A periodic table for liquid chromatography separation modes
Analytical Methods Committee, AMCTB No. 111
First published on 2nd August 2022
Abstract
The increasing trend for non-expert users to undertake analytical measurements using an expanding range of chromatographic approaches can lead to the use of unsuitable separation methods and the generation of poor-quality data. Technical Brief AMCTB No. 107 introduced liquid chromatography and guidance on selection of the appropriate separation modality. This publication aims to provide a quick and easy to use educational tool for optimizing the mode of liquid chromatographic separation. It will be of value to both expert and non-expert users across the natural, life and physical sciences.
Many modern-day applications requiring advanced separation techniques involve liquid chromatography (LC) instrumentation given its breadth of operational modes for substances across the polarity spectrum. Efficient and appropriate use of these separation techniques requires a degree of fundamental knowledge and experience to ensure successful separations and data validity. However, recent instrument developments focussing on ease of use as well as performance have led to an increasing trend for non-expert users to undertake this work, making accessible guidance increasingly important. Despite the existence of comprehensive user guides1–6 and online resources7 there remains a clear need to understand when and, importantly, why selected LC modes are used for the chosen sample analyte (and matrix) types. An introduction to method selection for LC formed the basis of a previous Technical Brief (AMCTB No. 107).8 However, whilst this provided users with a discrete selection of techniques as a starting point for method development, it did not indicate the degree of suitability of a separation mode for the different chemical substances that may be the target of the analysis. This current Technical Brief addresses this need by presenting a tool in the form of a periodic table (Fig. 1) for the optimal selection of common approaches in LC, along with relevant abbreviated terms. The table is not an exhaustive list of separation options as these are more widely covered within primary reference documents, such as that compiled by IUPAC.1 Rather, the techniques included are those typically used for modern-day applications, and their implementation will assume compatibility of sample conditions with applied technique.
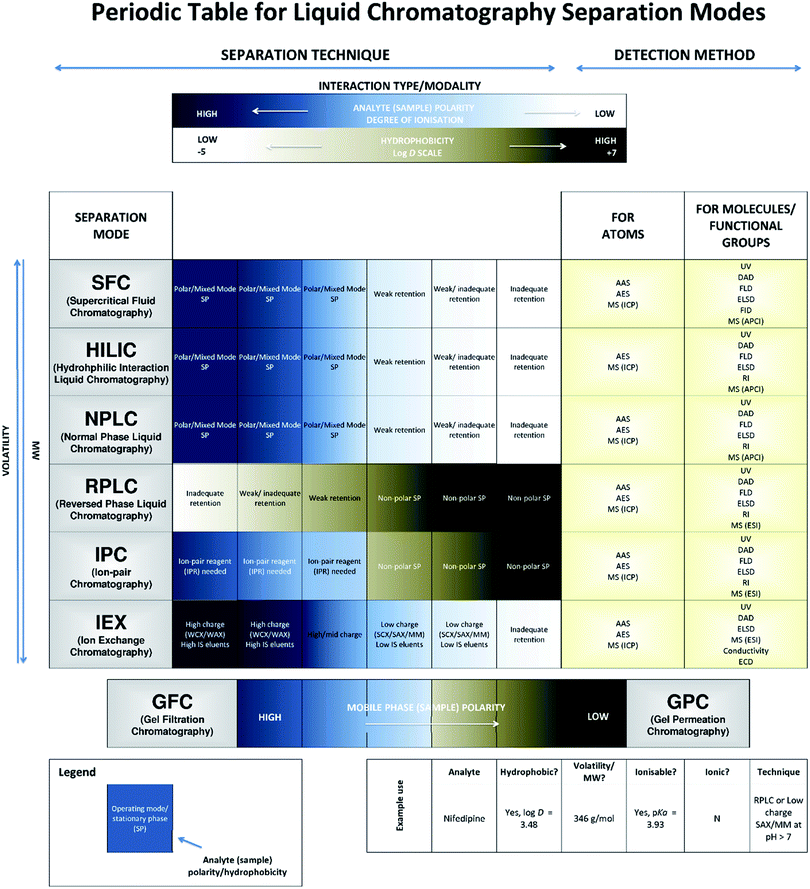 | ||
Fig. 1 A periodic table of liquid (fluid) phase chromatography detailing common separation modes in terms of partitioning (HILIC), adsorption (NPLC, SFC, RPLC, IPC and IEX) and size exclusion (gel permeation chromatography (GPC) and gel filtration chromatography (GFC)). This includes information regarding the suitability of the modality based on the physicochemical characteristics of the compound (i.e., log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D) and potential detectors (see Table 1). D) and potential detectors (see Table 1). | ||
Liquid chromatographic separations
The advances in stationary phases and separation modalities have enabled the separation of a wide range of analytes and chemicals by LC. However, to successfully separate a mixture an appropriate separation mode should be chosen that can enable the target analyte(s) to be discriminated from the remaining sample matrix components. This often requires consideration of the physicochemical characteristic(s) of the analyte, such as hydrophobicity, represented by log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D, and the acid dissociation constant (pKa). These characteristics have been discussed in more detail in the earlier Technical Brief8 and are generally available within repositories of chemical data for users to optimize the separation selectivity during method development. Specifically, log
