Open Access Article
Open Access ArticleRapid quantification of cannabidiol from oils by direct analysis in real time mass spectrometry†
Susanne
Huber
 a,
Klemens
Losso
a,
Klemens
Losso
 a,
Günther K.
Bonn
a,
Günther K.
Bonn
 ab and
Matthias
Rainer
ab and
Matthias
Rainer
 *a
*a
aInstitute of Analytical Chemistry and Radiochemistry, Leopold-Franzens University of Innsbruck, Innrain 80–82, A-6020 Innsbruck, Austria. E-mail: M.Rainer@uibk.ac.at
bADSI – Austrian Drug Screening Institute, Innrain 66a, A-6020 Insbruck, Austria
First published on 20th September 2022
Abstract
This work is the first to describe the use of Direct Analysis in Real Time Mass Spectrometry (DART-MS) for the rapid quantification of cannabidiol (CBD) in CBD oils. For this study, self-prepared samples spiked with CBD in hemp seed oil as well as commercial CBD oils from the Austrian market with different CBD contents were analyzed. CBD concentrations were between 5 and 30% (m/m) for the spiked samples as well as between 5 and 15% (m/m) for the real samples. The performance of quantification by means of DART-MS was assessed against a validated liquid chromatography-mass spectrometry (LC-MS) method. The correlation of the quantification results of both methods was high with a correlation factor greater than 0.98 and a maximum bias of 9.8%. Furthermore, the relative standard deviation values of the DART-MS measurments were below the tolerable limit of 12%. These results demonstrate that quantification of CBD by DART-MS is reliable and hence suitable as a rapid and cost-effective alternative method for quality control of CBD content in CBD oils.
1 Introduction
During the cannabidiol (CBD) boom in recent years, sales of CBD-containing consumer products, such as oils and cosmetics, have rapidly increased. One reason for this hype is the multiple positive effects on human health, though CBD has no psychoactive impact like the other major cannabinoid tetrahydrocannabidiol (THC).1The physiological actions of CBD are wide ranging and comprise for example anti-inflammatory, antioxidant, antidepressant, antianxiety, cardiovascular, neuroprotective, antiseizure and antispasticity effects.1–3 Due to these properties, the use of CBD is being researched for the treatment of various diseases in preclinical and clinical studies. These pathologies range from neurological diseases (epilepsy, Alzheimer's disease, Parkinson's disease, Huntington's disease, schizophrenia), autoimmune diseases (multiple sclerosis), inflammation (arthritis) as well as cancer and tumors (breast, lung, colon, brain and skin cancer) to chronic pain, anxiety disorders, sleep problems and depression.1–4
Due to the great media presence of the medical applicability of cannabinoids for various diseases with few and often inefficient conventional therapy options, the interest for Cannabis products for self-therapies has increased.2 CBD products are particularly in demand since they are not considered illegal drugs. In Europe, CBD products are subject to the novel food regulation, which in principle ensures safe consumption in terms of health, as well as no nutritional disadvantages of these products.5 Nevertheless, it can be a challenge for customers to classify the quality, proper dosage and effectiveness of each product from the numerous suppliers.
The initial issue is the distinction between hemp (seed) oil and CBD oil. Hemp (seed) oil is obtained from the seeds of the Cannabis plants and contains mainly omega-6 and omega-3 essential fatty acids, but no cannabinoids. Hemp (seed) oils are occasionally blended with other vegetable or medium-chain triglyceride (MCT) oils. These are sometimes sold as percent hemp oil, which can be very easily mistaken for CBD oils, which contain extracts of Cannabis flowers and leaves with cannabinoids and terpenoids. These extracts are added to hemp seed oil or other types of oil (e.g. sunflower or MCT oil), resulting in the declared CBD concentrations.6
The other major problem is the false labeling of the CBD content in the CBD oils. Studies7–11 on the declaration accuracy of CBD oils demonstrated that the CBD content frequently deviates from the advertised concentration. Only less than 50% of the products examined in these studies were accurately labelled. The majority of the incorrect declared CBD oils were under-labeled and in some products, CBD could not even be detected.7–11
These studies clearly demonstrate the need for quality control of CBD oils concerning the declared CBD content to prevent misleading and harming the health of consumers. A novel approach to rapid quantification of CBD in oils apart from conventional liquid chromatography (LC) analysis is provided by the method of Direct Analysis in Real Time Mass Spectrometry (DART-MS). Since its first publication in 2005 by Cody et al.,12 the use of DART-MS has been demonstrated for rapid quality control of a wide variety of analytes, e.g. spices,13,14 pesticides,15 explosives12,16 and polymers.17 Rapid quantification methods using DART-MS have already been developed for nicotine in cigars18 and melamine and cyanuric acid in milk powder.19 The process relevant to the DART-MS method is the so-called Penning ionization, in which electronically excited noble gas atoms react with the ambient atmosphere and subsequently ionize the analytes via several intermediate steps. Almost no fragmentation occurs and mainly [M + H]+ and [M − H]− ions are generated for the positive and negative mode, respectively.17,20 Due to the fast analysis time and easy applicability, DART-MS is a method for rapid analysis in quality control.
