
Beales
 b and
Ruth K.
Wilcox
b and
Ruth K.
Wilcox
 *a
*a
aInstitute of Medical and Biological Engineering, School of Mechanical Engineering, University of Leeds, UK LS2 9JT. E-mail: cm14mpc@leeds.ac.uk; R.K.Wilcox@leeds.ac.uk
bSchool of Chemistry, Astbury Centre for Structural Molecular Biology and Bragg Centre for Materials Research, University of Leeds, UK LS2 9JT
First published on 17th December 2021
Abstract
Back pain affects a person's health and mobility as well as being associated with large health and social costs. Lower back pain is frequently caused by degeneration of the intervertebral disc. Current operative and non-operative treatments are often ineffective and expensive. Nucleus augmentation is designed to be a minimally invasive method of restoring the disc to its native healthy state by restoring the disc height, and mechanical and/or biological properties. The majority of the candidate materials for nucleus augmentation are injectable hydrogels. In this review, we examine the materials that are currently under investigation for nucleus augmentation, and compare their ability to meet the design requirements for this application. Specifically, the delivery of the material into the disc, the mechanical properties of the material and the biological compatibility are examined. Recommendations for future testing are also made.
Introduction
Degeneration of the intervertebral disc (IVD) is one of the main causes of lower back pain, which is estimated to affect 80% of adults during their lifetimes,3 of which 10% will become chronically disabled.4 There is also a large economic burden associated with lower back pain, estimated to be as high as 1–2% of gross domestic product in many Western countries.5 This is attributed to the direct cost of treatment and indirect societal costs, such as the loss of active workforce. When non-surgical interventions such as physical exercise and analgesics are no longer effective, disc degeneration may be treated by spinal fusion.6,7 However, despite increasing numbers of procedures being performed, the clinical success of the surgery is relatively low compared to other orthopaedic interventions, with long recovery times and high rates of revision.8,9 Therefore, earlier-stage interventions that prevent or delay the need for spinal fusion are desirable. A number of biomaterials have been investigated as candidates to replace or augment the intervertebral disc; the characterisation methods for these purposes were recently reviewed by Schmitz et al.10 Here, we focus on the specific clinical application of nucleus augmentation as a treatment for intervertebral disc degeneration. We examine the state of the art in injectable materials and review candidate materials against the clinical requirements in terms of their delivery, mechanical properties and biocompatibility.The intervertebral disc
The healthy IVD (Fig. 1) is comprised of an outer annulus fibrosus of lamellae containing collagen fibres orientated anti-parallel to neighbouring lamellae and an inner gel-like nucleus pulposus.11 The discs are separated from the cranial and caudal vertebrae by thin (<1 mm) cartilaginous endplates.12,13 | ||
| Fig. 1 Structure of a healthy IVD under a compressive load with the nucleus distributing the loads into the annulus layers resulting in hoop stresses.2 Reproduced from ref. 1, licensed under CC BY 4.0. | ||
The ability of the disc to perform its function to transfer load comes from two main components, collagen and aggrecan, which make up the majority of the extracellular matrix composition.12,14,15
The collagen fibres provide tensile strength to the disc as well as securing the tissue to the endplates and bone.12 Aggrecan consists of sulfate-heavy glycosaminoglycans attached to a core protein. Aggrecans are bound to long-chain hyaluronan to form large proteoglycan networks. The negatively charged sulfate groups cause an influx of positively charged counterions which increases the osmotic pressure within the disc aiding in discal hydration.16
When load is applied, the internal pressure of the nucleus increases and its expansion is resisted by hoop stresses in the annulus, which are distributed through the network of collagen fibres.17 Due to the composition and hydrated nature of the disc tissue, the response to load is non-linear and both time and rate-dependent.18
Degeneration of the intervertebral disc
Non-traumatic degeneration of the IVD occurs mainly after skeletal maturity is reached.19 The nucleus pulposus becomes more fibrotic and less gel-like. Cell viability in the disc is reduced, with necrotic cells being reported in more than 50% of adult discs.20,21 Changes in the morphology of the disc allow fissures to form in the nucleus and the disc to lose height.13 Nerve and blood vessels start to grow into the disc and are thought to be a source of pain.22The pH inside the IVD varies with the stage of disc degeneration ranging from pH 7.4 in healthy discs to 5.7 in severely degenerated discs.23–26 The decreased pH can cause a decrease in cell proliferation and viability as well as an increase in the expression of proinflammatory cytokines and pain-related factors.23
As the degeneration progresses, the biochemistry of the disc changes. The proteoglycan networks break down into smaller aggrecan fragments through degradation at the hyaluronate binding region. The small, mobile fragments are then able to leach out of the disc. This reduces the osmotic pressure and reduces the level of hydration.13 Additionally, the ratio of collagen types changes and collagen type II can become denatured through enzymatic degradation.13 As the nucleus becomes more fibrotic with degeneration, it starts to behave more non-hydrostatically.17,27 These changes in the disc structure have a direct effect on the ability of the disc to carry out its load bearing function.
Nucleus augmentation
The relatively poor outcomes of late-stage treatments for intervertebral disc degeneration have led researchers to investigate the potential for earlier stage surgical interventions to delay or prevent the need for spinal fusion. Nucleus augmentation, in which a material is injected into the nucleus region of the IVD, has received considerable attention because it offers the potential to restore the natural biomechanics to the disc without the need for invasive surgery. The aim of the treatment is to augment the degenerated nucleus by restoring the disc height and improving the mechanical properties either directly through augmentation materials or additionally through the delivery of cells for tissue regeneration. As a concept still under development, the clinical indicators for nucleus augmentation have not yet been fully established, but would likely mirror those for nucleus replacement therapies, e.g. disc degeneration causing low back pain with or without leg pain.28 The treatment requires the annulus fibrosus to be sufficiently intact to prevent expulsion of the injected material. However, early stages of disc degeneration are often asymptomatic and therefore challenging to identify without MRI.29 As such, nucleus augmentation is likely best suited for intermediate to severe degeneration as a route to prevent or delay the need for spinal fusion.30There are multiple nucleus augmentation materials in development that use hydrogels or a polymeric suspension in water to form a gel. Therefore, the materials discussed will be referred to as ‘hydrogels’. Hydrogels can often be designed to be injected as a liquid that gels in situ by controlling the gelation trigger.31–34 Additionally, there a number of materials designed as cell scaffolds which have been used with the aim of successfully delivering cells into the disc. These materials differ from nucleus augmentation materials because they are not designed to restore the biomechanical properties to the disc but instead to deliver cells that go on to create extracellular matrix. This review focuses only on materials that are designed for nucleus augmentation, and not those designed purely for cell delivery.
Requirements for nucleus augmentation and testing processes
General requirements
Several authors10,31,32,35–37 have suggested requirements for a material to be successful as a nucleus augmentation device and be viable in a clinical setting, from which it can be summarised that the device must meet three key requirements:• Delivery: The delivery must be minimally invasive to avoid damage to the surrounding tissue and the injected material must form a gel rapidly in situ to minimise requirements for patients to remain immobile.
• Biological: The hydrogel must be biocompatible and administered aseptically without significant toxicity or carcinogenicity.
• Mechanical: The augmented disc must have similar mechanical properties to the healthy disc to be able to restore disc functionality and height.
All of these requirements are important and need to be taken into consideration for the development of a successful treatment. If the injected material does not cure or form a gel shortly after injection into the disc, then there is a heightened risk of leakage, preventing the desired mechanical restoration and potentially causing adverse side effects if the gel forms outside the intended treatment area. Similarly, the treatment needs to be administered through a minimally invasive technique to prevent further damage. This is commonly achieved by using small diameter needles. It is known that needle puncture to the annulus can affect the mechanical and biochemical properties of the disc over time. In a review of investigations where needles were used to puncture the IVD to either cause degeneration or to create control sham injections, Elliot et al.38 found that needle diameters greater than 40% of the disc height caused significant changes in mechanical and biochemical properties as well as changes in disc height. Below 40%, the results were more mixed and the authors concluded that there were minimal effects. On the other hand, work by Michalek et al.39 found that a needle diameter: disc height ratio as low as 16.4% can cause changes in the local structure of the annulus fibrosus and alter the mechanical properties.
Therefore, the size of the needle used to deliver the hydrogel needs to be considered to minimise the effect of causing damage to the human IVD when the treatment is translated into clinical use. Human lumbar disc heights are generally in the range of 7–11 mm.40–43 A 20G needle (0.91 mm diameter) has a needle diameter to disc height ratio of 8–15%44 and is, therefore, the largest needle that could be used whilst reducing the risk of damage to the annulus. If the same gauge of needle is used during in vitro or in vivo animal testing, where the disc size and height are likely to be less than a human IVD, it is likely more damage will be caused from the needle puncture. This allows animal testing to present a worst case scenario with regards to the effect of needle diameter.
As with all medical devices, safety standards must be met to minimise risk to the patient. Treatments must be produced and delivered aseptically to prevent infection. Additionally, the treatment must be biocompatible because any immune response that is caused by the treatment is likely to exacerbate the state of degeneration.23
In order to restore the biomechanical function to the disc, the treatment must have similar mechanical properties to the native tissue. The healthy nucleus pulposus has been found to have a complex modulus (G*) ranging from 7–20 kPa.45 The mechanical properties should be similar to the healthy disc because the treatment is being used to augment and restore the mechanical properties, rather than completely replacing the degenerated tissue. If the mechanical properties are dissimilar then the loads may not be distributed physiologically, or the natural movement of the spine will not be restored.