D, and the acid dissociation constant (pKa). These characteristics have been discussed in more detail in the earlier Technical Brief8 and are generally available within repositories of chemical data for users to optimize the separation selectivity during method development. Specifically, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D indicates the volatility and polarity of analytes that are capable of existing in a neutral and charged state, where polar substances typically have lower log
D indicates the volatility and polarity of analytes that are capable of existing in a neutral and charged state, where polar substances typically have lower log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D values than non-polar hydrocarbon molecules (see Fig. 1). Given that molecular weight can significantly affect the degree of volatility, substances within these categories typically exhibit a range of values, akin to a graduated scale, resulting in a range of chemical characteristics that can be used to facilitate the required selectivity. Similarly, analyses that do not consider the basicity or acidity of the substances via their pKa values can be less effective; by controlling pH in relation to this metric, the type (and degree of success) of separation modalities using charged, ion-exchange interactions, or neutral, hydrophobic interactions, can be specifically targeted.
D values than non-polar hydrocarbon molecules (see Fig. 1). Given that molecular weight can significantly affect the degree of volatility, substances within these categories typically exhibit a range of values, akin to a graduated scale, resulting in a range of chemical characteristics that can be used to facilitate the required selectivity. Similarly, analyses that do not consider the basicity or acidity of the substances via their pKa values can be less effective; by controlling pH in relation to this metric, the type (and degree of success) of separation modalities using charged, ion-exchange interactions, or neutral, hydrophobic interactions, can be specifically targeted.
In addition, to better meet the breadth of the separation range required for many complex matrices, stationary phase chemistries were developed that spanned non-polar hydrophobic interactions (e.g., reversed phase LC, RPLC), polar hydrophilic interactions (e.g., normal phase LC, NPLC) and ionic interactions (e.g., ion-exchange chromatography, IEX). However, these were often limited in their ability to separate on the basis of more subtle differences in analyte chemistries (resulting from the range of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D and pKa values encountered). This has led to the introduction of multi-modal stationary phases (e.g., ion-pair chromatography (IPC) or mixed-mode (MM) IEX), alternative mobile phases (e.g., supercritical fluid chromatography (SFC)) and additional mechanisms of separation (e.g., partition through the use of hydrophilic interaction chromatography (HILIC)) within the chromatographic platforms.
D and pKa values encountered). This has led to the introduction of multi-modal stationary phases (e.g., ion-pair chromatography (IPC) or mixed-mode (MM) IEX), alternative mobile phases (e.g., supercritical fluid chromatography (SFC)) and additional mechanisms of separation (e.g., partition through the use of hydrophilic interaction chromatography (HILIC)) within the chromatographic platforms.