In this study, we demonstrate the application of DART-MS for the quantitative analysis of CBD from CBD oils. The feasibility of the novel method is proven by reference to a validated LC-MS method for spiked and real samples. Therefore, the rapid DART-MS method is a well suitable alternative for quality control of CBD contents in CBD oils.
2 Material and methods
2.1 Reagents and materials
Methanol (MeOH) and acetonitrile (ACN) were in LC-MS grade (≥99.95%) and purchased from Th. Geyer (Renningen, Germany). Water was obtained from a MilliQ water purification system (Merck-Millipore, Billerica, MA, USA). Formic acid (FA, ≥98%) was purchased from Carl Roth (Karlsruhe, Germany). Cannabidiol standard (CBD, 1.0 mg in 1 mL MeOH) and cannabidiol-D3 standard (CBD-D3, 1 mg in 1 mL MeOH) were obtained from Cerilliant (Round Rock, TX, USA) and Lipomed (Arlesheim, Switzerland), respectively. Hemp oil native from Alnatura (Darmstadt, Germany) and cannabidiol CBD99 (>97.5%) from Candropharm (Helmond, Netherlands) were used to prepare the CBD oils. High purity helium (5.0) was purchased from Messer (Gumpoldskirchen, Austria). QuickStrip™ cards for DART analysis were obtained from IonSense (Saugus, MA, USA). Five CBD oils with concentrations of 5, 10 and 15% (m/m) were purchased from different Austrian online stores.Sample preparation was accomplished on an Ultrasonic Cleaner USC-TH from VWR (Radnor, PA, USA). Sample extraction was performed on a Thermomixer Comfort from Eppendorf (Hamburg, Germany) and centrifugation was carried out on a Centrifuge 5418 from Eppendorf (Hamburg, Germany).
2.2 Instrumentation
DART-MS analysis was performed on an ACQUITY QDa detector from Waters (Milford, MA, USA) equipped with a DART-SVP ion source and a QuickStrip™ module from IonSense (Saugus, MA, USA). The system was operated with helium as ionization gas at a temperature of 300 °C in positive ion mode. The distance between the MS inlet and the ion source was adjusted to 25 mm. The movement speed of the QuickStrip™ module was set to 1.00 mm s−1. The mass spectra were acquired in a full-scan between 100 and 500 m/z as well as a selective ion recording (SIR) at 315 m/z ([M + H]+ of CBD) and 318 m/z ([M + H]+ of CBD-D3) with a cone voltage of 10 V and a sampling frequency of 10 Hz.LC-MS analysis was performed on an ACQUITY Arc System coupled to an ACQUITY QDa detector from Waters (Milford, MA, USA). Separation was executed using an XSelect® CSH™ C18 (2.5 μm, 3.0 × 150 mm; Waters, Milford, MA, USA) column at a temperature of 60 °C. The mobile phase was composed of 0.1% (v/v) FA in water (eluent A) and ACN (eluent B). Elution was performed with the following gradient: 0 min/70% B, 5.5 min/70% B, 6 min/100% B, 7.5 min/100% B, 7.6 min/70% B, 12 min/70% B. The flow rate was set to 0.5 mL min−1 while the injection volume was 1 μL. To improve the ionization process in the ESI source, a mixture of 0.1% (v/v) FA and 70% (v/v) ACN in water was added immediately before the ESI source at a flow rate of 0.2 mL min−1. Detection was performed in positive electrospray ionization mode using a full-scan between 105 and 1000 m/z as well as SIR at 315 m/z ([M + H]+ of CBD) and 318 m/z ([M + H]+ of CBD-D3) with a cone voltage of 15 V and a sampling frequency of 2 Hz.