The nucleus augmentation materials reviewed in this article will be critiqued on whether they meet the requirements outlined above.
Testing process
The process for developing these technologies is shown in Fig. 2, and is similar to the regulatory approval pathway for other medical devices. The testing is split into mechanical and biological testing with a general progression from understanding the material properties to the application in humans. Most nucleus augmentation materials are yet to reach clinical trials and are currently progressing through the material testing and in vitro testing stages which allows assessment of the material's ability to meet the requirements. | ||
| Fig. 2 Generalised product development process with a focus on the earlier stages of testing for which the majority of hydrogels reviewed in this article are progressing through. | ||
Hydrogel systems and delivery
Table 1 summarises the hydrogels that have been investigated in this review as nucleus augmentation materials. The hydrogel material, gelation trigger and time are summarised as well as the needle gauge that was used to inject the hydrogel. The importance of the needle gauge, biocompatibility and mechanical properties are subsequently discussed.| Research group | Hydrogel | Gelation category | Gelation trigger | Gelation time | Needle gauge | Cellular |
|---|---|---|---|---|---|---|
| (A) Gelation caused by non-covalent interactions. (B) Gelation caused by polymerisation through covalent bonding. (C) Gelation caused by a combination of covalent and non-covalent interactions. See Fig. 3. | ||||||
| Sheffield Hallam, UK32 | LAPONITE® crosslinked poly-N-isopropylacrylamide, N,N′-dimethylacrylamide comonomer (pNIPAM-co-DMAc) | A | Thermal | <5 s | 26 | Cellular and acellular hydrogels investigated |
| Swiss Federal Institute of Technology in Lausanne (EPFL), Switzerland34 | Poly(ethyleneglycol)dimethacrylate (PEGDMA) | B | UV | 25 min | 19 | Acellular |
| Prince of Wales Hospital, Australia46 | Water in oil emulsion | A | Interaction of 2 liquids | <8 min | 18 | Acellular |
| University of Pennsylvania, USA33,47 | Oxidised dextran, N-carboxyethyl chitosan and teleostean (DCT) | C | Reaction between molecules | 10 hours | 22 | Cellular and acellular hydrogels investigated |
| City College of New York, USA31 | Carboxymethyl cellulose and methacrylated methylcellulose | B | Thermal | <4 min | 20 | Acellular |
| Sunnybrook Research Institute, Canada48,49 | Thiol-modified hyaluronan elastin-like polypeptide with polyethylene diacrylate | A | Thermal | 30 min | 18 | Acellular |
| Duke University, USA50 | NuCore silk and elastin copolymer | B | Reaction with crosslinking reagent | 5–30 min | NR | Acellular |
| University of Waterloo, Canada51,52 | pNIPAM and polyethylene glycol (PEG) copolymer | A | Thermal | NR | 18 | Acellular |
| University of Manchester, UK53 | Self-assembling peptide (FEFEFKFK) | A | Self-assembling | <6 s | 19 | Cellular |
| University of Manchester, UK54,55 | Methyl methacrylate (MMA), methacrylic acid (MAA), ethyleneglycol dimethacrylate (EGDMA) and 1,4-butanediol diacrylate BDDA co-monomers | A | pH | 5 min | NR | Acellular |
| Navy General Hospital, China56 | RADA16-I functionalised with bone morphogenetic protein-7 (BMP-7) | A | Self-assembling | NR | NR | Cellular |
| University of Leeds, UK57,58 | Self-assembling peptide with chondroitin sulfate | A | Self-assembling | Seconds | 25 | Acellular |
| University of Quebec, Canada59 | Chitosan hydrogel with β-glycerophosphate, sodium hydrogen carbonate, or phosphate buffer | A | Thermal | <15 s | 25 | Cellular and acellular hydrogels investigated |
| Donghau University, China60,61 | Oxidised dextran, amino-modified gelatin and polyethylene glycol (PEG) | C | UV | <1 min | 19 | Cellular |
| Tehran University of Medical Sciences, Iran62 | Chitosan, β-glycerophosphate, chondroitin sulfate, collagen, gelatin, fibroin silk hydrogel | A | Thermal | 30 min | NR | Acellular |
| Indian Institute of Technology, India63 | Silk fibroin composite | A | Thermal | <20 min | NR | Acellular |
| Rowan University, USA64 | Poly(N-isopropylacrylamide)-graft-chondroitin sulfate with calcium crosslinked alginate microparticles | A | Thermal | <5 min | NR | Acellular |
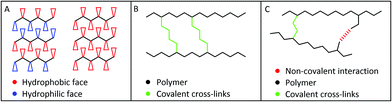
The different materials used for the hydrogels results in different classes of interactions that allow the hydrogel to form within the IVD. The chemical structure of the materials determines these interactions and the gelation trigger. The gelation trigger can be a change in the environment such as pH, temperature or light which causes a change in the non-covalent interactions between molecules (Fig. 3A), a reaction or interaction with another molecule that causes covalent bonds to be formed (Fig. 3B) or a combination of the two (Fig. 3C).31–34
A large proportion of the hydrogels discussed in this article undergo gelation via non-covalent interactions (Fig. 3A). The interactions are determined by the structure of the molecules but can include hydrogen bonding, hydrophobic interactions, π–π stacking and electrostatic interactions. Whilst these interactions are relatively weak and subject to environmental conditions such as pH, temperature and the solvent used, the number of interactions is often large and therefore determines the properties of the hydrogel.65,66 The dependence on environmental conditions allows the use of gelation triggers such as temperature and pH. Whilst the non-covalent interactions arise from the chemical structure itself, modifying the structure allows the number and in turn strength of the non-covalent interactions to be altered to adapt the mechanical and chemical properties of the hydrogel as required.
The hydrogels that undergo gelation via covalent interactions (Fig. 3B) form much stronger and irreversible bonds compared to non-covalent interactions.67 These require a chemical reaction between two molecules, usually a polymer and a cross-linker, whilst avoiding any cross-reaction with native biomolecules in the disc. The cross-linker may spontaneously form covalent bonds or an external input may be required such as UV light when using a photoinitiator as the cross-linking agent.33,34 The stronger bond formation may provide the hydrogel with stronger mechanical properties and enhance its durability and lifetime.
Alternatively, the hydrogels may utilise a combination of both covalent and non-covalent interactions for gelation. This may provide a method of increasing the strength of the hydrogel through the use of covalent interactions whilst maintaining adaptability through the non-covalent interactions.
Clinical delivery discussion
Five hydrogels have been injected using a 20G or smaller diameter needle (20G–26G), as shown in Table 1, which would therefore minimise the damage caused to the disc during the injection. However, this does not mean that the other hydrogels cannot be injected through narrow gauge needles, only that they have been investigated with wider gauge needles thus far. For example, the EPFL hydrogel uses UV light to trigger gelation. In this study a 19G needle was used but the authors stated that with updated UV technology a needle as small as a 25G or a 26G would be possible.34 The extrusion testing showed that the hydrogel requires much less pressure to extrude through a 0.5 mm hole compared to the native nucleus pulposus tissue. The current 19G needle is therefore large enough to cause annulus damage and create a hole that permits extrusion of the hydrogel which makes this treatment currently unsuitable.44 The use of a 25G needle, with upgraded UV technology, would reduce the level of annulus damage and may reduce the propensity for hydrogel extrusion.The hydrogel developed at Sunnybrook Research Institute was investigated using two different injection methods. A modified kyphoplasty technique was investigated where the hydrogel is inserted into a cavity within the IVD, created by the inflation of a balloon, through an 11G needle.48 Additionally, a direct injection through an 18G needle was also used. This shows that modification of the injection method can reduce the size of the needle required, however an 18G needle is still likely to cause damage to the annulus.