The plethora of techniques has, therefore, often become a source of confusion in selecting the most appropriate modality for an application, particularly when new users of chromatographic technology are attempting method development and where more than one separation option may apply. AMCTB No. 107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) provides a decision tree to help select an appropriate modality. Building on these principles, we have prepared a periodic table for liquid chromatography separation modes (Fig. 1) that provides a guide as to the general applicability of LC modes and stationary phases in a recognisable scientific format, helping users to refine the method options for the range of analyte chemistries detailed above. To help explain the detector terms, a glossary has been included (Table 1). However, users should note that the application of the optional separation mode will be dependent on available laboratory instrumentation and should be considered as part of method selection.
8) provides a decision tree to help select an appropriate modality. Building on these principles, we have prepared a periodic table for liquid chromatography separation modes (Fig. 1) that provides a guide as to the general applicability of LC modes and stationary phases in a recognisable scientific format, helping users to refine the method options for the range of analyte chemistries detailed above. To help explain the detector terms, a glossary has been included (Table 1). However, users should note that the application of the optional separation mode will be dependent on available laboratory instrumentation and should be considered as part of method selection.
| Abbreviation | Term | Description |
|---|---|---|
| a These descriptions have been abbreviated or adapted from the text in the cited reference. | ||
| AAS | Atomic Absorption Spectroscopy | Measures the amount of a chemical element based on the measurement of the absorption of characteristic electromagnetic radiation by atoms in the vapour phasea.9 |
| AES | Atomic Emission Spectroscopy | Measures the amount of a chemical element based on the measurement of the intensity of characteristic electromagnetic radiation emitted by atoms or moleculesa.9 |
| ICP-MS | Inductively Coupled Plasma-Mass Spectrometry | Mass spectrometry technique based on coupling a mass spectrometer with an inductively coupled plasma as an ion source that both atomizes samples into their constituent atoms and ionizes them to form atomic cationsa.10 |
| UV | Ultraviolet Spectroscopy | Molecular absorption spectroscopy in the ultraviolet (UV) and visible (VIS) is concerned with the measured absorption of radiation in its passage through a gas, a liquid or a solida.11 |
| DAD | Diode Array Detector | A UV spectrophotometry detector that uses an arrangement of a number of photodiodes on a single chipa.12 |
| FLD | Fluorescence Detector | Measures the fluorescence of a molecule/sample.13 |
| ELSD | Evaporative Light Scattering Detector | Measures the light scattered by non-volatile molecules/particles following desolvation.14 |
| FID | Flame Ionization Detection | Technique that uses a hydrogen flame to ionize gaseous molecules to measure the resulting change in electrical current.15 |
| APCI-MS | Atmospheric Pressure Chemical Ionization-Mass Spectrometry | Chemical ionization of a sample that is a gas or nebulized liquid, using an atmospheric pressure corona discharge or beta emitter such as 63Nia.10 |
| RI | Refractive Index | Measures the change in the direction of light when passing through media that have a different refractive index based on alternative (bio)chemical compositions.14 |
| ESI-MS | Electrospray Ionization-Mass Spectrometry | Spray ionization process in which either cations or anions in solution are transferred to the gas phase via formation and desolvation at atmospheric pressure of a stream of highly charged droplets that result from applying a potential difference between the tip of the electrospray needle containing the solution and a counter electrodea.10 |
| — | Conductivity Detection | Measures the electrolyte concentration of a solution via its conductivity.14 |
| ECD | Electrochemical Detection | Methods in which either current or potential is measured during an electrochemical reaction. The gas or liquid containing the trace impurity to be analysed is sent through an electrochemical cell containing a liquid or solid electrolyte and in which an electrochemical reaction specific to the impurity takes placea.15 |
Use of the periodic table
Two case studies will illustrate the application of the periodic table to specific analytes. The examples include an explanation of optional separation modes.Case study 1: separation of nifedipine in a liquid extract
This is a small molecule with moderate hydrophobicity and several acidic functional groups (see Fig. 2). | ||
| Fig. 2 Chemical structure of nifedipine with relevant physicochemical properties for the separation. | ||
From the decision tree in AMCTB No. 107,8 the analyte is in a liquid sample and could require LC for separating nifedipine from the remaining matrix components. Using the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D for nifedipine it is clear from the periodic table (Fig. 1), that RP-LC or IPC could be used to successfully retain the drug via the use of a non-polar stationary phase (providing the sample is relatively polar). Furthermore, by using the chemical structure information (e.g., weakly acidic, negatively charged, functional groups), IEX-LC may also be used to retain the drug providing a low ionic strength (IS) eluent is employed to ensure retention where the analyte is expected to elute when an appropriate shift in pH exceeds the drug pKa of 3.93 (see AMCTB No. 107
D for nifedipine it is clear from the periodic table (Fig. 1), that RP-LC or IPC could be used to successfully retain the drug via the use of a non-polar stationary phase (providing the sample is relatively polar). Furthermore, by using the chemical structure information (e.g., weakly acidic, negatively charged, functional groups), IEX-LC may also be used to retain the drug providing a low ionic strength (IS) eluent is employed to ensure retention where the analyte is expected to elute when an appropriate shift in pH exceeds the drug pKa of 3.93 (see AMCTB No. 107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) for further explanation of pKa). Given nifedipine is a small molecule, a possible eight detection options may be explored, each with their own degree of selectivity and sensitivity, and should be chosen according to the needs of the analysis.