2.3 Preparation of CBD oils
Hemp seed oil was spiked with solid CBD, to obtain CBD oils with concentrations between 5% and 30% (m/m). Therefore, 50, 100, 150, 200, 250 and 300 mg CBD were dissolved in 950, 900, 850, 800, 750 and 700 mg hemp seed oil, respectively. To support the dissolving process, the mixtures were sonicated at 30 °C for 20 minutes.2.4 Sample preparation
For the liquid-phase extraction of CBD, 250 mg of CBD oil was weighed into a reaction tube. Additionally, 1.5 mL MeOH was added and the mixture was shaken at 1200 rpm and 25 °C for 5 min. Afterwards, the two liquid phases were separated by centrifugation at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 873 rcf for 10 min. The upper MeOH phase was transferred into a fresh reaction tube. The extraction was repeated once and the supernatants were merged. The extract was diluted in two steps, whereby the second was depending on the concentration of CBD oil. After a dilution of 1
873 rcf for 10 min. The upper MeOH phase was transferred into a fresh reaction tube. The extraction was repeated once and the supernatants were merged. The extract was diluted in two steps, whereby the second was depending on the concentration of CBD oil. After a dilution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 (v/v), the lower concentrations (5%, 10% and 15% (m/m)) were diluted 4
49 (v/v), the lower concentrations (5%, 10% and 15% (m/m)) were diluted 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (v/v) and the higher concentrations (20%, 25% and 30% (m/m)) were diluted 1
6 (v/v) and the higher concentrations (20%, 25% and 30% (m/m)) were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (v/v). In the same step, CBD-D3 was spiked to the samples to obtain a final concentration of 40 mg L−1 CBD-D3 in each extract. Each concentration and each consumer product was processed in triplicate.
9 (v/v). In the same step, CBD-D3 was spiked to the samples to obtain a final concentration of 40 mg L−1 CBD-D3 in each extract. Each concentration and each consumer product was processed in triplicate.
2.5 Quantification
In order to quantify the samples, standard solutions of 20, 40, 60, 80 and 100 mg L−1 CBD, each containing 40 mg L−1 CBD-D3 were prepared in MeOH. For quantification by DART-MS, each sample had its own calibration line using one QuickStrip™ card per sample. 3 μL of each solution was pipetted in duplicate following the pattern shown in Fig. 1. The QuickStrip™ cards were dried at room temperature (RT) before measurements. Three QuickStrip™ cards were prepared for each sample to ensure reliable measurement.Regarding the reference quantification by LC-MS, a linear calibration model was used for all samples in which each concentration level was measured three times. Every sample solution was injected in triplicate.
2.6 Method validation
A method validation for the performance of the LC-MS method was executed to ensure a reliable reference method. The parameters linearity, limit of detection (LOD), limit of quantification (LOQ), repeatability, accuracy and stability were investigated according to international guidelines.21–24The calibration model with five different CBD concentration levels was selected in the range from 20 to 100 mg L−1 (20, 40, 60, 80 and 100 mg L−1). In order to determine the linearity of the calibration model, each concentration level was prepared and measured three times, to obtain a total of nine measurement results for each level. Bias and RSD were calculated for each concentration level to evaluate the linearity.
LOD and LOQ were determined with a separate five point calibration line from 2 to 10 mg L−1 (2, 4, 6, 8, 10 mg L−1). All concentration levels were also prepared and measured three times. The calculation of LOD and LOQ was executed according to German industry norm DIN32645: 2008-11.23
Repeatability was examined by the measurement of two CBD standard solutions of 30 and 90 mg L−1 for three consecutive days with ten injections per concentration per day. Intra-day and inter-day RSD were determined for both concentration levels.
To demonstrate the accuracy, extraction solutions of hemp seed oil were spiked with CBD standard to obtain three concentration levels of 30, 60 and 90 mg L−1. All three solutions were prepared and measured three times, to obtain a total of nine measurement results for each concentration. Recovery and RSD were calculated for each level.
To investigate the stability of the analyte, spiked extraction solutions of hemp seed oil with concentration of 30 and 90 mg L−1 CBD were stored at RT, 4 °C and −20 °C. After one, two, three, seven and fourteen days, three aliquots of each concentration and storage temperature were measured. The percent changes in peak area ratios between the analyte and the internal standard compared to the reference measurements on day zero were determined.