Biological considerations
The aim of biocompatibility testing is to determine the body's response to medical device implants, to ensure that the implants are non-toxic and will not be rejected by the immune system. Toxicity may be caused by the material itself, its degradation products or contaminants incorporated during the synthesis. The stages of biocompatibility testing aim to minimise the risk to animals and humans, firstly testing in vitro cytotoxicity, followed by in vivo biocompatibility prior to first-in-human safety trials.10,37Prior to in vitro/in vivo testing, all materials must be rendered sterile to prevent the risk of microbial contamination of in vitro cultures or in vivo infection in animals and patients. A range of sterilisation processes have been adopted for nucleus augmentation devices and the choice of sterilant depends on the material used as discussed by Schmitz et al.10 The materials covered in this review use sterilisation protocols involving UV light,53 filter sterilisation34,49,56 and gamma irradiation.57
Cytotoxicity testing (in vitro)
Most hydrogels are investigated using contact, indirect contact and/or extract cytotoxicity assays. These provide different methods of interaction between the cells and the hydrogel. Contact and indirect contact assays are used to monitor cell growth surrounding the hydrogel. Extract assays assess the effect of smaller, more soluble components of the hydrogel. The assays can be evaluated using different methods such as quantitative assays or qualitative imaging. A successful result will show cell growth uninhibited by the material and similar to a known non-cytotoxic material.68 Additionally, for quantitative methods, the viable cell count should not reduce by more than 30% which is classified as being cytotoxic according to ISO 10993-5.69Biocompatibility testing (in vivo)
In vivo biocompatibility testing provides an opportunity for long-term investigations within an appropriate biological environment.70 Initial biocompatibility testing involves the injection of or implantation of the hydrogel within a live animal for a set period of time as discussed by Schmitz et al.10 The animal is then sacrificed, and the effect of the treatment is observed. This includes histology to observe any inflammatory response such as the presence of lymphocytes or fibrous scar tissue. A positive outcome will show minimal inflammation, no production of fibrous scar tissue and the presence of type 2 macrophages migrating into the gel. Initial biological safety testing is usually conducted in a generic small animal model, such as subcutaneous implantation in mice. Following this, implantation into a large animal model is performed to assess safety at the relevant anatomical site. Additional testing can be conducted as the material progresses through the testing timeline.37,70Additional considerations for cell-seeded hydrogels
Some hydrogels have been developed with the additional purpose of acting as a cell scaffold. Here, the aim is to use the hydrogel to implant viable cells which can effectively maintain and regenerate the tissue over time. It is expected that implanted mesenchymal stem cells (MSCs) will differentiate into nucleus pulposus cells which produce and turn over extracellular matrix. However, the challenge of controlling MSC differentiation can complicate the treatment.71 The differentiation of the MSCs into nucleus pulposus cells is dependent on the correct mechanical loading and environment. If the hydrogel leaks out of the disc then the MSCs will be in a different environment, and in some cases this has been shown to cause unwanted bone formation.72Additionally, acellular scaffolds are class III medical devices whereas treatments that utilise cells are classified as advanced therapy medicinal products (ATMPs).73,74 This has the implication that the ATMP testing is more in depth to ensure the safety of the treatment and may extend the time and cost needed to progress the treatment to market. Biocompatibility testing, in addition to demonstrating the safety of the hydrogel, should evaluate the safety of encapsulated cells. Cell phenotype, migration, viability and immune compatibility should be considered as outlined in more detail by Schmitz et al.10
Table 2 summaries the cytotoxicity and biocompatibility testing that has been published for the nucleus augmentation hydrogels.
| Author | Method | Cells/marker | Significant difference to control | Results |
|---|---|---|---|---|
| a Papers detailing methods and results of biocompatibility testing are not accessible, so they are not discussed further. | ||||
| Sheffield Hallam, UK32 | Immunohistochemistry on bovine nucleus pulposus tissue explants in vitro | Caspase 3 as apoptosis marker | N | Low levels of apoptosis observed in media injected control. No significant difference from control with acellular hydrogel, MSC injection or hydrogel with MSCs across all time points for 6 weeks. |
| EPFL, Switzerland34 | No biocompatibility testing | N/A | N/A | N/A |
| Prince of Wales Hospital, Australia46 | No biocompatibility testing | N/A | N/A | N/A |
| University of Pennsylvania, USA33,36,47,75 | Qualitative cytocompatibility | Nucleus pulposus bovine cells with DAPI staining | N | Live-dead staining showed that the majority of cells remained viable after 2 weeks of culture. DAPI-stained sections showed that cells adhered to the hydrogel surface but did not infiltrate. |
| Extract cytotoxicity assay | Mouse dermal fibroblast (III8C) with MTS assay | N | The DCT sample showed cell proliferation over 28 days at a similar magnitude to controls. Hydrogels without teleostean resulted in reduced cellular activity compared to the controls. | |
| MSC survival and differentiation determined by DNA content | Bovine MSCs in media with or without TGF-β3 | N | DNA content in hydrogels with or without TGF-β3 was not significantly different to control after 14 days. At 42 days there was no significant difference without TGF-β3 but with TGF-β3 caused a significant increase in DNA content compared to the control and the sample without TGF-β3. | |
| In vivo subcutaneous implant | Mouse model | N/A | Fibrous tissue formation around the hydrogel with cell infiltration into the hydrogel. | |
| City College of New York, USA31 | Contact cytotoxicity | Human dermal fibroblasts | N | DNA content of the CMC control sample significantly reduced over 6 days. The CMC-MC hydrogel showed no significant difference to the control. |
| In vivo subcutaneous implant | Rat model | Y | Hydrogel samples containing the crosslinking reagent showed formation of a fibrous capsule with macrophages present within the fibrous capsule. Hydrogel samples without the crosslinker showed no fibrous capsule formation but did not have suitable mechanical properties. | |
| Sunnybrook Research Institute, Canada49 | Cell scaffold evaluation with live/dead imaging | Human IVD cells | Y | Modified hyaluronan was used as a cell scaffold with or without the elastin-like peptide. Imaging was conducted at 1 and 3 weeks of culture. There was a significant decrease in the number of viable cells from week 1 to week 3 for both scaffolds. |
Duke University, USAa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
Biocompatibility and toxicology testing followed ISO 10993 | Acute tests include cytotoxicity, sensitisation (Guinea pig), intracutaneous reactivity (rabbit), systemic toxicity (mouse), pyrogenicity, muscle implant evaluation and genotoxicity. Chronic toxicity testing was conducted with a subcutaneous rat model. Neurofunctional testing conducted in a rat model. | N | The material was shown to be non-cytotoxic, non-irritating and non-toxic in all tests. The chronic toxicity testing was assessed at time points to 1 year and then beyond and showed no toxicity. Neurofunctional testing showed no neurotoxicity. |
| University of Waterloo, Canada51,52 | No biocompatibility testing | N/A | N/A | N/A |
| University of Manchester SAP, UK53 | Cell viability | Bovine nucleus pulposus cells used with Live/Dead assay on days 1, 3, 7 and 14 after cell encapsulation into hydrogel | N (concentration dependent) | Different peptide concentrations showed significantly different percentages of viable cells compared to the alginate control over 7 days. 30 mg ml−1 was the least cytotoxic and showed no significant difference at day 3, 7 or 14. A concentration of 25 mg ml−1 showed a cell viability of 68.2% which is cytotoxic according to ISO 10993. |
| Total viable cell numbers | Bovine nucleus pulposus cells using Cytotox 96 assay on days 1, 3, 7 and 14 | N (concentration dependent) | There was no significant difference for the 30 mg ml−1 hydrogel in cell number from days 1 to 7. There was no significant difference when compared to the alginate control at all time points. | |
| University of Manchester, UK54,55 | Cytotoxicity | MTT assay at 2 and 5 days using human nucleus pulposus cells | N | A hydrogel prepared using a different method resulted in a decrease in percentage cell viability to 79.2% after 5 days. The use of different hydrogel preparation methods means the results aren't applicable to all of their hydrogels. |
| Cell viability | Live/Dead assay at 1, 4 and 7 days using human nucleus pulposus cells | N | Live/Dead staining showed no evidence of cell death with no change in cell morphology. | |
| Navy General Hospital, China56,76 | Cytotoxicity | Human degenerated nucleus pulposus cells cultured in hydrogel scaffolds were imaged at 1, 4 and 7 days to identify live and dead cells | N | All hydrogel scaffolds had greater than 90% cell survival at all time points. |
| 3D cell migration assessment | Human degenerated nucleus pulposus cells seeded on top of scaffolds. At 1, 4 and 7 days a fluorescence label was used to determine the extent of migration. | N/A | All scaffolds resulted in cell migration. RAD-SNV and RAD-KPS scaffolds resulted in a greater number of cell clusters and a longer distance of migration compared to RADA16-I and RAD-KAI. | |
| Cell proliferation | MTT assay after 7 days using rabbit bone marrow mesenchymal stem cells investigating RAD-KPS. | N | RADA-KPS concentrations of 0.1%, 0.05% and 0.025% increased the proliferation of BMSCs. | |