8) for further explanation of pKa). Given nifedipine is a small molecule, a possible eight detection options may be explored, each with their own degree of selectivity and sensitivity, and should be chosen according to the needs of the analysis.
Case study 2: separation of captopril in a liquid extract
This is a small molecule with low hydrophobicity and several polar, ionizable functional groups (see Fig. 3).From the decision tree in AMCTB No. 107,8 the analyte is in a liquid sample and could require LC for separating the drug from the remaining matrix components. Given captopril is strongly polar as indicated by the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D, the use of RP would likely result in weak retention (see Fig. 1). Hence, NP-LC, or even a weak IEX sorbent given the ionizable functional groups, may be preferable to retain captopril, however, the latter would require a high to moderate IS eluent via an appropriate counter ion or shift in pH to exceed the drug pKa (see AMCTB No. 107 8 for further explanation of pKa) to ensure the release of captopril from the retention mechanism. Similarly, IPC could be employed using a relevant ion-pair that is first loaded onto the RP stationary phase prior to the sample matrix that contains captopril. As with nifedipine, a possible eight detection options for this small molecule may be explored, each with their own degree of selectivity and sensitivity, and should be chosen according to the needs of the analysis.
D, the use of RP would likely result in weak retention (see Fig. 1). Hence, NP-LC, or even a weak IEX sorbent given the ionizable functional groups, may be preferable to retain captopril, however, the latter would require a high to moderate IS eluent via an appropriate counter ion or shift in pH to exceed the drug pKa (see AMCTB No. 107 8 for further explanation of pKa) to ensure the release of captopril from the retention mechanism. Similarly, IPC could be employed using a relevant ion-pair that is first loaded onto the RP stationary phase prior to the sample matrix that contains captopril. As with nifedipine, a possible eight detection options for this small molecule may be explored, each with their own degree of selectivity and sensitivity, and should be chosen according to the needs of the analysis.
Concluding remarks
The plethora of techniques and modalities within LC provides the capability to establish a more detailed picture of complex mixtures and for analyte(s) to be measured without significant interference. For new users of LC, navigating this breadth of technology can be challenging and can often lead to inappropriate technique choice, and incoherent and unreliable data. Therefore, there remains a need for tools to guide these users in better selecting techniques for successful separations, particularly where several options may be available. The periodic table of liquid chromatography separation modes is intended to help address this need, providing some assistance in understanding the impact of hydrophobicity and ionizability on the separation choice, and introduces relevant terminology as a starting point for method development by non-expert users.Ruth Godfrey 0000-0002-8830-3625 (Swansea University), and Scott Fletcher and Robert Boughtflower (RSC Separation Sciences Group (SSG)).
This Technical Brief was prepared for the Analytical Methods Committee (AMC), with contributions from members of the AMC Instrumental Analysis Expert Working Group and the RSC Separation Sciences Group (SSG) and approved by the AMC on 28 May 2022.
Further reading
- T. A. Maryutina, E. Y. Savonina, P. S. Fedotov, R. M. Smith, H. Siren and D. B. Hibbert, Terminology of separation methods (IUPAC Recommendations 2017), Pure Appl. Chem., 2018, 90(1), 181–231 CrossRef CAS.