3 Results and discussion
3.1 Quantification
The concentrations of CBD in the spiked samples determined by means of both LC-MS and DART-MS are listed in Table 1. In comparison of the two methods, relatively high RSD values were observed with DART-MS quantification. This deviation among individual measurements can be explained by the open ion source of the DART-MS system, since even small variations in ambient conditions (e.g. air humidity) will lead to changes in ionization.12,20 However, with one exception, the RSD values are less than 12% and are within the quality criterion of 15%.25 Therefore, the deviations of the DART-MS measurements are acceptable.| Sample code | LC-MS | DART-MS | |||
|---|---|---|---|---|---|
| Content of CBD/% (m/m) | RSD/% | Content of CBD/% (m/m) | RSD/% | Bias/% | |
| CBD_5_01 | 4.45 | 0.97 | 4.59 | 11.01 | 3.2 |
| CBD_5_02 | 4.90 | 1.28 | 4.91 | 10.59 | 0.4 |
| CBD_5_03 | 5.28 | 1.02 | 5.02 | 8.97 | −5.0 |
| CBD_10_01 | 10.27 | 0.42 | 11.14 | 8.16 | 8.5 |
| CBD_10_02 | 9.90 | 1.77 | 9.74 | 3.98 | −1.6 |
| CBD_10_03 | 10.87 | 1.02 | 10.65 | 6.77 | −1.9 |
| CBD_15_01 | 16.56 | 0.24 | 17.70 | 10.23 | 6.9 |
| CBD_15_02 | 15.29 | 2.91 | 15.69 | 3.99 | 2.6 |
| CBD_15_03 | 16.00 | 0.94 | 16.76 | 11.87 | 4.8 |
| CBD_20_01 | 19.72 | 0.77 | 19.08 | 8.98 | −3.2 |
| CBD_20_02 | 20.16 | 0.30 | 21.04 | 11.62 | 4.4 |
| CBD_20_03 | 18.12 | 1.52 | 17.90 | 11.45 | −1.2 |
| CBD_25_01 | 22.66 | 0.55 | 21.79 | 6.77 | −3.9 |
| CBD_25_02 | 26.98 | 1.34 | 27.64 | 8.47 | 2.4 |
| CBD_25_03 | 24.54 | 0.73 | 24.88 | 6.54 | 1.4 |
| CBD_30_01 | 27.62 | 0.13 | 29.09 | 11.00 | 5.3 |
| CBD_30_02 | 31.02 | 0.84 | 31.26 | 19.56 | 0.8 |
| CBD_30_03 | 34.18 | 1.58 | 33.40 | 12.72 | −2.3 |
Furthermore, a high correlation of the CBD content between the methods was confirmed with a maximum bias of 8.5%. A correlation plot (Fig. 2), in which the quantification results of DART-MS analysis were plotted against the results of the LC-MS analysis, further supports this correlation. Thus, the correlation coefficient of the spiked samples in the range of 5% to 30% (m/m) CBD is 0.9973. Due to the high correlation coefficient between both methods and the acceptable RSD values for the DART-MS determinations, the DART-MS method is principal suitable for rapid quantification of CBD content in oils.
 | ||
| Fig. 2 Correlation plot of the CBD concentrations of spiked hemp seed oils determined by LC-MS and DART-MS. The correlation coefficient is 0.9973. | ||
Subsequently, the applicability of the DART-MS quantification to real samples was examined. Therefore, five different CBD oils with concentrations of 5, 10 and 15% (m/m) were obtained in several Austrian online stores. These concentrations were chosen because the low concentration oils are more commonly purchased and are especially popular with inexperienced new users because they are significantly less expensive. The samples vary in the oil base (hemp seed oil, medium-chain triglyceride oils, sunflower oil) as well as in the preparation and addition of the hemp extracts. The CBD content of the real samples determined by both, LC-MS and DART-MS, are listed in ESI Table S1.† The corresponding correlation plot is illustrated in Fig. 3. Example mass spectra of the real samples and one spiked sample are shown in ESI Fig. S1–S3.†
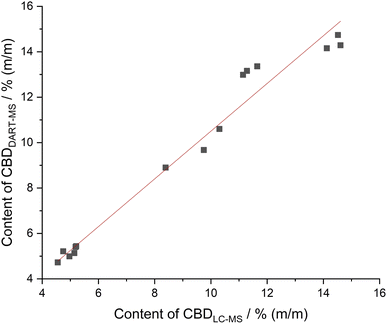 | ||
| Fig. 3 Correlation plot of the CBD concentration of five different real samples determined by LC-MS and DART-MS. The correlation coefficient is 0.9846. | ||
The RSD values below 12% for all DART-MS measurements of the real sample are similar to the results from the spiked hemp seed oils samples and are therefore satisfactory. Moreover, the correlation factor of the LC-MS and DART-MS determinations of the real-word samples in the decreased concentration range from 5 to 15% (m/m) is 0.9846. This value is slightly lower than the factor of spiked hemp seed oils. This minor degradation in performance can be related to the real sample with sample code RWS3_10. This sample has a maximum bias of 16.6%, which is still acceptable. All other samples have a bias smaller than 9.8%, which corresponds to the values of the spiked hemp seed oils samples.