| Cell proliferation | CCK-8 assay. Human degenerated nucleus pulposus cells cultured in hydrogel scaffolds with or without BMP7. Number of cells counted out 1, 3, 5 and 7 days. | Y | Proliferation rates of the cells cultured in the hydrogel scaffolds were significantly higher than the cells cultured in the positive and negative control groups. | |
| In vivo subcutaneous mouse model | RAD-KPS hydrogel was used. Mice sacrificed at 3, 14 and 28 days after injection and analysed with H&E staining. | N/A | At 3 and 14 days an inflammatory response was observed with infiltration of inflammatory cells and degradation of the marginal area of the scaffold. At 28 days the number of macrophages was reduced, and the number of fibroblasts had increased. The scaffold was partially degraded and was being replaced with connective tissue. | |
| University of Leeds, UK57 | Contact cytotoxicity | L929 cells grown in contact with peptide hydrogel samples. | N | No evidence of contact cytotoxicity, all cells grew up to the hydrogel samples. |
| Cell proliferation | MTT assay after 14 days of cell culture in the peptide hydrogels. | N (formulation dependent) | P11-8 hydrogels were shown to support cell growth compared to a collagen control gel over 14 days. P11-12, P11-16 and P11-18 did not appear to support cell growth. | |
| University of Quebec, Canada59,77 | Cell viability | Bovine nucleus pulposus cells mixed with hydrogel solution to give encapsulated cells and LIVE/DEAD assay performed after 14 days of culture. | Y | After 14 days the hydrogel with β-glycerophosphate resulted in only 16% cell viability, hydrogels with sodium hydrogen carbonate and low concentrations of phosphate buffer resulted in the highest cell viability (>80%). Increasing the phosphate buffer lowered the cell viability. |
| Cell metabolic activity | Encapsulated bovine nucleus pulposus cells measured using Alamar Blue assay after 3, 7 and 14 days of culture. | Y | Hydrogel with β-glycerophosphate had a cytotoxic effect resulting in no metabolic activity. The hydrogels with sodium hydrogen carbonate and phosphate buffer at all concentrations showed no loss in metabolic activity over the 14 days. | |
| Cell metabolic activity | L929 cells were encapsulated in the hydrogels and evaluated by Alamar Blue assay at 1, 3 and 7 days. | Y | Hydrogels with β-glycerophosphate showed a reduced metabolic activity at all time points. The addition of sodium hydrogen carbonate improved metabolic activity. Hydrogels with sodium hydrogen carbonate and phosphate buffer without β-glycerophosphate showed the highest cell metabolic activity at 3 and 7 days. | |
| Donghau University, China60,61 | Cell viability | Porcine nucleus pulposus cells were encapsulated in the hydrogel. A LIVE/DEAD assay was conducted after 14 days of culture. | Y | The hydrogel containing all three components showed high cell viability after 14 days, the two hydrogels containing only dextran/gelatin or just PEG showed a reduced cell viability. The PEG hydrogel had a higher number of dead cells where as the dextran/gelatin hydrogel degraded causing a large number of cells to flow out. |
| Cell proliferation | MTT assay conducted on hydrogels with encapsulated cells after 4 and 8 days. | N (formulation dependent) | The optimised hydrogel containing all three components showed the highest cell metabolic activity after 8 days and there was also an increase seen from day 4 to day 8. The dextran/gelatin hydrogel had the highest activity at day 4 but dropped to the lowest value at day 8. The PEG only hydrogel cell activity remained low at both timepoints. | |
| In vivo subcutaneous rat model | Hydrogel samples were transferred into the subcutaneous pocket. The rats were sacrificed after 8 weeks and analysed with H & E staining. | N/A | There was no acute inflammatory response present after 1 week for any of the gels. However all gels exhibited a chronic inflammatory response after 8 weeks with the presence of macrophages or lymphocytes. Additionally, there was evidence of a fibrotic collagen surrounding the hydrogels but it was severe for the PEG hydrogel. | |
| Tehran University, Iran62 | Cell Proliferation | MTT assay conducted on rabbit nucleus pulposus cells after culture with the hydrogel at day 0, 3, 14 and 21. | N | The cell viability with the hydrogel was not significantly lower than the control when comparing the same timepoints across 21 days. |
| Cell viability | Trypan blue was added to rabbit nucleus pulposus cells grown with the hydrogel at day 0, 3, 14, and 21. | N | The hydrogel cell count was not significantly lower than the control when comparing the same timepoints across the 21 days. | |
| Indian Institute of Technology, India63 | Cell proliferation | Porcine nucleus pulposus cells were seeded on the hydrogels and used for an Alamar blue dye reduction assay at day 1, 3 and 7. | N/A | All hydrogel formulations resulted in an increase in cell proliferation over 7 days. |
| Cell viability | Porcine nucleus pulposus cells were encapsulated in the hydrogels and assessed with a LIVE/DEAD assay after 7 days. | N/A | All hydrogel formulations showed that cells were viable after 7 days. | |
| Rowan University, USA64 | Cell viability | Human adipose derived mesenchymal stem cells were encapsulated in the hydrogels and a LIVE/DEAD assay was conducted at day 14. | N/A | After 14 days the two hydrogel formulations investigated showed high levels of cell viability. |
| Cell metabolic activity | Human adipose derived mesenchymal stem cells were encapsulate in the hydrogels and an Alamar blue cell viability assay was conducted over 14 days. | N/A | Both hydrogel formulations investigated showed significant increases in reagent reduction at day 14 compared to day 0 showing cell proliferation. The cell metabolic activity was higher for the formulation without calcium-crosslinked alginate microparticles compared to the formulation with the microparticles. | |
Cytotoxicity and biocompatibility discussion
Most hydrogels were found to be non-cytotoxic, as quantified by cell viability or proliferation, although different tests, cell types and viability assays were used across the different research groups. Some hydrogels were found to be cytotoxic at particular concentrations or in certain combinations, and while a non-cytotoxic variant was possible for all, this potentially compromised the properties of the material.53,57Some of the cellular hydrogels were investigated to determine the effect of including cells on the extracellular matrix production. An increase in extracellular matrix components such as collagen type II, aggrecan and chondroitin sulfate was seen with some hydrogels32 whereas other hydrogels saw an increase in aggrecan but not collagen type II.36 These increases in extracellular matrix production cannot be wholly attributed to the injected cells as the native nucleus pulposus cells may also have produced some of the extracellular matrix.
Favourable conditions are used as standard in the pre-clinical evaluation of cellular hydrogels which do not reflect the pathophysiological environment of the degenerate disc.32,78,79 Degenerate tissue is more acidic and contains matrix degrading enzymes and pro-inflammatory cytokines that will affect the cell viability and net extracellular matrix production.23,80–82 Additional testing of cell-seeded hydrogels in conditions that better simulate those seen in the disc would aid the understanding of how cellular hydrogels perform in a degenerated disc environment. Tao et al.56 achieved this by using human degenerated nucleus pulposus cells for cytotoxicity testing. Additionally, it can be achieved by decreasing the pH, decreasing the glucose concentration, increasing the osmolarity and including cytokines such as Interleukin 1 and tumour necrosis factor alpha.83,84 When grown under degenerate conditions, MSC proliferation and viability were shown to decrease, and extracellular matrix production was prohibited. However, all four hydrogels that act as cell scaffolds were able to show good results for cell proliferation/survival.32,36,53,56
Some research groups have started in vivo small animal biocompatibility testing with in vivo subcutaneous implants of the hydrogels.31,50,56,75 Two of the four hydrogels that were tested as a subcutaneous implant showed formation of fibrous tissue,31,75 and one also resulted in an inflammatory response.56 The use of a crosslinker with one of the hydrogels was shown to cause the formation of a fibrous capsule.31 The same hydrogel without the crosslinker did not result in a fibrous capsule formation. Some of these hydrogels exhibited positive cytotoxicity results and therefore emphasises the importance of in vivo biocompatibility testing to understand the safety of the hydrogels.
Mechanical considerations
A nucleus augmentation material needs to be injectable as a liquid and then cure in situ to form a gel with the required mechanical properties to restore the disc function. Within the native healthy disc, the fluid content of the nucleus plays a major role in its performance. Some of the hydrogels have been designed to mimic this activity and increase the osmotic pressure of the disc once injected allowing the influx of water into the disc and helping to restore the biomechanics of the healthy disc. To determine if the mechanical properties have been restored, a suitable and physiologically relevant testing protocol is required. Different factors influence the mechanical properties of the IVD and can relate to either the environment that the sample is tested in, such as temperature, the level of hydration and how much tissue is retained as well as factors relating to the mechanical loading.18 While standards exist for testing nucleus devices (e.g. ASTM F2789-1085), these require either the device to be tested in isolation or within a synthetic annulus. Consequently, these tests are not necessarily suitable for nucleus augmentation where hydrogels interact with the natural tissue and there is fluid transfer between them. For this reason, many hydrogels have instead been tested using ex vivo disc specimens. To date, a range of mechanical testing protocols have been employed meaning only limited comparison can be undertaken between studies.The mechanical testing of the hydrogels used as nucleus augmentation materials (Table 1) is summarised in Table 3.