- World Health Organization, Good Chromatography Practices. Working Document QAS/19.791/Rev.1, 2019 Search PubMed.
- Guide to Achieving Reliable Quantitative LC-MS Measurements, ed. M. Sargent, RSC Analytical Methods Committee, 2013, ISBN 978-0-948926-27-3 Search PubMed.
- Eurachem, The Fitness for Purpose of Analytical Methods: A Laboratory Guide to Method Validation and Related Topics, 2nd edn, 2014 Search PubMed.
- European Medicines Agency, ICH Guideline M10 on Bioanalytical Method Validation: Draft Version, 2019 Search PubMed.
- Food and Drug Administration, Bioanalytical Method Validation: Guidance for Industry, 2018 Search PubMed.
- https://www.chromacademy.com/, accessed 29/04/2022.
- RSC Analytical Methods Committee, Method selection for liquid chromatography, Anal. Methods, 2021, 13, 3205–3208 RSC.
- H. Goenaga Infante, J. Warren, J. Chalmers, G. Dent, J. L. Todoli, J. Collingwood, N. Telling, M. Resano, A. Limbeck, T. Schoenberger, D. B. Hibbert, A. LeGresley, K. Adams and D. Craston, Glossary of methods and terms used in analytical spectroscopy (IUPAC Recommendations 2019), Pure Appl. Chem., 2021, 93(6), 647–776 CrossRef CAS.
- K. K. Murray, R. K. Boyd, M. N. Eberlin, G. J. Langley, L. Li and Y. Naito, Definitions of terms relating to mass spectrometry (IUPAC Recommendations 2013), Pure Appl. Chem., 2013, 85(7), 1515–1609 CrossRef CAS.
- K. Laqua, W. H. Melhuish and M. Zander, Nomenclature, symbols, units and their usage in spectrochemical analysis-VII. Molecular absorption spectroscopy, ultraviolet and visible (UV/VIS) (IUPAC Recommendations 1988), Pure Appl. Chem., 1988, 60(9), 1449–1460 CrossRef CAS.
- K. Laqua, B. Schrader, G. G. Hoffmann, D. S. Moore and T. Vo-Dinh, Nomenclature, symbols, units and their usage in spectrochemical analysis-XI. Detection of radiation (IUPAC Recommendations 1995), Pure Appl. Chem., 1995, 67(10), 1745–1760 CrossRef CAS.
- W. H. Melhuish, Nomenclature, symbols, units and their usage in spectrochemical analysis-VI: Molecular luminescence spectroscopy (IUPAC Recommendations 1983), Pure Appl. Chem., 1984, 56(2), 231–245 CrossRef.
- S. Moldoveanu and V. David, Essentials in Modern HPLC Separations, Elsevier, 2012 Search PubMed.
- J. G. Calvert, Glossary of atmospheric chemistry terms (Recommendations 1990), Pure Appl. Chem., 1990, 62(11), 2167–2219 CrossRef CAS.
- L. Xu, L. Li, J. Huang, S. Yu, J. Wang and N. Li, Determination of the lipophilicity (log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P o/w) of organic compounds by microemulsion liquid chromatography, J. Pharm. Biomed. Anal., 2015, 102, 409–416 CrossRef CAS PubMed.
P o/w) of organic compounds by microemulsion liquid chromatography, J. Pharm. Biomed. Anal., 2015, 102, 409–416 CrossRef CAS PubMed. - C. Osei-Asare, S. L. Kipo, K. Ofori-Kwakye and M. El Boakye-Gyasi, Comparative in vitro dissolution of commercially available sustained release of nifedipine tablet brands in the Kumasi Metropolis, Ghana, J. Appl. Pharm. Sci., 2015, 5(8), 054–060 CrossRef CAS.
- M. Remko, Acidity, Lipophilicity, Solubility, Absorption, and Polar Surface Area of Some ACE Inhibitors, Chem. Pap., 61(2), 133–141 CAS.
| This journal is © The Royal Society of Chemistry 2022 |