Consequently, DART-MS quantification is an appropriate alternative to rapidly determine the CBD content in oils.
3.2 Method validation
In order to guarantee the reliability of the LC-MS method, several validation parameters were investigated.The calibration model ranging from 20 to 100 mg L−1 CBD, showed a good linearity with a coefficient of determination of 0.9965, bias between −1.8 and 2.1% and a maximal RSD value of 3.4%. All results are summarized in ESI Table S2.†
The determined values for LOD and LOQ were 0.657 and 1.63 mg L−1 CBD, respectively, which are excellent for quantification of CBD in the range of 20 to 100 mg L−1. Validation parameters of the calibration line for the calculation of LOD and LOQ are listed in ESI Table S3.†
Repeatability was verified on three consecutive days with two concentrations levels. RSD values of intra-day and inter-day repeatability were less than 1.6% for 30 mg L−1 CBD and less than 1.3% for 90 mg L−1 CBD.
Good accuracy for three different analyte concentration levels was shown with recoveries higher than 95% and RSD values lower than 1.7%. Detailed results of determining the accuracy are presented in Table 2.
| CBD concentration/mg L−1 | Recovery/% | RSD/% |
|---|---|---|
| 30 | 95.3 | 1.7 |
| 60 | 97.7 | 1.6 |
| 90 | 98.6 | 1.2 |
CBD concentration in hemp seed oil extracts is stable over a period of 14 days at the storage conditions of RT, 4 °C and −20 °C, as illustrated in Fig. 4. The recovery values of concentration at each day compared to day zero were 99 to 101%. The peak areas of CBD and CBD-D3 decreased by a maximum of 10% by day 14. This demonstrates the stability of the analyte in the CBD oils extracts over a period of 14 days.
4 Conclusions
The present work describes a novel DART-MS approach for rapid quantification of the CBD content of oils. Satisfactory results were obtained compared to LC-MS measurements as a reference method. Since, there is a strong correlation between the quantification results of both methods, as shown by a maximum bias of 9.8% (except for an outlier in the real samples with a bias of 16.6%) and correlation coefficients of 0.9973 and 0.9846 for the spiked samples and real samples, respectively. RSD values of less than 12% for all samples are also within an acceptable level for reliable determination of CBD concentration.The DART-MS method is especially suitable for the lower contents between 5 and 15% CBD, which are also the most common concentrations of traded CBD oils, due to the fact that these concentrations could be definitely classified by both DART-MS and LC-MS. To enhance the method for CBD oils with CBD content higher than 20%, optimization of the extraction procedure of CBD from the oil is required to obtain results with reduced deviations. In consecutive studies, further real samples can be investigated to prove applicability for various oil compositions and to find causes for possible outliers. These steps were not considered in this work since the focus was on the proof of principle for the quantification of CBD by means of DART-MS.
In summary, DART-MS is an excellent alternative for quality control in the quantification of CBD in oils, since it offers several advantages. The method is cost effective and resource efficient as no solvents are required for the measurements. In addition, DART-MS is user-friendly and can be operated even by inexperienced users.
Author contributions
Susanne Huber: conceptualization, methodology, validation, investigation, writing – original draft, visualization. Klemens Losso: methodology, writing – review & editing. Günther K. Bonn: resources, writing – review & editing, supervision. Matthias Rainer: resources, writing – review & editing, supervision.Conflicts of interest
There are no conflicts to declare.References
- C. Rong, Y. Lee, N. E. Carmona, D. S. Cha, R.-M. Ragguett, J. D. Rosenblat, R. B. Mansur, R. C. Ho and R. S. McIntyre, Pharmacol. Res., 2017, 121, 213–218 CrossRef CAS PubMed.