| Author | Sample | Degeneration model | Test | Parameters | Restorationa | Comments |
|---|---|---|---|---|---|---|
| a Measured property in the treated sample restored to the value seen in the native healthy sample with a significant difference to the degenerated sample. | ||||||
| Sheffield Hallam, UK32 | In vitro whole bovine tail IVDs | Collagenase digestion | Cyclic compression | 0.53–0.65 MPa @ 2Hz for 100 seconds | Y | Stiffness and strain values of treated discs restored to control and significantly different to degenerate discs. |
| EPFL, Switzerland34 | In vitro bovine IVDs | None | Confined compression | 10 mm min−1 | N/A | Both samples withstood 3.5 MPa but no degenerate model used. |
| Extrusion pressure | 1 mm min−1 through 0.5–2.0 mm holes | N | Native tissue was always extruded at a higher pressure than the hydrogel. Significant difference between tissue and hydrogel samples for 0.75 and 1.0 mm holes. | |||
| Swelling pressure | Y | No significant difference between nucleus pulposus and hydrogel. | ||||
| Papain digestion | Cyclic compression | 7 days split into 16 hours of 0.15 ± 0.05 MPa at 0.2 Hz followed by 8 hours of 0.0625 ± 0.0125 MPa | Y | Disc height was the only dependent variable. Degenerated state was significantly lower than the healthy state. The treated state was significantly larger than the degenerate and not significantly different to the healthy state. | ||
| Prince of Wales Hospital, Australia46 | In vitro ovine functional spinal units (FSUs) | 18G needle degeneration | Cyclic compression | Axial rotation, lateral bending and flexion/extension applies at 1 Nm s−1 to 5 Nm for 4 cycles in each mode. Axial compression 100–1000 N @ 100 N s−1 for 4 cycles. | N | Degenerate model did not produce significantly different mechanical properties to the control. Disc height significantly different at all stages. |
| University of Pennsylvania, USA33,47 | Human lumbar FSUs | Nucleotomy | Cyclic compression | 0.12–0.96 MPa (1482 ± 224 N) @ 2 Hz for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
N | Implant samples showed similar results to the sham injection. |
| Goat lumbar FSUs | Chondroitinase-ABC digestion | Cyclic compression | −230 N to 115 N @ 0.5 Hz | N | Compressive modulus and neutral zone range of motion (ROM) were not restored after degeneration. Total ROM and neutral zone modulus were restored within significance. | |
| City College of New York, USA31 | In vitro bovine FSUs | Discectomy | Cyclic compression | 0.5 MPa (compression)–0.25 MPa (tension) @ 0.1 Hz for 25 cycles. Followed by ramp compression from 0 to 170 N at 1 N s−1 | Y | Compressive, tensile, neutral zone and slow ramp stiffness all restored. ROM and neutral zone length restored. Sham control did not restore any measures. |
| Sunnybrook Research Institute, Canada48,49 | In vitro Yorkshire Boar lumbar FSUs | Chondroitinase-ABC | Cyclic compression | Axial compression of 400 N at 1 mm s−1. Continuous axial rotation, lateral bending and flexion/extension with a bending moment of 5 Nm at a rate of 2° s−1. Loads applied for 5 cycles. | Y | Direct injection restored axial compression and axial rotation neutral zone stiffness as well as axial rotation ROM. The modified kyphoplasty injection restored axial rotation neutral zone stiffness. There was no significant difference to the degenerate samples for axial compression stiffness. |
| In vivo rabbit lumbar IVDs | N/A | 12 weeks in vivo test | Disc volume and mean signal intensity were determined from MRI scans before surgery and 6 and 12 weeks after surgery. | N | Disc volume and signal intensity both significantly decreased following hydrogel treatment when compared to the non-punctured control. However, this does not necessarily represent the mechanical performance of the hydrogel in vivo. | |
| Duke University, USA50 | In vitro silicone annulus | N/A | Cyclic compression | 100–1000 N @ 3 Hz for 10 million cycles. Axial torsion with a 250 N pre-load and a torque ±2 Nm @ 3 Hz for 10 million cycles. | Y | Material reported to withstand the testing. |
| In vitro cadaveric FSUs | Annulotomy & partial nucleotomy | Extrusion force | Axial compression applied with column in neutral posture and hyperflexion. | Y | No extrusion prior to bony and/or endplate failure. Average failure load was 3555 N (neutral) and 2637 N (hyperflexion). | |
| University of Waterloo, Canada51,52 | In vitro porcine cervical FSUs | None | Cyclic compression | 8000 cycles of flexion/extension, injection of hydrogel or sham then 8000 cycles of flexion/extension. | N | There is no degenerate control, the only degeneration is caused by insertion of the needle for injection. The disc stiffness of the sham injection returns to similar values to those seen pre-sham injection. The disc is pulled apart using a 5 kg weight which makes the injection protocol harder to translate into clinic. |
| In vitro gel samples | N/A | Unconfined compression | Hydrogel samples after 7, 30, 60 and 90 days in PBS. Compressed at a 100% min−1 strain rate. | N/A | Compressive modulus at 15% strain, 90 days was 141 ± 36 kPa. | |
| In vitro porcine cervical spines | 12G needle puncture and compression to extrude nucleus | Compression and bending moment | 10 cycles of flexion/extension under 1000 N of compression. | Y | Relative displacement was significantly different after nucleotomy and then restored after hydrogel injection. However, 10 cycles is not enough to show the ability of the hydrogel to restore biomechanics. | |
| University of Manchester SAP, UK53 | Hydrogel at different concentrations | N/A | Rheology at 37 °C | Frequency sweep at 1% strain | N/A | There was no significant difference between cellular and acellular hydrogels. All concentrations showed significant reductions in G′ over 14 days. G* was not significantly different to G* for a non-degenerate nucleus pulposus. |
| Recovery cycle experiment to mimic injection process. 160% strain which was then reduced to 1%. | Y | G′ was recovered within approximately 3 minutes. | ||||
| University of Manchester, UK55 | In vitro bovine IVDs | Collagenase digestion | Non-cyclic compression | A stress of 1 MPa was applied and the strain was measured. | Y | One hydrogel sample was significantly different to the sample injected with PBS and not significantly different to the healthy control. |
| Navy General Hospital, China56 | Hydrogel with different functional BMP-7 groups | N/A | Rheology at 37 °C | Frequency sweep from 0.1 rad s−1 to 100 rad s−1 at a constant shear stress of 1 Pa | N/A | Functionalisation with BMP-7 groups slightly lowered the G′ and G′′ compared to RADA16-I. All functionalised peptides had similar moduli. |
| University of Leeds, UK58 | In vitro bovine IVDs | Nucleotomy | Non-cyclic compression | Axial compression from 0–9 kN at a load rate of 1 mm min−1. | Y | The degenerate sample is significantly different to the healthy control. Treatment with some peptide and chondroitin sulfate ratios is able to restore the normalised stiffness so that it is significantly different to the degenerate sample but not significantly different to the healthy sample. |
| University of Quebec, Canada59 | Hydrogel formulations | N/A | Rheology at room temperature and 37 °C | Storage and loss moduli measured within the linear viscoelastic region at 5% strain and a frequency of 1 Hz. | N/A | All hydrogels measured at 37 °C resulted in storage moduli between 1000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa and loss moduli between 10 and 1000. The only change at 25 °C was a decrease in the rate at which the storage moduli increased. 000 Pa and loss moduli between 10 and 1000. The only change at 25 °C was a decrease in the rate at which the storage moduli increased. |
| Hydrogel formulations and human lumbar nucleus pulposus tissue (Thompson grade 3) | None | Incremental stress relaxation during unconfined compression | Compression applied in increments of 5% strain at 5% s−1 followed by 5 min of relaxation until reaching 25% strain. Samples in PBS during the test. | N/A | Two hydrogel formulations containing sodium hydrogen carbonate and low concentrations of phosphate buffer without β-glycerophosphate had equilibrium moduli that were not significantly different to human nucleus pulposus. | |
| Donghau University, China60 | Hydrogel formulations | N/A | Unconfined compression | All samples were conducted at room temperature, preloaded for 1 min then compressed at a strain rate of 1 mm min−1. | N/A | The hydrogel exhibited higher ductility compared to the PEG hydrogel and much higher stiffness compared to the dextran/gelatin hydrogel. |
| N/A | Cyclic compression | Uniaxial compression. Samples placed in a PBS bath at 37 °C for 3 days. Samples were loaded at 1 Hz between 5% and 20% strain for 8 hours. Heights of hydrogels were measured without load applied before and after loading durations of 7 and 14 days. | N/A | The hydrogel was able to maintain disc height after 14 days. The control hydrogels containing PEG or dextran and gelatin were not able to maintain disc height at 14 days. | ||
| Tehran University, Iran62 | Hydrogel | N/A | Rheology at 37 °C | Storage, loss and complex moduli measured within the linear viscoelastic region at 5% strain at a frequency range from 0.1 to 100 rad s−1. | N/A | The hydrogel exhibits frequency dependent behaviour, behaving more liquid like at frequencies below 20 rad s−1 and more solid like at higher frequencies. |
| Indian Institute of Technology, India63 | Hydrogel Formulations | N/A | Unconfined compression | Compressive strain was applied at 5 mm min−1 up to 80% or until the construct fractured. | N/A | Changing the ratios of the two silk components altered the compressive modulus of the material ranging from 21.85 kPa to 11.37 kPa. |
| N/A | Cyclic compression | Uniaxial compression. Hydrogels were compressed to an axial strain of 15% for 50 cycles at a rate of 5 mm min−1. | N/A | Some ratios showed permanent deformation at higher strain rates above 25%. The two ratios with the lowest compressive moduli did not show any deformation up to 80% applied strain. | ||
| Ex vivo porcine lumbar discs | Collagenase and protease digestion | Cyclic compression | Uniaxial compression. 5 cycles at 2% strain at a rate of 1 mm min−1. | Y | The degenerated disc was treated with PBS and showed a reduced compressive stress after 5 cycles. This was restored to native healthy levels when the hydrogel was injected. | |
| Rowan University, USA64 | Hydrogel formulations | N/A | Unconfined compression | Hydrogels were compressed at a rate of 1 mm min−1 and compressive moduli measured at 25% strain. | N/A | Hydrogel formulations with the calcium crosslinked alginate microparticles had a higher compressive modulus than hydrogel formulations without. Increasing the microparticle concentration increased the compressive modulus. |
| N/A | Confined compression | Hydrogels were compressed within a silicone surrogate annulus fibrosus at a rate of 1 mm min−1 and compressive moduli were measured at 25% strain. | N/A | Hydrogel formulations with the microparticles had a higher compressive modulus and increasing the microparticle concentration increased the compressive modulus. | ||
| In vitro porcine IVDs | Nucleotomy and mechanical degeneration | Cyclic compression | Uniaxial compression. 10 cycles of 1000 N (compression)–100 N (tension) at a rate of 0.1 Hz. | Y | Degenerated samples had a significantly different neutral zone stiffness and compressive stiffness but not a significantly different range of motion. The treated samples did not have a significantly different neutral zone stiffness, compressive stiffness or range of motion compared to the native control. | |
Mechanical discussion
Table 3 illustrates the wide range of mechanical testing protocols that have been adopted, including differences in the loads and how they are applied as well as the methods employed to represent disc degeneration. Cadaveric samples are less readily available than animal tissue, and generally have higher levels of specimen-to-specimen variation. Bovine IVDs have been established as a suitable alternative that mimics the physiology of the human IVD and therefore, have been used by a number of authors.86 Caprine and ovine IVDs are also commonly used.46,47Whilst cyclic compression is a common method to determine the mechanical properties of the IVD before and after treatment, the parameters used vary between research groups. A comparison of the parameters for cyclic compression testing shows that many research groups apply physiological loads, usually less than 1 MPa, between 1–2 Hz (Table 3). The variation is mainly seen within the number of cycles that the samples are subject to. Many of the mechanical tests use a small number of cycles which only allows for the immediate restorative effect of the treatment to be investigated, for which the hydration of the specimen needs to be well controlled because specimens can differ in their initial behaviour until a fluid equilibrium is achieved. When studies use thousands of cycles, the change in mechanical properties over time is evident.51 Therefore, it is important to be cautious when reviewing mechanical testing results of nucleus augmentation materials that are only tested using a small number of cycles, particularly where tissue hydration has not been controlled. On the other hand, it is important to recognise that early stage testing of nucleus augmentation materials is not designed to test a treatment for the extent of the clinical lifetime but instead to help further the understanding of the mechanical properties of the treatment and its ability to restore the disc's functional performance.
Another important factor to consider when evaluating the ability of nucleus augmentation to restore the discs mechanical properties is the model of degeneration that is used. There are different methods that aim to replicate the degeneration seen in the native disc. One method is by the insertion of a large diameter needle which, as shown in the previous section, can cause the tissue to mechanically degrade.46 Alternatively, some research groups use enzymatic degradation which involves injection of an enzyme that breaks down the extracellular matrix of the disc. This a more controlled measure as the quantity of enzyme and the length of time it is used for will determine the extent of the degradation. Alternatively, some research groups use discectomy or nucleotomy which involves manually removing the tissue. This provides a degenerated disc but also requires a more invasive surgery and repair prior to the simulated treatment, lending itself more to the assessment of nucleus replacement than nucleus augmentation.
The successful mechanical testing protocols can test the hydrogel within the IVD over a number of cycles with physiological loading and compare the measured property to the degenerate and/or healthy control. Some hydrogels are at the beginning of the mechanical testing process and only have rheology data published. Rheological profiles of hydrogels can provide valuable insight into how they will perform in vitro.
Similar to the biological testing, some hydrogels showed different mechanical properties when a concentration or component was changed as part of the investigation. This emphasises that any hydrogel that has not shown restoration of the mechanical properties may be able to be altered in order to improve the mechanical properties.
A comparison of cellular and acellular hydrogels shows that both are able to restore the mechanical properties to the disc.32 This suggests that cells may not influence the initial mechanical properties, none-the-less they may maintain such properties over time in vivo through tissue regeneration. In which case, the degradation rate of the hydrogel should be matched to the production rate of new extracellular matrix by cells, to ensure that mechanical properties remain constant throughout the lifetime of the implant. Indeed, some hydrogels use extracellular matrix materials such as glycosaminoglycans as a component of the hydrogel to aid restoration of the biochemical properties.58
Future perspectives
The standardisation of cytotoxicity and biocompatibility testing is shown in the similar test lengths for the hydrogels. However, the mechanical testing highlighted the differences in testing protocols between research groups, potentially as a result of a lack of standardisation. A comparison of cyclic compression protocols and results show that a larger number of cycles increases the applicability and understanding of how the hydrogel will perform over time, however a lot of the reported testing to date has utilised a smaller number of cycles which only provides results on the immediate restorative properties of the hydrogel. Rheological measurements provide information on the gel stiffness and offer the potential for testing that utilises a high number of cycles to assess the longer term gel stability. Rheological measurements could be utilised to test the hydrogels under more extreme conditions such as a lower pH to assess the durability and possible failure under harsh conditions. Additionally, the duration or disassembly of the hydrogel can be assessed using rheology to examine the longevity of the hydrogels. However, in vitro tests are necessary to evaluate the risk of gel expulsion or diffusion over time as well as the restorative effect of the gel.Recommendations for future mechanical testing, including both longer term cyclic loading regimes and those designed to assess the risk of expulsion, were recently proposed by Dixon et al.1 and provide a potential framework for future evaluation. The length of testing is likely to depend on the failure mechanisms being investigated. Hydrogels injected via large diameter needles may be more likely to be expelled from the disc whereas hydrogels that are in equilibrium with a disassembled state may gradually diffuse through the disc.
The majority of the hydrogels reviewed were biocompatible. Where different concentrations or different hydrogel components were used, different levels of cytotoxicity were seen with some being non-cytotoxic. Some hydrogels showed signs of cytotoxicity with fibrous tissue formation or inflammatory responses and further testing would be needed to identify the likely cause.
Conclusion
There are different methods in development for the treatment of degenerated IVDs. In this article, the hydrogels were reviewed and critiqued against the criteria that were set out for a successful nucleus augmentation material. It was found that some hydrogels have been injected using needles with a diameter sufficiently small to limit annulus fibrosus damage. The use of a small gauge needle is partly dependent on the gelation trigger. Hydrogels that have an internal trigger (self-assembling, thermal and pH triggers) are less invasive compared to those that require an external input (UV trigger).The gel stiffness should be similar to the healthy nucleus. Hydrogels that are too stiff result in an increased risk of adversely affecting the load transfer. These stiffer materials are more similar to total nucleus or total disc replacement materials. Softer hydrogels are more likely to disperse and leach through the annulus over time, therefore, failing to adequately restore the mechanical properties. Multiple hydrogels were able to restore the mechanical properties of degenerated IVDs in vitro. However, making comparisons between the hydrogels and the mechanical testing is difficult due to the differences in methods such as the applied load and the number of cycles. From the current published studies, with different hydrogels being at different stages of testing, there is not sufficient evidence that any of the hydrogels are more superior than others in terms of their mechanical performance.
The nucleus augmentation materials discussed in this review show the variety of technologies and approaches that can be applied to augmentation of a degenerated disc. Future testing will further the understanding of the hydrogels and their potential to restore biomechanics to a degenerated disc. The testing conducted to date has shown that there are a range of promising candidate materials for nucleus augmentation, offering an exciting new approach to effectively treating back pain in the future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support of the UK Engineering and Physical Sciences Research Council through grants EP/L014823/1, EP/R513258/1 and EP/P001076/1 and the National Institute for Health Research (NIHR) Leeds Biomedical Research Centre (BRC).References
- A. R. Dixon, J. P. Warren, M. P. Culbert, M. Mengoni and R. K. Wilcox, Review of in vitro mechanical testing for intervertebral disc injectable biomaterials, J. Mech. Behav. Biomed. Mater., 2021, 123, 104703 CrossRef CAS PubMed.
- E. Saker and R. S. Tubbs, Anatomy of the Lumbar Intervertebral Discs, in Lumbar Spine Textbook, [Online], ed. S. D. Boden, Data Trace Publishing Company, 2021. Available from: https://www.wheelessonline.com/issls/lumbar-intervertebral-discs-anatomy/ Search PubMed.
- P. M. Kent and J. L. Keating, The epidemiology of low back pain in primary care, Chiropr. Osteopat., 2005, 13, 13–13 CrossRef PubMed.
- N. Maniadakis and A. Gray, The economic burden of back pain in the UK, Pain, 2000, 84(1), 95–103 CrossRef PubMed.
- C. J. Phillips, Economic burden of chronic pain, Expert. Rev. Pharmacoecon. Outcomes Res., 2006, 6(5), 591–601 CrossRef PubMed.
- K. Malik and A. Nelson, Chapter 24 – Overview of Low Back Pain Disorders. in Essentials of Pain Medicine, ed. H. T. Benzon, et al., Elsevier, 4th edn, 2018, pp. 193–206 Search PubMed.
- NHS, Lumbar Decompression Surgery – How it's performed, [Online], 2020. [Accessed 27 January]. Available from: https://www.nhs.uk/conditions/lumbar-decompression-surgery/what-happens/ Search PubMed.
- J. I. Brox, O. P. Nygaard, I. Holm, A. Keller, T. Ingebrigtsen and O. Reikeras, Four-year follow-up of surgical versus non-surgical therapy for chronic low back pain, Ann. Rheum. Dis., 2010, 69(9), 1643–1648 CrossRef PubMed.
- C. K. Lee and N. A. Langrana, A review of spinal fusion for degenerative disc disease: need for alternative treatment approach of disc arthroplasty?, Spine J., 2004, 4(6 Suppl.), 173s–176s CrossRef PubMed.
- T. C. Schmitz, E. Salzer, J. F. Crispim, G. T. Fabra, C. LeVisage, A. Pandit, M. Tryfonidou, C. L. Maitre and K. Ito, Characterization of biomaterials intended for use in the nucleus pulposus of degenerated intervertebral discs, Acta Biomater., 2020, 114, 1–15 CrossRef CAS PubMed.
- M. Nikkhoo, J.-L. Wang, M. Abdollahi, Y.-C. Hsu, M. Parnianpour and K. Khalaf, A regenerative approach towards recovering the mechanical properties of degenerated intervertebral discs: Genipin and platelet-rich plasma therapies, Proc. Inst. Mech. Eng., Part H, 2016, 231(2), 127–137 CrossRef PubMed.
- P. P. Raj, Intervertebral Disc: Anatomy-Physiology-Pathophysiology-Treatment, Pain Pract., 2008, 8(1), 18–44 CrossRef PubMed.
- J. P. G. Urban and S. Roberts, Degeneration of the intervertebral disc, Arthritis Res. Ther., 2003, 5(3), 120–130 CrossRef PubMed.
- D. R. Eyre and H. Muir, Quantitative analysis of types I and II collagens in human intervertebral discs at various ages, Biochim. Biophys. Acta, Protein Struct., 1977, 492(1), 29–42 CrossRef CAS.
- B. Johnstone and M. T. Bayliss, The large proteoglycans of the human intervertebral disc. Changes in their biosynthesis and structure with age, topography, and pathology, Spine, 1995, 20(6), 674–684 CrossRef CAS PubMed.