- S. Pisanti, A. M. Malfitano, E. Ciaglia, A. Lamberti, R. Ranieri, G. Cuomo, M. Abate, G. Faggiana, M. C. Proto and D. Fiore, Pharmacol. Ther., 2017, 175, 133–150 CrossRef CAS PubMed.
- S. A. Millar, N. L. Stone, A. S. Yates and S. E. O'Sullivan, Front. Pharmacol., 2018, 9, 1365 CrossRef CAS.
- P. F. Whiting, R. F. Wolff, S. Deshpande, M. Di Nisio, S. Duffy, A. V. Hernandez, J. C. Keurentjes, S. Lang, K. Misso and S. Ryder, Jama, 2015, 313, 2456–2473 CrossRef CAS PubMed.
- Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No. 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No. 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No. 1852/2001.
- H. J. VanDolah, B. A. Bauer and K. F. Mauck, Mayo Clin. Proc., 2019, 94, 1840–1851 CrossRef CAS PubMed.
- M. O. Bonn-Miller, M. J. Loflin, B. F. Thomas, J. P. Marcu, T. Hyke and R. Vandrey, Jama, 2017, 318, 1708–1709 CrossRef.
- A. Hazekamp, Medical Cannabis and Cannabinoids, 2018, 1, 65–72 CrossRef.
- R. Pavlovic, G. Nenna, L. Calvi, S. Panseri, G. Borgonovo, L. Giupponi, G. Cannazza and A. Giorgi, Molecules, 2018, 23, 1230 CrossRef.
- E. Johnson, M. Kilgore and S. Babalonis, J. Cannabis Res., 2022, 4, 28 CrossRef PubMed.
- J. P. Liebling, N. J. Clarkson, B. W. Gibbs, A. S. Yates and S. E. O'Sullivan, Cannabis Cannabinoid Res., 2022, 7, 207–213 CrossRef PubMed.
- R. B. Cody, J. A. Laramée and H. D. Durst, Anal. Chem., 2005, 77, 2297–2302 CrossRef CAS PubMed.
- K. Losso, K. B. Bec, S. Mayr, J. Grabska, S. Stuppner, M. Jones, T. Jakschitz, M. Rainer, G. K. Bonn and C. W. Huck, Spectrochim. Acta, Part A, 2022, 265, 120347 CrossRef CAS PubMed.
- B. Antal, A. Kuki, L. Nagy, T. Nagy, M. Zsuga, M. M-HAMVAS, G. Vasas and S. Keki, Anal. Sci., 2016, 32, 1111–1116 CrossRef CAS PubMed.
- E. Crawford and B. Musselman, Anal. Bioanal. Chem., 2012, 403, 2807–2812 CrossRef CAS PubMed.
- J. Frazier, V. Benefield and M. Zhang, Forensic Chem., 2020, 18, 100233 CrossRef CAS.
- J. H. Gross, Anal. Bioanal. Chem., 2013, 405, 8663–8668 CrossRef CAS PubMed.
- K. Losso, J. Cardini, S. Huber, C. Kappacher, T. Jakschitz, M. Rainer and G. K. Bonn, Talanta, 2022, 238, 123057 CrossRef CAS PubMed.
- L. Vaclavik, J. Rosmus, B. Popping and J. Hajslova, J. Chromatogr. A, 2010, 1217, 4204–4211 CrossRef CAS.
- J. H. Gross, Mass Spectrometry: A Textbook, Springer, Cham, 2017 Search PubMed.
- G. A. Shabir, J. Valid. Technol., 2005, 10, 314–325 Search PubMed.
- F. Peters, M. Hartung, M. Herbold, G. Schmitt, T. Daldrup and F. Mußhoff, Toxichem Krimtech, 2009, 76, 185–208 Search PubMed.
- DIN 32645. Chemical Analysis – Decision Limit, Detection Limit and Determination Limit under Repeatability Conditions – Terms, Methods, Evaluation, Beuth Verlag GmbH, Berlin, 2008 Search PubMed.
- I. H. T. Guideline, Q2 (R1), 2005, vol. 1, p. 5 Search PubMed.
- F. Peters, M. Hartung, M. Herbold, G. Schmitt, T. Daldrup and F. Mußhoff, Toxichem Krimtech, 2009, 76, 185 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Three further tables with experimental results and DART mass spectra are included. See https://doi.org/10.1039/d2ay01229d |
| This journal is © The Royal Society of Chemistry 2022 |