- S. S. Sivan, E. Wachtel and P. Roughley, Structure, function, aging and turnover of aggrecan in the intervertebral disc, Biochim. Biophys. Acta, Gen. Subj., 2014, 1840(10), 3181–3189 CrossRef CAS PubMed.
- G. Lyons, S. M. Eisenstein and M. B. Sweet, Biochemical changes in intervertebral disc degeneration, Biochim. Biophys. Acta, 1981, 673(4), 443–453 CrossRef CAS.
- N. Newell, J. P. Little, A. Christou, M. A. Adams, C. J. Adam and S. D. Masouros, Biomechanics of the human intervertebral disc: A review of testing techniques and results, J. Mech. Behav. Biomed. Mater., 2017, 69, 420–434 CrossRef CAS PubMed.
- J. A. Buckwalter, Aging and degeneration of the human intervertebral disc, Spine, 1995, 20(11), 1307–1314 CrossRef CAS PubMed.
- J. J. Trout, J. A. Buckwalter and K. C. Moore, Ultrastructure of the human intervertebral disc: II. Cells of the nucleus pulposus, Anat. Rec., 1982, 204(4), 307–314 CrossRef CAS PubMed.
- H. E. Gruber and E. N. Hanley Jr., Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls, Spine, 1998, 23(7), 751–757 CrossRef CAS PubMed.
- S. Roberts, S. M. Eisenstein, J. Menage, E. H. Evans and I. K. Ashton, Mechanoreceptors in intervertebral discs. Morphology, distribution, and neuropeptides, Spine, 1995, 20(24), 2645–2651 CrossRef CAS PubMed.
- H. T. J. Gilbert, N. Hodson, P. Baird, S. M. Richardson and J. A. Hoyland, Acidic pH promotes intervertebral disc degeneration: Acid-sensing ion channel -3 as a potential therapeutic target, Sci. Rep., 2016, 6, 37360 CrossRef CAS PubMed.
- K. Ichimura, H. Tsuji, H. Matsui and N. Makiyama, Cell culture of the intervertebral disc of rats: factors influencing culture, proteoglycan, collagen, and deoxyribonucleic acid synthesis, J. Spinal Disord., 1991, 4(4), 428–436 CrossRef CAS PubMed.
- B. Diamant, J. Karlsson and A. Nachemson, Correlation between lactate levels and pH in discs of patients with lumbar rhizopathies, Experientia, 1968, 24(12), 1195–1196 CrossRef CAS PubMed.
- A. Nachemson, Intradiscal measurements of pH in patients with lumbar rhizopathies, Acta Orthop. Scand., 1969, 40(1), 23–42 CrossRef CAS PubMed.
- J. S. Shah, The Lumbar Spine and Back Pain, Pitman Medical, London, 1976 Search PubMed.
- P. M. Klara and C. D. Ray, Artificial nucleus replacement: clinical experience, Spine, 2002, 27(12), 1374–1377 CrossRef PubMed.
- S. D. Boden, D. O. Davis, T. S. Dina, N. J. Patronas and S. W. Wiesel, Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation, J. Bone Jt. Surg., Am. Vol., 1990, 72(3), 403–408 CrossRef CAS.
- D. G. Ju, L. E. Kanim and H. W. Bae, Intervertebral Disc Repair: Current Concepts, Global Spine J., 2020, 10(2 Suppl), 130s–136s CrossRef PubMed.
- D. M. Varma, H. A. Lin, R. G. Long, G. T. Gold, A. C. Hecht, J. C. Iatridis and S. B. Nicoll, Thermoresponsive, redox-polymerized cellulosic hydrogels undergo in situ gelation and restore intervertebral disc biomechanics post discectomy, Eur. Cells Mater., 2018, 35, 300–317 CrossRef CAS PubMed.
- A. A. Thorpe, G. Dougill, L. Vickers, N. D. Reeves, C. Sammon, G. Cooper and C. L. Le Maitre, Thermally triggered hydrogel injection into bovine intervertebral disc tissue explants induces differentiation of mesenchymal stem cells and restores mechanical function, Acta Biomater., 2017, 54, 212–226 CrossRef CAS PubMed.
- B. L. Showalter, D. M. Elliott, W. Chen and N. R. Malhotra, Evaluation of an In Situ Gelable and Injectable Hydrogel Treatment to Preserve Human Disc Mechanical Function Undergoing Physiologic Cyclic Loading Followed by Hydrated Recovery, J. Biomech. Eng., 2015, 137(8), 081008 CrossRef PubMed.
- A. Schmocker, A. Khoushabi, D. A. Frauchiger, B. Gantenbein, C. Schizas, C. Moser, P. E. Bourban and D. P. Pioletti, A photopolymerized composite hydrogel and surgical implanting tool for a nucleus pulposus replacement, Biomaterials, 2016, 88, 110–119 CrossRef CAS PubMed.
- D. E. Miles, Self-assembling peptide/glycosaminoglycan hydrogels for spinal therapies, Thesis, University of Leeds, 2012 Search PubMed.
- L. J. Smith, D. J. Gorth, B. L. Showalter, J. A. Chiaro, E. E. Beattie, D. M. Elliott, R. L. Mauck, W. Chen and N. R. Malhotra, In vitro characterization of a stem-cell-seeded triple-interpenetrating-network hydrogel for functional regeneration of the nucleus pulposus, Tissue Eng., Part A, 2014, 20(13–14), 1841–1849 CrossRef CAS PubMed.
- ISO, ISO 10993-1:2020, ISO, 2020.
- D. M. Elliott, C. S. Yerramalli, J. C. Beckstein, J. I. Boxberger, W. Johannessen and E. J. Vresilovic, The effect of relative needle diameter in puncture and sham injection animal models of degeneration, Spine, 2008, 33(6), 588–596 CrossRef PubMed.
- A. J. Michalek, M. R. Buckley, L. J. Bonassar, I. Cohen and J. C. Iatridis, The effects of needle puncture injury on microscale shear strain in the intervertebral disc annulus fibrosus, Spine J., 2010, 10(12), 1098–1105 CrossRef PubMed.
- J. C. Beckstein, S. Sen, T. P. Schaer, E. J. Vresilovic and D. M. Elliott, Comparison of Animal Discs Used in Disc Research to Human Lumbar Disc: Axial Compression Mechanics and Glycosaminoglycan Content, Spine, 2008, 33(6), E166–E173 CrossRef PubMed.
- D. M. Elliott and J. J. Sarver, Young investigator award winner: validation of the mouse and rat disc as mechanical models of the human lumbar disc, Spine, 2004, 29(7), 713–722 CrossRef PubMed.
- L. A. Monaco, S. J. DeWitte-Orr and D. E. Gregory, A comparison between porcine, ovine, and bovine intervertebral disc anatomy and single lamella annulus fibrosus tensile properties, J. Morphol., 2016, 277(2), 244–251 CrossRef PubMed.
- L. Twomey and J. Taylor, Age changes in lumbar intervertebral discs, Acta Orthop. Scand., 1985, 56(6), 496–499 CrossRef CAS PubMed.
- Merck, Syringe Needle Gauge Chart, [Online], 2020. [Accessed 14 January]. Available from: https://www.sigmaaldrich.com/chemistry/stockroom-reagents/learning-center/technical-library/needle-gauge-chart.html Search PubMed.
- J. C. Iatridis, M. Weidenbaum, L. A. Setton and V. C. Mow, Is the nucleus pulposus a solid or a fluid? Mechanical behaviors of the nucleus pulposus of the human intervertebral disc, Spine, 1996, 21(10), 1174–1184 CrossRef CAS PubMed.
- M. H. Pelletier, C. S. Cohen, P. Ducheyne and W. R. Walsh, Restoring Segmental Biomechanics Through Nucleus Augmentation: An In Vitro Study, Clin. Spine Surg., 2016, 29(10), 461–467 CrossRef PubMed.
- S. E. Gullbrand, T. P. Schaer, P. Agarwal, J. R. Bendigo, G. R. Dodge, W. Chen, D. M. Elliott, R. L. Mauck, N. R. Malhotra and L. J. Smith, Translation of an injectable triple-interpenetrating-network hydrogel for intervertebral disc regeneration in a goat model, Acta Biomater., 2017, 60, 201–209 CrossRef CAS PubMed.
- A. E. Leckie, M. K. Akens, K. A. Woodhouse, A. J. Yee and C. M. Whyne, Evaluation of thiol-modified hyaluronan and elastin-like polypeptide composite augmentation in early-stage disc degeneration: comparing 2 minimally invasive techniques, Spine, 2012, 37(20), E1296–E1303 CrossRef PubMed.
- I. L. Moss, L. Gordon, K. A. Woodhouse, C. M. Whyne and A. J. Yee, A novel thiol-modified hyaluronan and elastin-like polypetide composite material for tissue engineering of the nucleus pulposus of the intervertebral disc, Spine, 2011, 36(13), 1022–1029 CrossRef PubMed.
- L. M. Boyd and A. J. Carter, Injectable biomaterials and vertebral endplate treatment for repair and regeneration of the intervertebral disc, Eur. Spine J., 2006, 15(Suppl. 3), S414–S421 CrossRef PubMed.
- C. Balkovec, J. Vernengo and S. M. McGill, The use of a novel injectable hydrogel nucleus pulposus replacement in restoring the mechanical properties of cyclically fatigued porcine intervertebral discs, J. Biomech. Eng., 2013, 135(6), 61004–61005 CrossRef PubMed.
- J. Vernengo, G. W. Fussell, N. G. Smith and A. M. Lowman, Evaluation of novel injectable hydrogels for nucleus pulposus replacement, J. Biomed. Mater. Res., Part B, 2008, 84(1), 64–69 CrossRef CAS PubMed.
- S. Wan, S. Borland, S. M. Richardson, C. L. R. Merry, A. Saiani and J. E. Gough, Self-assembling peptide hydrogel for intervertebral disc tissue engineering, Acta Biomater., 2016, 46, 29–40 CrossRef CAS PubMed.
- T. J. Freemont and B. R. Saunders, pH-Responsive microgel dispersions for repairing damaged load-bearing soft tissue, Soft Matter, 2008, 4(5), 919–924 RSC.
- A. H. Milani, A. J. Freemont, J. A. Hoyland, D. J. Adlam and B. R. Saunders, Injectable Doubly Cross-Linked Microgels for Improving the Mechanical Properties of Degenerated Intervertebral Discs, Biomacromolecules, 2012, 13(9), 2793–2801 CrossRef CAS PubMed.
- H. Tao, Y. Wu, H. Li, C. Wang, Y. Zhang, C. Li, T. Wen, X. Wang, Q. He, D. Wang and D. Ruan, BMP7-Based Functionalized Self-Assembling Peptides for Nucleus Pulposus Tissue Engineering, ACS Appl. Mater. Interfaces, 2015, 7(31), 17076–17087 CrossRef CAS PubMed.
- S. Maude, D. E. Miles, S. H. Felton, J. Ingram, L. M. Carrick, R. K. Wilcox, E. Ingham and A. Aggeli, De novo designed positively charged tape-forming peptides: self-assembly and gelation in physiological solutions and their evaluation as 3D matrices for cell growth, Soft Matter, 2011, 7(18), 8085–8099 RSC.
- D. E. Miles, E. A. Mitchell, N. Kapur, P. A. Beales and R. K. Wilcox, Peptide:glycosaminoglycan hybrid hydrogels as an injectable intervention for spinal disc degeneration, J. Mater. Chem. B, 2016, 4(19), 3225–3231 RSC.
- Y. Alinejad, A. Adoungotchodo, M. P. Grant, L. M. Epure, J. Antoniou, F. Mwale and S. Lerouge, Injectable Chitosan Hydrogels with Enhanced Mechanical Properties for Nucleus Pulposus Regeneration, Tissue Eng., Part A, 2019, 25(5–6), 303–313 CrossRef CAS PubMed.
- Y. Gan, P. Li, L. Wang, X. Mo, L. Song, Y. Xu, C. Zhao, B. Ouyang, B. Tu, L. Luo, L. Zhu, S. Dong, F. Li and Q. Zhou, An interpenetrating network-strengthened and toughened hydrogel that supports cell-based nucleus pulposus regeneration, Biomaterials, 2017, 136, 12–28 CrossRef CAS PubMed.
- X. Geng, X. Mo, L. Fan, A. Yin and J. Fang, Hierarchically designed injectable hydrogel from oxidized dextran, amino gelatin and 4-arm poly(ethylene glycol)-acrylate for tissue engineering application, J. Mater. Chem., 2012, 22(48), 25130–25139 RSC.
- M. Ghorbani, J. Ai, M. R. Nourani, M. Azami, B. Hashemibeni, S. Asadpour and S. Bordbar, Injectable natural polymer compound for tissue engineering of intervertebral disc: In vitro study, Mater. Sci. Eng., C, 2017, 80, 502–508 CrossRef CAS PubMed.
- B. K. Bhunia and B. B. Mandal, Exploring Gelation and Physicochemical Behavior of in Situ Bioresponsive Silk Hydrogels for Disc Degeneration Therapy, ACS Biomater. Sci. Eng., 2019, 5(2), 870–886 CrossRef CAS PubMed.
- T. Christiani, K. Mys, K. Dyer, J. Kadlowec, C. Iftode and A. J. Vernengo, Using embedded alginate microparticles to tune the properties of in situ forming poly(N-isopropylacrylamide)-graft-chondroitin sulfate bioadhesive hydrogels for replacement and repair of the nucleus pulposus of the intervertebral disc, JOR Spine, 2021, e1161 Search PubMed.
- J. Wang, K. Liu, R. Xing and X. Yan, Peptide self-assembly: thermodynamics and kinetics, Chem. Soc. Rev., 2016, 45(20), 5589–5604 RSC.
- X. Zhao, F. Pan, H. Xu, M. Yaseen, H. Shan, C. A. E. Hauser, S. Zhang and J. R. Lu, Molecular self-assembly and applications of designer peptide amphiphiles, Chem. Soc. Rev., 2010, 39(9), 3480–3498 RSC.
- J. M. Berg, Chemical Bonds in Biochemistry, in Biochemistry, ed. J. L. Tymoczko and L. Stryer, W. H. Freeman, New York, 5th edn, 2002 Search PubMed.
- W. Li, J. Zhou and Y. Xu, Study of the in vitro cytotoxicity testing of medical devices, Biomed. Rep., 2015, 3(5), 617–620 CrossRef CAS PubMed.
- ISO, ISO10993-5:2009, 2009.
- R. Giardino, M. Fini, N. N. Aldini and A. Parrilli, 15 – Testing the in vivo biocompatibility of biocomposites, in Biomedical Composites, ed. L. Ambrosio, Woodhead Publishing, 2nd edn, 2017, pp. 357–374 Search PubMed.
- F. L. Acosta, L. Metz, H. D. Adkisson, J. Liu, E. Carruthers-Liebenberg, C. Milliman, M. Maloney and J. C. Lotz, Porcine Intervertebral Disc Repair Using Allogeneic Juvenile Articular Chondrocytes or Mesenchymal Stem Cells, Tissue Eng., Part A, 2011, 17(23–24), 3045–3055 CrossRef CAS PubMed.
- G. Vadalà, G. Sowa, M. Hubert, L. G. Gilbertson, V. Denaro and J. D. Kang, Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation, J. Tissue Eng. Regener. Med., 2012, 6(5), 348–355 CrossRef PubMed.
- E. Parliament, Regulation (EU) 2017/745, Official Journal of the European Union, 2017 Search PubMed.
- European Parliament, Council of the European Union, Regulation on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004 2007, Off. J. Eur. Union, 2007, 124–125 Search PubMed.
- H. Zhang, A. Qadeer, D. Mynarcik and W. Chen, Delivery of rosiglitazone from an injectable triple interpenetrating network hydrogel composed of naturally derived materials, Biomaterials, 2011, 32(3), 890–898 CrossRef CAS PubMed.
- Y. Wu, Z. Jia, L. Liu, Y. Zhao, H. Li, C. Wang, H. Tao, Y. Tang, Q. He and D. Ruan, Functional Self-Assembled Peptide Nanofibers for Bone Marrow Mesenchymal Stem Cell Encapsulation and Regeneration in Nucleus Pulposus, Artif. Organs, 2016, 40(6), E112–E119 CrossRef CAS PubMed.
- C. Ceccaldi, E. Assaad, E. Hui, M. Buccionyte, A. Adoungotchodo and S. Lerouge, Optimization of Injectable Thermosensitive Scaffolds with Enhanced Mechanical Properties for Cell Therapy, Macromol. Biosci., 2017, 17(6), 1600435 CrossRef PubMed.
- C. Neidlinger-Wilke, F. Galbusera, H. Pratsinis, E. Mavrogonatou, A. Mietsch, D. Kletsas and H.-J. Wilke, Mechanical loading of the intervertebral disc: from the macroscopic to the cellular level, Eur. Spine J., 2014, 23(3), 333–343 CrossRef PubMed.
- J. P. G. Urban, S. Smith and J. C. T. Fairbank, Nutrition of the Intervertebral Disc, Spine, 2004, 29(23), 2700–2709 CrossRef PubMed.
- C. L. Le Maitre, A. Pockert, D. J. Buttle, A. J. Freemont and J. A. Hoyland, Matrix synthesis and degradation in human intervertebral disc degeneration, Biochem. Soc. Trans., 2007, 35(4), 652–655 CrossRef CAS PubMed.
- J. A. Hoyland, C. Le Maitre and A. J. Freemont, Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc, Rheumatology, 2008, 47(6), 809–814 CrossRef CAS PubMed.
- C. L. Le Maitre, J. A. Hoyland and A. J. Freemont, Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1β and TNFα expression profile, Arthritis Res. Ther., 2007, 9(4), R77 CrossRef PubMed.
- C. L. Le Maitre, A. Pockert, D. J. Buttle, A. J. Freemont and J. A. Hoyland, Matrix synthesis and degradation in human intervertebral disc degeneration, Biochem. Soc. Trans., 2007, 35(4), 652–655 CrossRef CAS PubMed.
- K. Wuertz, K. Godburn, C. Neidlinger-Wilke, J. Urban and J. C. Iatridis, Behavior of mesenchymal stem cells in the chemical microenvironment of the intervertebral disc, Spine, 2008, 33(17), 1843–1849 CrossRef PubMed.
- A. Internation, ASTM F2789-10(2020), ASTM, West Conshohocken, PA, 2020 Search PubMed.
- N. Newell, G. Grigoriadis, A. Christou, D. Carpanen and S. D. Masouros, Material properties of bovine intervertebral discs across strain rates, J. Mech. Behav. Biomed. Mater., 2017, 65, 824–830 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |