 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Assessing the effectiveness of microplastic extraction methods on fishmeal with different properties
Chloe
Way
 *a,
Malcolm D.
Hudson
a,
Ian D.
Williams
*a,
Malcolm D.
Hudson
a,
Ian D.
Williams
 b,
G. John
Langley
c and
Robert
Marsh
d
b,
G. John
Langley
c and
Robert
Marsh
d
aFaculty of Environmental and Life Sciences, University of Southampton, Highfield Campus, University Road, Southampton SO17 1BJ, UK. E-mail: C.J.Way@soton.ac.uk
bFaculty of Engineering and Physical Sciences, University of Southampton, Highfield Campus, University Road, Southampton SO17 1BJ, UK
cSchool of Chemistry, University of Southampton, Highfield Campus, University Road, Southampton SO17 1BJ, UK
dFaculty of Ocean and Earth Science, National Oceanography Centre, European Way, Southampton, SO14 3ZH, UK
First published on 19th January 2022
Abstract
Microplastic presence in fishmeal is an emerging research area because of its potential to enter food chains, and the importance of fishmeal within global food security. However, fishmeal is a complex medium dependant on fish composition. This study measured properties (organics, carbonates, protein and density) of five fishmeal types (trimmings, sardine and anchovy, krill, tuna and salmon), sourced from locations worldwide (Norway, South America, Antarctica, Spain and Scotland). Microplastic recovery rates were compared for existing methodologies using sodium chloride overflows and potassium hydroxide digestions and then compared to newly developed methods. These methods included dispersants and calcium chloride density separations which were developed and designed to be environmentally conscious and affordable, which we argue should become an international standard approach for researchers. A calcium chloride overflow with dispersant and potassium hydroxide digestion provided the highest recovery rate in sardine and anchovy fishmeal (66.3%). Positive correlations with recovery rate were found with protein content, and negative correlations with organic content. Low recovery rates found here suggest microplastics in fishmeal reported in the literature are underestimated. With complex media such as fishmeal, attention must be paid to variation between types and composition when choosing methods and interpreting results.
Introduction
Plastic pollution is a concern worldwide. Tides, rivers and currents such as the North Atlantic current,1 the Norwegian Coastal current (NCC),1 the Humboldt current,2 the Canary current,3 and the melting of sea ice around the Antarctic peninsula4 provide pathways for plastics to enter the marine environment. It is thought than an estimated 1.15–2.41 million tonnes of plastic enter the ocean from rivers alone.5 Once in the marine environment, plastic debris is subject to fragmentation into secondary microplastics by ultraviolet radiation, and mechanical and microbial degradation.6 Other forms of microplastics include primary microplastics that enter the marine environment as a small size, such as those in toiletries, cosmetics, tyre wear particles and synthetic fibres from washing clothes.7 A definition of microplastics which includes their physiochemical properties was proposed by Frias and Nash (2019):8 “Microplastics are any synthetic solid particle or polymeric matrix, with regular or irregular shape and with size ranging from 1 μm to 5 mm, of either primary or secondary manufacturing origin, which are insoluble in water”. However, others believe large microplastics are between 1–5 mm.9Due to the widespread nature of marine microplastics, there is a high potential for them to infiltrate the human food chain. Many studies have identified microplastics in the gastrointestinal tract10–12 and gills13,14 of marine life; however, few have studied either the whole fish or the tissue used as food for humans. Ribeiro et al. (2020)15 investigated the edible sections of commonly eaten seafood such as oysters, prawns, squid, crabs and sardines, and found sardines had the highest amount of microplastic in mass (0.3 mg g−1 tissue). Similarly, Karami et al. (2017)16 found more MP in the flesh of dried fish than the organs. There are many avenues microplastics may enter this pathway. For example, in areas where microplastics concentrations are high, it is more likely that some will be ingested by organisms (non-selective feeding).17 Moreover, some marine organisms have shown an ability to selectively ingest microplastics of certain sizes.18 Many marine organisms exposed to microplastics are harvested for fishmeal production, which indicates the potential for microplastic-contaminated fishmeal to get into the human food chain.
Fishmeal is a foodstuff made of whole fish or fish trimmings that is broken down, cooked, strained and milled.19 It has a high nutritional content including proteins, omega-3 fatty acids, amino acids and vitamins, that can support the diet of many animals.20 The majority of landings in certain fisheries around the world supply primarily to the fishmeal sector. For example, 98% of landings of Peruvian anchovies are used to produce fishmeal and fish oil.21 Fishmeal is mainly used as feed in aquaculture, pig and poultry farming.22 Furthermore, aquaculture provided 171 million tonnes of fish in 2016, with 88% being used as food for humans.23 The fish provided by aquaculture are a cheap source of protein and in 2018, aquaculture was the main supply of fish for 52% of the world's population,24 which showcases the importance of aquaculture with respect to global food security.25 Fishmeal is of considerable economic value, with Peruvian fishmeal pellets alone selling for £1126 per metric tonne in 2009.26 Therefore, in the light of growing public concern surrounding microplastics, it is necessary to evaluate the production of fishmeal and food as a potential exposure pathway.
Fishmeal is a considerably complex medium, which will bring about issues when creating a method to isolate the microplastics within. Previously, other media including: seawater;10,27,28 freshwater;28,29 estuaries;30,31 sediments;10,32,33 soils;34–36 sewage/wastewater;35,37 and biota10,11,38,39 have been assessed for microplastics using various different methods. Studies use density separation techniques involving saline solutions,37,40 and acidic and basic solutions to digest a media, making the polymers more easily available for extraction.41,42 An aim of many of these studies is to develop and standardise methodologies within each medium. Fishmeal is yet to be studied in much depth, with few studies at present being able to isolate and identify microplastics, and few validating methods with a recovery study to show how effective they are at recovering microplastics. Underwood et al. (2017) also noted this issue of many studies not validating methods with a recovery experiment.43 Moreover, studies that have extracted microplastics from fishmeal, have used widely different methods applied to different kinds of fishmeal, which vary considerably with regard to source material and composition.
Hanachi et al. (2019)44 and Karbalaei et al. (2020)45 have reported similar methodologies (potassium hydroxide (KOH) digestion) albeit with slight differences in amounts of sample and spectroscopic method used. Also, the fishmeal used is different, with Hanachi et al. (2019)44 using fishmeal from Iran, composed of salmon, sardines and kilka caught in the Persian Gulf and Caspian Sea, whereas the study by Karbalaei et al. (2020)45 used Malaysian fishmeal containing Indian mackerel (Rastrelliger kanagurta) and fish waste from the China Sea. Thiele et al. (2021)46 investigated microplastics in fishmeal but used a very different method than the previous studies; concluding that a sodium chloride (NaCl) soak and density separation was the most suitable method to extract microplastics from fishmeal, as applied to whitefish fishmeal, and sardine and anchovy fishmeal. This study was the only fishmeal focused study that undertook a recovery study (producing recoveries between 49 and 71%). More recently, Gündoğdu et al. (2021)47 assessed 26 different fishmeal types including fishmeal composed of; pilchard, blue whiting, sandeel, krill, anchovy, sprat, sardines, and mixed fish. They separated the microplastics from the fishmeal using a 30% KOH:NaClO solution as a way to digest the organic material before using NaI as a density separation.
Research into microplastics is fundamentally about studying its effects in/on the environment. Therefore we believe the study of this pollutant should not contribute harm to the environment either, including the use of chemicals. Many chemicals are known to be toxic to aquatic life, for example, zinc chloride can affect the growth of fish embryos.48 Similarly, we believe the cost of studying microplastics should be kept to a minimum where possible to maximise opportunities for research and monitoring globally. Microplastic research is evolving at such as rate that standardisation should be of high importance so that studies can be comparable. However, for many researchers, this cannot be adhered to if the cost of equipment/chemicals used are high. Therefore, we aim to use equipment and chemicals in this study that are affordable, environmentally friendly and easily accessible.
What is clear from the literature is that many methodologies are being investigated on many types of fishmeal, with no clear reason as to why certain methods are being chosen over others. Fishmeal has a range of different properties, from protein and oil content, to organic content, carbonate content and different bulk densities. Consequently, it could prove difficult to apply one universally effective method to all different types of fishmeal to extract microplastics reliably and consistently. Therefore, this study aims to: (i) investigate whether different methods used to extract microplastics (density separation, chemical digestion and dispersants) are more suited to fishmeal with certain characteristics (protein content, organic content, carbonate content and bulk density) and (ii) considers practicality, environmental impact and cost-effectiveness.
Methods
Methods from previously published studies looking into microplastics into fishmeal45–47 were gathered and assessed with regard to the effectiveness of extracting microplastics from fishmeal, while remaining cost effective and using environmentally friendly reagents. We refer to high cost methods as those which use a reagent that is over USD$100 per litre (Table 1). Environmentally friendly methods are those which do not have a report of aquatic toxicity on the respective safety data sheets (Table 1). The method by Gündoğdu et al. (2021)47 was investigated but ruled out due to the inclusion of large amounts of high-cost reagents which are not environmentally friendly. The method by Karbalaei et al. (2020)45 was tested as only a small amount of expensive reagent (NaI) is required. The method by Thiele et al. (2021)46 was tested, and due to it being the most environmentally friendly and cost-effective method, it was further developed using commonly used methods in microplastic extraction such as chemical digestion with KOH, the use of a dispersant (sodium hexametaphosphate), and an increased density saline solution of low-cost calcium chloride (Table 1). These methods are detailed in Table 3. The effectiveness of each method on each fishmeal was assessed by determining the recovery of spiked microplastics. Polymers were not assessed for signs of degradation: KOH at a temperature of 40 °C was the only digestion solution used and has already been tested for its ability to degrade polymers at this temperature, with no effect found.49| Separating solution | Density of salt in solutione (g cm−3) | Solution density in literaturec (g cm−3) | Environmentally friendly?b | Approx. costa (USD per kg) | Approx. cost per litree (USD per L) |
|---|---|---|---|---|---|
| a Cost per kg listed on Fisher scientific,50 in US Dollars (USD). b Sodium iodide hazards includes aquatic toxicity. Zinc chloride hazards include chronic aquatic toxicity. Sodium bromide should not be released into the environment. Sodium polytungstate may cause long term adverse effects in the aquatic environment. c Literature: a (ref. 46), b (ref. 32), c (ref. 51), d (ref. 52), e (ref. 53), f (ref. 54), g (ref. 55), h (ref. 56). d N/A (not applicable). e Approximate cost per litre of salt solution at specific density. | |||||
| Seawater | 1.02 | N/A | Y | N/A | N/A |
| Sodium chloride (NaCl) | 1.19 (26 wt% @ 25 °C) | 1.2a | Y | ∼$60.54 | ∼$15.74 |
| Calcium chloride (CaCl2) | 1.39 (40 wt% @ 20 °C) | 1.46b, 1.4c | Y | ∼$60.69 | ∼$24.27 |
| Sodium bromide (NaBr) | 1.41 (40 wt% @ 20 °C) | 1.37d, 1.55e | N | ∼$96.14 | ∼$38.45 |
| Zinc chloride (ZnCl2) | 1.7 (60 wt% @ 20 °C) | 1.5f | N | ∼$87.31 | ∼$52.38 |
| Sodium iodide (NaI) | 1.8 (60 wt% @ 20 °C) | 1.566d, 1.8g | N | ∼$533.98 | ∼$320 |
| Sodium polytungstate | 3.1 (85 wt% @ 20 °C) | 1.5h | N | ∼$623.42 | ∼$497.94 |
Spiking microplastics
Microplastic polymer types, sizes and amounts used for spiking were based on the methods used by Radford et al. (2021).70 Materials used to create the spiking plastics were from common consumer products and consisted of the main six plastic resin codes57 (Table 2). Each polymer was either sorted into fibres and fragments (PET and PP) or sorted into two size categories (0.25–0.5 mm and 0.5–1 mm) (HDPE, PVC, LDPE and PS). Plastic fragments were sized using a household coffee grinder and sized metal sieves (1 mm, 0.5 mm, 0.25 mm), and fibres were manually cut. The spiking plastics were chosen due their specific characteristic and/or colours to aid straightforward identification when mixed with a sample, and included polymers that could be broadly categorised as high (>1 g cm−3: PET, PVC) and low (<1 g cm−3: HDPE, LDPE, PP, PS) density. The spiking plastic polymer types were confirmed with high matches (>85% for all polymers) using Attenuated Total Reflectance Fourier-Transform Infrared spectroscopy (ATR FTIR) (Frontier, PerkinElmer). Each fishmeal sample was spiked with a total of 60 microplastic particles (five of each type of spiking plastic created).| Resin code | Abbreviation | Shape | Size (mm) | Colour | Original product | Densitya (g cm−3) |
|---|---|---|---|---|---|---|
| a Densities of plastics gathered from British Plastics Federation (2020).58 | ||||||
| 1 | PET | Fragment | 0.5–1 | Blue | Drinks bottle | 1.37 |
| Fibre | 1–5 | Green | Craft ribbon | |||
| 2 | HDPE | Fragment | 0.25–0.5 | Pink | Cleaning product bottle | 0.944–0.965 |
| Fragment | 0.5–1 | |||||
| 3 | PVC | Fragment | 0.25–0.5 | Red | Tablecloth | 1.38 |
| Fragment | 0.5–1 | |||||
| 4 | LDPE | Fragment | 0.25–0.5 | Purple | Carrier bag | 0.917–0.930 |
| Fragment | 0.5–1 | |||||
| 5 | PP | Fragment | 0.5–1 | White | Storage bottle | 0.905 |
| Fibre | 1–5 | Purple | Carpet | |||
| 6 | PS | Fragment | 0.25–0.5 | White | Packaging | 0.028–0.045 |
| Fragment | 0.5–1 | |||||
| Thiele et al. (2021)46 (method 1) | - 40 g fishmeal to 550 ml glass jar |
| - Add NaCl (1.2 g cm−3) (99.5%, Acros Organics) to sample up to a cm (50 ml) from top of 550 ml jar | |
| - Add lid and agitate for 30 seconds | |
| - Leave for a minimum of 30 minutes | |
| Overflow method | |
| - Place jar in larger container and remove lid | |
| - Slowly pour NaCl into jar to allow supernatant to overflow into container | |
| - Rinse outside of jar and inside of lid with pure water into overflow liquid | |
| - Repeat overflow three times for each sample, filtering each overflow separately | |
| - Filter supernatant through 20–25 μm filter paper and place in Petri dish | |
| NaCl density separation and KOH digestion (method 2) | - 40 g fishmeal to 550 ml glass jar |
| - Add NaCl (1.2 g cm−3) to sample up to a cm (50 ml) from top of 550 ml jar | |
| - Add lid and agitate for 30 seconds | |
| - Leave for a minimum of 30 minutes | |
| - Follow overflow method | |
| - Filter supernatant onto 25 μm metal mesh | |
| - Place metal mesh in 200 ml 10% KOH (>85%, Fisher Scientific) and heat to 40 °C at 100 rpm for 1 hour | |
| - Filter over 20–25 μm filter paper | |
| Dispersant, NaCl density separation and KOH digestion (method 3) | - 40 g fishmeal to glass 550 ml jar |
| - Add NaCl (1.2 g cm−3) and 50 ml dispersant (5% sodium hexametaphosphate) (general purpose grade, Fisher Scientific) to sample up to a cm (50 ml) from top of jar | |
| - Add lid and agitate for 30 seconds | |
| - Leave for a minimum of 30 minutes | |
| - Follow overflow method | |
| - Filter supernatant onto 25 μm metal mesh | |
| - Place metal mesh in 200 ml 10% KOH and heat to 40 °C at 100 rpm for 1 hour | |
| - Filter over 20–25 μm filter paper | |
| Karbalaei et al. (2020)45 (method 4) | - Place 20 g fishmeal sample into 250 ml DURAN glass bottle |
| - Add 200 ml KOH to each sample | |
| - Incubate sample at 40 °C for 72 hours | |
| - Filter sample over 149 μm filter paper | |
| - Place 149 μm filter paper in 10–15 ml NaI (≥99.5%, Sigma-Aldrich) and sonicate for 5 min at 50 Hz by ultrasonic bath | |
| - Remove filter papers and repeat sonication process | |
| - Centrifuge solution at 500 × g for 2 min at room temperature | |
| - Filter the supernatant though 8 μm filter paper and place in Petri dish | |
| Dispersant, CaCl2 density separation and KOH digestion (method 5) | - 40 g fishmeal to 550 ml glass jar |
| - Add CaCl2 (1.4 g cm−3) (93%, Fisher Scientific) and 50 ml dispersant (5% sodium hexametaphosphate) to sample up to a cm (50 ml) from top of jar | |
| - Add lid and agitate for 30 seconds | |
| - Leave for a minimum of 30 minutes | |
| - Follow overflow method | |
| - Filter supernatant onto 149 μm metal mesh | |
| - Place metal mesh in 200 ml 10% KOH and heat to 40 °C at 100 rpm for 1 hour | |
| - Filter over 20–25 μm filter paper |
Fishmeal
Commercial fishmeal samples were bought from online UK suppliers, with focus on collecting fishmeal made from various fish caught from different locations around the world. Fishmeal collected included Norwegian LT94 fishmeal, South American sardine and anchovy fishmeal, Antarctic krill meal, Spanish tuna fishmeal and Scottish salmon fishmeal. Properties of the fishmeal are detailed in Table 4. Protein and oil content of fishmeal was listed on their product specification sheets. The organic matter content was calculated using loss-on-ignition (LOI) at 550 °C and carbonate content was calculated using LOI at 950 °C. Bulk density of the fishmeal was calculated by weighing 1 cm3 of dried fishmeal.| Fishmeal | Type of fish used | Organic content (%) | Carbonate content (%) | Bulk density (g cm−3) | Protein (%) | Oila (%) |
|---|---|---|---|---|---|---|
| a N/A: not available in fishmeal specification sheet. | ||||||
| Norwegian LT94 | Species unknown, mix of whole fish and trimmings | 81.75 ± 0.04 | 5.47 ± 0.03 | 0.74 ± 0.01 | 71 | 12 |
| S American sardine & anchovy | Whole sardines and anchovies | 74.69 ± 0.05 | 3.419 ± 0.006 | 0.827 ± 0.007 | 68 | N/A |
| Antarctic krill | Antarctic krill | 87.49 ± 0.01 | 3.554 ± 0.004 | 0.47 ± 0.01 | 56 | N/A |
| Spanish tuna | Whole tuna | 77.89 ± 0.23 | 3.46 ± 0.04 | 0.69 ± 0.01 | 60 | 12 |
| Scottish salmon | Whole salmon | 76.49 ± 3.41 | 5.38 ± 0.58 | 0.752 ± 0.009 | 66 | 9 |
Each fishmeal sample was weighed in triplicate according to the amount needed for each method (Table 3). Methods used include those from existing literature45,46 and new methods based on steps commonly used for other media (density separation (NaCl) with digestion and two density separations (NaCl and CaCl2) with dispersant and digestion), which use environmentally friendly chemicals and solutions, with minimal steps to avoid loss of microplastics.
Method by Thiele et al. (2021)46 (method 1)
Glass jars (550 ml) were used to accurately weigh 40 g of fishmeal in triplicate. NaCl (1.2 g cm−3) was added to the fishmeal in 550 ml jars up to approximately 1 cm (50 ml) from the top, the lid was added, and the jar was shaken for 30 seconds. Thiele et al. (2021)46 stated jars must be left to stand to settle for a minimum of 30 minutes, in the case of this study, samples were left for 24 hours. Once settled, the jar was placed in a larger beaker and lid was removed. NaCl was slowly poured into the jar to allow the supernatant to overflow into beaker. The outside of the jar and the lid was rinsed with pure water into the overflow liquid. This “overflow method” was repeated three times for each sample, filtering each overflow separately. The supernatant was vacuum filtered through 20–25 μm filter paper and stored in a Petri dish for analysis.NaCl density separation with KOH digestion – (method 2)
This method was created with similarities to the steps used by Thiele et al. (2021),46 to maintain levels of standardisation. 40 g of fishmeal was placed in 550 ml jars in triplicate and NaCl was added up to 1 cm (50 ml) from the top, before being shaken for 30 seconds and left to settle for 24 hours. The overflow method was applied; however, supernatant was filtered on to 25 μm metal filters. The metal filter was placed in glass jars with 200 ml 10% KOH and heated to 40 °C and agitated at 100 rpm for 1 hour. The sample was then vacuum filtered through a 25 μm filter paper and stored in a Petri dish for analysis.NaCl density separation with dispersant and KOH digestion – (method 3)
This method was followed the same as the density separation with KOH digestion (method 2), with one difference. Before NaCl is added to the sample, 50 ml dispersant (5% sodium hexametaphosphate) was added.Method by Karbalaei et al. (2020)45 (method 4)
This method was followed as closely as possible to the method reported. Glass jars were used to accurately weigh out 20 g of each fishmeal, in triplicate. Following this, 200 ml of 10% KOH was added to the glass jars, which were then incubated at 40 °C for 72 hours. The contents of the jar were then vacuum filtered through 149 μm metal filters. This metal filter was then placed in 10 ml of 4.4 M sodium iodide (NaI) and sonicated at 50 Hz for 5 minutes, before the filter was removed, and the sonication step was repeated once more. The mixture was centrifuged at 500 × g for two minutes before allowing the supernatant to be filtered through an 8 μm filter membrane.CaCl2 density separation with dispersant and KOH digestion – (method 5)
This method was followed the same as the density separation with dispersant and KOH digestion (method 3), with one difference; the saline solution was changed to a higher density (1.4 g cm−3) solution of calcium chloride. Note the solution was filtered through a larger pore size filter (149 μm) due to the viscosity of the calcium chloride solution.Calculating spiked plastic recovery rates
Recovered microplastic particles were manually counted under a Nikon SMZ100 microscope (×40 magnification) and percentage of microplastics recovered (recovery rate) was calculated.Statistics
Statistical analysis was undertaken via RStudio (1.3.1093). Distribution of data were shown using histograms and Shapiro–Wilks normality tests. Non-normal distributions were observed in all data sets. Therefore, Kruskal–Wallis tests were used for the recovery rates of microplastics using different methods, and Dunn's test to look for pairwise comparisons between fishmeal types. Kruskal–Wallis tests were used to analyse recovery rates of specific polymers between methods, and to analyse the recovery rates of different size and shape microplastics between methods used, followed by post hoc analysis with Dunn's test. Correlations between recovery rate and all four fishmeal properties were estimated using Spearman's rank.Results
Fishmeal properties
Fishmeal properties measured include organic content (%), carbonate content (%), bulk density (g cm−3), protein (%) and oil (%) (Table 4). Antarctic krill meal had the highest organic content (87.5%), the lowest bulk density (0.47 g cm−3) and lowest protein content (56%). The South American sardine and anchovy fishmeal had the lowest organic content (74.7%), the lowest carbonate content (3.4%) and the highest bulk density (0.83%).Recovery rates of polymers in fishmeal
The five methods used to extract the spiked microplastics from each fishmeal type produced significantly different recovery rates (p < 0.05, Kruskal Wallis). The NaCl density separation method (method 1), the density separation with KOH digestion method (method 2), the NaCl density separation with dispersant and digestion method (method 3) and the CaCl2 method (method 5) all recovered significantly more spiked microplastics overall than the method outlined by Karbalaei et al. (2020)45 (method 4) (p < 0.05, Dunn's Test) (Fig. 1).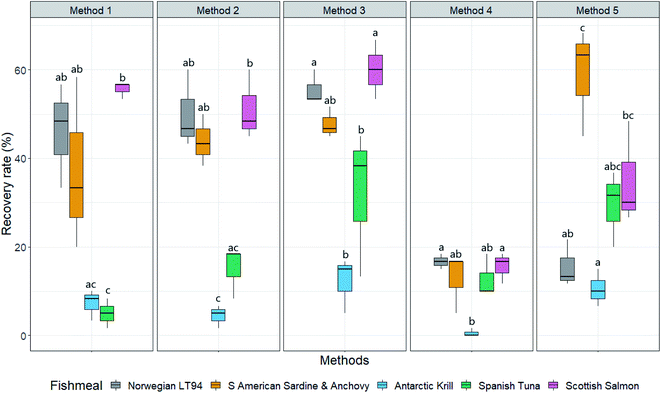 | ||
| Fig. 1 Spiked microplastic recovery rate (%) from five fishmeal types (Norwegian LT94, South American Sardine and Anchovy, Antarctic Krill, Spanish Tuna and Scottish Salmon), using five extraction methods (NaCl density separation (method 1), NaCl density separation followed by a KOH digestion (method 2), NaCl density separation with sodium hexametaphosphate dispersant followed by KOH digestion (method 3), a previously published method by Karbalaei et al. (2020)45 (method 4) and a calcium chloride density separation with sodium hexametaphosphate dispersant followed by KOH digestion (method 5)). Boxes represent median values with the interquartile range, whiskers represent min and max values. Boxes with different letters are significantly different (Dunn's test, p < 0.05). | ||
The NaCl density separation (method 1) recovered significantly different amounts of microplastics from the five different fishmeal types (p < 0.05, Kruskal Wallis). This method was more effective at recovering microplastics from the Norwegian LT94 (48.3% (11.7 IQR) RR (recovery rate)) and sardine and anchovy (33.3% (19.2 IQR) RR) than the Spanish tuna (5% (3.3 IQR) RR) (p < 0.05, Dunn's Test), and more effective at recovering microplastics from the Scottish salmon (56.7% (1.7 IQR) RR) than the Antarctic krill (8.33% (3.3 IQR) RR) and Spanish tuna (5% (3.3 IQR) RR) fishmeal (p < 0.05, Dunn's Test).
The method using a NaCl density separation with a KOH digestion (method 2) recovered significantly different amounts of spiked microplastics from the five fishmeal types (p < 0.05, Kruskal Wallis). This method recovered significantly more microplastics from Norwegian LT94 and Sardine and anchovy fishmeal (46.7% (8.3 IQR) RR and 43.3% (5.8 IQR) RR respectively), than Antarctic krill meal (5% (2.5 IQR) RR) (P < 0.05, Dunn's Test), and this method was more effective at recovering spiked microplastics from Scottish salmon fishmeal (48.3% (7.5 IQR) RR) than Antarctic krill meal and Spanish tuna meal (18.3% (5 IQR) RR) (p < 0.05, Dunn's Test).
The addition of a dispersant (sodium hexametaphosphate) to NaCl density separation and KOH digestion (method 3) resulted in significant differences between the recovery rate of spiked microplastics extracted from the five fishmeal types (p < 0.05 Kruskal Wallis). Using this method, significantly more spiked microplastics were recovered from the Scottish salmon fishmeal (60% (6.6 IQR) RR) and the Norwegian LT94 fishmeal (53.3% (3.3 IQR) RR) than the Antarctic krill meal (15% (5.8 IQR) RR) and the Spanish tuna fishmeal (38.3% (15.8 IQR) RR) (p < 0.05, Dunn's Test).
The method developed by Karbalaei et al. (2020)45 (method 4) did not affect the recovery rate of spiked microplastics between the fishmeal types (p > 0.05, Kruskal Wallis). However, the Norwegian LT94 fishmeal, the sardine and anchovy fishmeal and the Scottish salmon fishmeal had the same median recovery rate of 16.7%.
When using an increased density saline solution of calcium chloride with a dispersant and a KOH digestion (method 5) (Fig. 1), a significant difference in the recovered microplastics was found between the five fishmeal types (p < 0.05, Kruskal Wallis). Significantly more microplastics were extracted from the sardine and anchovy fishmeal (66.3% (11.6 IQR) RR) than the Norwegian LT94 fishmeal (13.33% (5 IQR) RR) and the Antarctic krill meal (10% (4.2 IQR) RR) (p < 0.05, Dunn's Test). Also significantly more microplastics were recovered from the Scottish salmon fishmeal (30% (10.8 IQR) RR) than the Antarctic krill meal using this method (p < 0.05, Dunn's Test).
Effect of fishmeal properties on recovery rates
All methods but the method by Karbalaei et al. (2020)45 (method 4) produced strong significant positive correlations between spiked microplastic recovery rates and bulk density (rs = 0.71 (method 1), rs = 0.73 (method 2), rs = 0.63 (method 3), rs = 0.75 (method 5), p < 0.05, Spearman's rank) (Fig. 2). The NaCl density separation with added KOH digestion method (method 2), the density separation with dispersant and KOH digestion method (method 3) and the method by Karbalaei et al. (2020)45 (method 4) all had the strongest significant positive correlation between spiked microplastic recovery rate and protein content (rs = 0.76, 0.71, 0.59 (respectively), p < 0.05 Spearman's rank) (Fig. 2). These three methods and the method with CaCl2 used as a saline solution (method 5) shared the strongest significant negative correlation between recovery rate and organic content (rs = -0.52, −0.38, −0.41, −0.89 (respectively), p < 0.05 Spearman's rank). Moreover, there was no significant correlation between spiked microplastic recovery rate and organic content when using the NaCl density separation (method 1) (rs = −0.46, p > 0.05, Spearman's rank) (Fig. 2). | ||
| Fig. 2 Correlogram showing Spearman Rho correlation coefficients between fishmeal properties (organic content, carbonate content, protein content and bulk density) and spiked microplastic recovery rate. −1 indicates strong negative correlation, +1 indicates strong positive correlation. Squares including a black cross represent those correlations with no significance (p > 0.05). The five methods include: NaCl density separation (method 1), NaCl density separation followed by a KOH digestion (method 2), NaCl density separation with sodium hexametaphosphate dispersant followed by KOH digestion (method 3), a previously published method by Karbalaei et al. (2020)45 (method 4) and a calcium chloride density separation with sodium hexametaphosphate dispersant followed by KOH digestion (method 5). | ||
Recovery of individual polymers
All five methods used recovered significantly different amounts of spiked microplastic polymer types (p < 0.05 for all, Kruskal Wallis) (Fig. 3). The NaCl density separation method (method 1) extracted significantly more low-density polymers such as HDPE (48% RR), LDPE (56.7% RR) and PS (42.7% RR) than high-density polymers such as PET (4.7% RR) and PVC (0.7% RR) (p < 0.05 for all, Dunn's test). This method also extracted significantly more LDPE than PP (28.7% RR) (<0.05, Dunn's test). | ||
| Fig. 3 Average recovery rates (%) of 6 common microplastic polymers (first six plastic resin codes), extracted from fishmeal, using five separation/digestion methods used in existing literature (NaCl density separation (method 1), NaCl separation with a KOH digestion (method 2), NaCl separation with sodium hexametaphosphate dispersant followed by KOH digestion (method 3), a previously published method by Karbalaei et al. (2020)45 (method 4) and a calcium chloride density separation with sodium hexametaphosphate dispersant followed by KOH digestion (method 5)). Error bars represent standard error of the mean. Bars with different letter notations within each method are significantly different (Dunn's test, p < 0.05). | ||
The methods with added KOH digestion (method 2) and added dispersant (method 3) recovered significantly more low-density polymers [such as HDPE (KOH: 57.3% RR, dispersant: 70.7% RR), LDPE (KOH: 60% RR, dispersant: 75.3% RR), PP (KOH: 32% RR, dispersant: 44.7% RR) and PS (KOH: 41.3%, dispersant: 50.7% RR)] than high-density PET [(KOH: 4% RR, dispersant: 6% RR) and PVC (KOH: 2.7% RR, dispersant: 2% RR)] (p < 0.05, Dunn's test).
The method by Karbalaei et al. (2020)45 (method 4) recovered significantly more low-density polymers [such as HDPE (14.7% RR), LDPE (32.7% RR), PP (7.3% RR) and PS (10.7% RR)] than high-density PET (0.7% RR) (p < 0.05 Dunn's test). However, this method only found significantly more low-density HDPE and LDPE than high-density PVC (4% RR) (<0.05, Dunn's test). This method also recovered significantly more LDPE polymers than any other polymer (p < 0.05, Dunn's test).
The method with an increased density saline solution of calcium chloride, a dispersant and a KOH digestion (method 5) also recovered significantly more low-density polymers of HDPE (62% RR) and LDPE (60.6% RR) than the higher density polymers of PET (11.3% RR) and PVC (20.6% RR) (p < 0.05, Dunn's test). However, polystyrene (15.3% RR), which has the lowest density, was recovered significantly less than the other low-density polymers of LDPE and HDPE (p < 0.05, Dunn's test). This method also recovered the highest amount of the high-density polymers such as PET and PVC compared to the other four methods, with recovery rates of 11.3% and 20.6% respectively (Fig. 3).
Individual polymer properties
All methods that include a NaCl (methods 1, 2 and 3) or a CaCl2 density separation (method 5) recovered significantly more big (0.5–1 mm) microplastics (41.7%, 42%, 51.3%, 47% RR respectively) than the method by Karbalaei et al. (2020)45 (14.3% RR) (p < 0.05, Dunn's test) (Fig. 4A). These four methods also recovered significantly more fragments (RR = method 1: 32%, method 2: 34.8%, method 3: 43%, method 5: 31.3%) than the method by Karbalaei et al. (2020)45 (method 4) (RR = 10.7%) (p < 0.05, Dunn's test for both) (Fig. 4B). | ||
| Fig. 4 Average recovery rate (%) of big (0.5–1 mm) (A), small (0.25–0.5 mm) (A), fibres(B) and fragments (B) spiked microplastics extracted from fishmeal, using five different methods (NaCl density separation (method 1), NaCl separation with a KOH digestion (method 2), NaCl separation with sodium hexametaphosphate dispersant followed by KOH digestion (method 3), a previously published method by Karbalaei et al. (2020)45 (method 4) and a calcium chloride density separation with sodium hexametaphosphate dispersant followed by KOH digestion (method 5)). Bars with different letter notations are significantly different (Dunn's test, p < 0.05), different case of letters represents different tests in each plot. | ||
However, method 4 (Karbalaei et al. 2020) recovered on average more small (0.25–0.5 mm) microplastics (16.7% RR) than big microplastics (14.3% RR) which is an opposite trends to all other methods which recovered more big microplastics than small.
Discussion
When investigating microplastics in a new medium, it is paramount to understand the properties of the medium and whether these will have an effect on extraction of plastic particles. Here, we measured four properties of five commercially available types of fishmeal and subjected them to five different methods to establish recovery rate of spiked microplastics. We found the method of CaCl2 density separation with dispersant and KOH digestion recovered the most microplastics in the sardine and anchovy fishmeal. However, the NaCl density separation with dispersant and a KOH digestion stage recovered the most microplastics from the four other fishmeal types. Moreover, the organic content of fishmeal was found to be negatively correlated with microplastic recovery rate. Overall, recovery rates varied across fishmeal types when using the same method (Fig. 1), suggesting that the properties of the fishmeal could influence the amount of microplastics recovered. In addition, recovery rates were also low (0–66.3%), suggesting a potential for general underestimation of microplastics reported in fishmeal literature.Sodium chloride density separation has been used as a method to separate microplastics from a matrix for a long time.59 More recently, it has been utilised to recover microplastics from fishmeal. Thiele et al. (2021)46 used a NaCl density separation ‘Overflow’ method (Table 3) to extract microplastics from two fishmeal types. They found a recovery rate of 49.3 ± 1.2% in sardine and anchovy fishmeal, whereas this study found 33.3% recovery rate with the same fishmeal type (but obtained from a different source). This difference in recovery rate suggests there is a variability in the same fishmeal when manufactured in different places, or that the fish is sourced from different locations. This in turn may influence the effectiveness of the method. The study by Thiele et al. (2021)46 used different spiking polymers consisting of PS, PP, PET, PA and rayon, which have different densities than the polymers used in this study (PET, HDPE, LDPE, PVC, PS and PP), making it difficult to compare recovery rates. Sodium chloride is frequently used when studying microplastics. For example, Hanvey et al. (2017)60 compared studies looking into microplastics in sediments, and almost half (19/43) used NaCl as a saline solution. Similarly, a meta-analysis looking into recovery rate studies by Way et al. (2022)61 found that 16 out of the 71 studies included used NaCl, which was the most frequently used reagent in the analysis. Using NaCl as a density separation is also recommended by the Marine Strategy Framework Directive (MSFD).62 There are several reasons as to why this method is widely used and accepted: ease of use, affordability, and its non-toxic properties (Table 1). Although the studies which use zinc chloride (ZnCl2)63 and NaI64 have found high recovery rates (95.5–100% and >98% respectively), the use of the more expensive and hazardous saline solutions involve multiple steps to reduce sample mass, allowing for less of the solution to be used.40 Moreover, many studies do not use these higher-density, expensive saline solutions at the highest density the salt can reach at 20 °C (Table 1), suggesting that it is much more economically viable to use the lower-density, lower expense saline solutions. For these reasons, this study used and developed methods with NaCl over other more expensive and toxic reagents such as ZnCl2 and NaI, in order to encourage replication and standardisation from others.
This study combined NaCl with KOH to facilitate digestion and found recovery rates of between 5% and 48.3%, depending on the fishmeal type. Many studies have reported KOH an effective digestion reagent, which depending on the incubation temperature, it can have little effect on the polymer properties. For example, Karami et al. (2017)16 found that using KOH at 40 °C had no effect on the polymers and was effective at digesting fish tissues. Thiele et al. (2021)46 trialled the use of KOH in recovering microplastics and found fishmeal that was digested in 10% KOH was not filterable through 25 μm filter papers. This study used KOH to digest residual fishmeal after density separation with 5% sodium hexametaphosphate as a dispersant, allowing for easier filtration. This proved to be an effective method in extracting the spiked microplastics with recovery rates between 15% and 60%. Other studies have used various surfactants/dispersants as an effective way of dispersing microplastics in a solution.65–67
When a method was trialled using a higher density salt solution (CaCl2) with added dispersant and a KOH digestion (method 5), spiking plastics were recovered at a higher rate of between 10–66.3%. Similar recoveries of 69% and 55.5% have been found when using calcium chloride to recover microplastics from sediment.32,51 The calcium chloride solution has a higher density than sodium chloride, so is expected to recover plastics with a higher density. However, it was observed that using this solution often caused the lower density fishmeal to rise in the beaker, which caused issues with the overflow technique and following filtration (Fig. 5). This could explain how the highest recovery (66.6%) was found in the sardine and anchovy fishmeal which also has the highest bulk density (0.83 g cm−3) (Table 4) and thus less likely to float in the calcium chloride solution. Moreover, this method did recover more high-density polymers such as PET and PVC than other methods using NaCl. Using this method, significantly less PS was recovered than other polymers. Crichton et al. (2017),32 who also used calcium chloride as a density separation similarly found higher recovery rates of PVC (86.6%) than the category of polymers containing polystyrene (42.2%). They explained that the low recovery rates could be due to the calcium chloride settling overnight.
The chemistry/properties of calcium chloride may provide another explanation for the behaviour of the fishmeal in the beakers and the results found. Unlike sodium chloride, calcium chloride is hygroscopic meaning it can absorb the moisture from air, and is deliquescent, so the salt will readily dissolve from the moisture absorbed from the air.68,69 In solution calcium chloride may attract more water until equilibrium is reached between the ambient and solution vapor pressure. Having properties that readily absorbs water from the surroundings could provide an opportunity for water to be drawn out from the fishmeal, allowing the fishmeal to rise – thus causing the issues found with overflowing and filtering mentioned previously. Moreover, the calcium chloride solution at a density of 1.4 g cm−3 has a viscous texture, making the solution difficult to filter. Although this method recovered the highest recovery rate, we would not recommend the use of this solution, due to the issues of overflowing and filtering, making it difficult to locate the recovered spiking plastics. However, if the aim of a study is to recover high density microplastics, this method may prove useful if large pore-sized filters are used.
Microplastics were more difficult to recover from the fishmeal with the highest organic content, shown with a significant negative correlation with the recovery rate of the spiked microplastics (rs = −0.52, −0.38, −0.41, −0.89) (Antarctic krill organic content = 87.5%) using all methods. Similar trends are found with other media. For example, Radford et al. (2021)70 found lower recovery rates of microplastics from soils with higher organic matter. Hurley et al. (2018)35 mostly found higher extraction efficiencies in soils with lower organic content than in the higher organic content sludge samples. Some studies have succeeded in removing large amounts of organic matter, thus achieving high recovery rates, by using digestion steps.71 However, this often entails using hazardous/toxic reagents such as hydrogen peroxide or Fenton's reagent.
Bulk density (g cm−3) often refers to the density of polymers and the saline solution. We measured the bulk density of the fishmeal types (Table 4). Significant correlations were found between the bulk density of fishmeal and recovery rate of spiked microplastics (rs = 0.71, 0.73, 0.63, 0.49, 0.75). In this study, the fishmeal with the highest bulk density (sardine and anchovy: bulk density = 0.83 g cm−3) sank in NaCl solution, making it easier for the microplastics to rise and overflow the glass jar. However, it is known that microplastics have the ability to lower the bulk density of a matrix, such as soil.72 If this is the case, it may become more difficult to extract microplastics from a sample that is highly contaminated with the particles.
Some studies have investigated the use of enzymes to digest material when extracting microplastics,73–75 as they can be effective for reducing fats and proteins. However, this study found a significant positive correlation between fishmeal with a high protein content (Norwegian LT94 fishmeal) and the recovery rate of spiked microplastics (rs = 0.66, 0.76, 0.71, 0.59), showing that a reduction in protein content may not benefit the extraction of microplastics from fishmeal. Furthermore, the use of some enzymes, such as proteinase-K can be expensive due to the high purification.74
Here, more low density polymers (HDPE, LDPE, PS and PP) were extracted than the high density polymers (PET and PVC). Similar findings have been found by Thiele et al. (2021),46 who extracted more spiked PS fragments than PET and rayon from sardine and anchovy fishmeal. This finding is comparable across other media. For example, Radford et al. (2021)70 found PET had the lowest recovery rates in soil, whereas LDPE had the highest recovery rates. In some cases, the high-density polymers can be recovered with the higher-density solutions, such as zinc bromide (ZnBr2).52 However, this study did not utilise these solutions due to their hazardous nature and expense, but a slightly higher density, non-toxic reagent of CaCl2 was tested and found high recovery rates of PET and PVC than the methods using NaCl. Attention must be noted when comparing recovery rates of polymers between studies as polymer densities and thus their floatability can be affected by the addition of plasticisers and additives.76 If the aim of a study is to target high density polymers, for example in bottom feeder fish/invertebrates, then using high density saline solutions may be beneficial. To avoid the high cost of these saline solutions, some researchers have begun looking into recycling saline solutions.55 However, recycling the solutions by evaporation could be energy-intensive and very time-consuming, depending on the number of samples and amount of solution used.
This study showed that when using a NaCl or CaCl2 density separation method, more ‘big’ (0.5–1 mm) microplastics were recovered than the ‘small’ (0.25–0.5 mm) microplastics, and more fragments than fibres. The opposite trend was found when utilising the method by Karbalaei et al. (2020).45 With few recovery studies published using fishmeal as a medium, it is difficult to compare trends. Other studies have shown that smaller microplastics are easier to find than large when using NaCl and water,52 whereas large microplastics are easier to recover when using higher density solutions such as ZnCl2.63
The shape and size of microplastics recovered could depend on the number of steps used during the methodology. The method by Karbalaei et al. (2020)45 had several steps, with different equipment, ultimately giving higher chance of losing microplastics between stages. This could be a reason for finding less of the larger spiking plastics, which may have been lost through the multiple stages of the method. Alternative methods that minimise stages of preparation include the use of pyrolysis-GC-MS. Pyrolysis-GC-MS involves heating (pyrolysis) a small sample which produces pyrolysates which move into a gas chromatography (GC) column, are separated and then detected by a mass spectrometer (MS).77 Pyrolysis-GC-MS has the benefits of being able to detect the presence of additives and phthalates of microplastics, is less restricted by the size of the microplastic to be identified, has lower chance of contamination and is more reproducible given access to equipment.77 This technique is emerging as an option for identifying microplastics in environmental samples. For example, Ribeiro et al. (2020)15 used a KOH digestion followed by accelerated solvent extraction and then pyrolysis to identify microplastics in common seafood. If this technique could be adopted to identify microplastics in fishmeal, large numbers of samples could be processed, with higher accuracy and with less chance of contamination.
For future applications of these methods it would be worth evaluating the reproducibility between different operators and different laboratory settings to see whether similar results could be reproduced.
When developing a method to extract microplastics from an environmental medium, there must be a fine balance between performance (recovery rate), cost and environmental impact. Although calcium chloride and sodium chloride are usually reported as having lower performance than other high density saline solutions, the significantly lower cost and environmental impact make them a preferred solution to use in most investigations of fishmeal samples. Seeing as microplastics are a pollutant themselves, this balance is something all microplastic researchers should consider when developing a method they hope to be universally accepted.
Conclusions
Fishmeal is a globally important feed in aquaculture and agriculture. Consequently, microplastic presence in fishmeal is concerning and analytical methodologies are emerging. This study highlights the variability of fishmeal media, the complexity this brings when attempting to extract microplastics, and the importance of using environmentally conscious and affordable methods.We recommend using a dispersant with NaCl density separation and a KOH digestion; and analysing the fishmeal properties: lower recoveries may be anticipated from fishmeal types with higher organic and lower protein content. This method is of low cost and is environmentally friendly, which is a balance we argue should become an international standard approach for researchers to allow for a method that is widely accepted (philosophically and scientifically) and easy to replicate. The low recovery rates found in this study highlight the possibility of variable underestimation of microplastics being reported in fishmeal. This is an issue that probably applies to other complex media and must also be accounted for if the method is used for microplastic extraction in the future.
Conflicts of interest
The authors receive no third-party funding for this related work and have no affiliation to the fishmeal or food industry.Credit author statement
Chloe Way: conceptualisation, methodology, validation, formal analysis, investigation, data curation, writing-original draft, visualisation. Malcolm Hudson: supervision, project administration, funding acquisition, writing-review and editing. Ian Williams: supervision, writing-review and editing. John Langley: supervision, writing-review and editing. Robert Marsh: supervision, writing-review and editing.Data availability
Data supporting this study are openly available from the University of Southampton repository at: https://doi.org/10.5258/SOTON/D2102Acknowledgements
This work was supported by the School of Geography and Environmental Science at the University of Southampton, and the Leverhulme Trust Southampton Marine and Maritime Institute. We would like to thank Freya Radford for help with sourcing and identifying the spiking microplastics, and the School Laboratory Manager Peter Morgan for his support and input.References
- N. G. Winther and J. A. Johannessen, J. Geophys. Res. C Oceans, 2006, 111 Search PubMed.
- J. Kämpf and P. Chapman, in Upwelling Systems of the World: A Scientific Journey to the Most Productive Marine Ecosystems, Springer International Publishing, Cham, 2016, pp. 161–201, DOI: DOI:10.1007/978-3-319-42524-5_5.
- J. Kämpf and P. Chapman, in Upwelling Systems of the World: A Scientific Journey to the Most Productive Marine Ecosystems, Springer International Publishing, Cham, 2016, pp. 203–250, DOI: DOI:10.1007/978-3-319-42524-5_6.
- S. Nicol, J. Foster and S. Kawaguchi, Fish Fish., 2012, 13, 30–40 CrossRef.
- L. C. M. Lebreton, J. van der Zwet, J.-W. Damsteeg, B. Slat, A. Andrady and J. Reisser, Nat. Commun., 2017, 8, 15611 CrossRef CAS PubMed.
- A. L. Andrady, in Marine Anthropogenic Litter, ed. M. Bergmann, L. Gutow and M. Klages, Springer International Publishing, Cham, 2015, pp. 57–72, DOI: DOI:10.1007/978-3-319-16510-3_3.
- J. Boucher and D. Friot, Primary Microplastics in the Oceans: a Global Evaluation of Sources, Iucn Gland, Switzerland, 2017 Search PubMed.
- J. P. G. L. Frias and R. Nash, Mar. Pollut. Bull., 2019, 138, 145–147 CrossRef CAS PubMed.
- International Organization for Standardization, ISO/TR 21960:2020(en) Plastics - Environmental aspects - State of knowledge and methodologies, https://www.iso.org/standard/72300.html, (accessed April 2020, 2020) Search PubMed.
- O. Guven, K. Gokdag, B. Jovanovic and A. E. Kideys, Environ. Pollut., 2017, 223, 286–294 CrossRef CAS PubMed.
- A. L. Lusher, M. McHugh and R. C. Thompson, Mar. Pollut. Bull., 2013, 67, 94–99 CrossRef CAS PubMed.
- S. Roch and A. Brinker, Environ. Sci. Technol., 2017, 51, 4522–4530 CrossRef CAS PubMed.
- D. Brennecke, E. Ferreira, T. Costa, D. Appel, B. Da Gama and M. Lenz, Mar. Pollut. Bull., 2015, 96, 491–495 CrossRef CAS PubMed.
- D. Fabbri, A. G. Rombolà, I. Vassura, C. Torri, S. Franzellitti, M. Capolupo and E. Fabbri, J. Anal. Appl. Pyrolysis, 2020, 149, 104836 CrossRef CAS.
- F. Ribeiro, E. D. Okoffo, J. W. O'Brien, S. Fraissinet-Tachet, S. O'Brien, M. Gallen, S. Samanipour, S. Kaserzon, J. F. Mueller, T. Galloway and K. V. Thomas, Environ. Sci. Technol., 2020, 54, 9408–9417 CrossRef CAS PubMed.
- A. Karami, A. Golieskardi, Y. B. Ho, V. Larat and B. Salamatinia, Sci. Rep., 2017, 7, 5473 CrossRef PubMed.
- C. Scherer, N. Brennholt, G. Reifferscheid and M. Wagner, Sci. Rep., 2017, 7, 17006 CrossRef PubMed.
- M. Cole, P. Lindeque, E. Fileman, C. Halsband, R. Goodhead, J. Moger and T. S. Galloway, Environ. Sci. Technol., 2013, 47, 6646–6655 CrossRef CAS PubMed.
- Food and Agriculture Organization of the United Nations, The Production of Fishmeal and Oil, Food and Agriculture Organisation of the United Nations, Rome, FAO Fisheries Technical Paper 142, 1986 Search PubMed.
- IFFO The Marine Ingredients Organisation, Usage/Destination, https://www.iffo.net/usage-destination, (accessed April, 2020).
- U. N. Wijkstrom, Presented in Part at the Farming the Waters for People and Food, Phuket, Thailand., 2010 Search PubMed.
- C. J. Shepherd and A. J. Jackson, J. Fish. Biol., 2013, 83, 1046–1066 CrossRef CAS PubMed.
- Food and Agriculture Organization of the United Nations, The State of World Fisheries and Aquaculture 2018 - Meeting the Sustainable Development Goals, Food and Agriculture Organization of the United Nations, Rome., 2018 Search PubMed.
- FAO, The State of World Fisheries and Aquaculture 2020, Sustainability in Action, Rome, 2020 Search PubMed.
- J. A. Pradeepkiran, Transl. Anim. Sci., 2019, 3, 903–910 CrossRef CAS PubMed.
- World Bank Commodity Price, Data, Fishmeal Monthly Price, https://www.indexmundi.com/commodities/?commodity=fish-meal%26months=12%26currency=gbp, (accessed March, 2020) Search PubMed.
- M. Cole, H. Webb, P. K. Lindeque, E. S. Fileman, C. Halsband and T. S. Galloway, Sci. Rep., 2014, 4, 8 Search PubMed.
- J. Grbic, B. Nguyen, E. Guo, J. B. You, D. Sinton and C. M. Rochman, Environ. Sci. Technol. Lett., 2019, 6, 68–72 CrossRef CAS.
- M. O. Rodrigues, A. M. M. Goncalves, F. J. M. Goncalves, H. Nogueira, J. C. Marques and N. Abrantes, Ecol. Indicat., 2018, 89, 488–495 CrossRef CAS.
- Z. T. Anderson, A. B. Cundy, I. W. Croudace, P. E. Warwick, O. Celis-Hernandez and J. L. Stead, Sci. Rep., 2018, 8, 9428 CrossRef PubMed.
- J. L. Stead, A. B. Cundy, M. D. Hudson, C. E. L. Thompson, I. D. Williams, A. E. Russell and K. Pabortsava, Sci. Rep., 2020, 10, 14147 CrossRef CAS PubMed.
- E. M. Crichton, M. Noel, E. A. Gies and P. S. Ross, Anal. Methods, 2017, 9, 1419–1428 RSC.
- R. Nakajima, M. Tsuchiya, D. J. Lindsay, T. Kitahashi, K. Fujikura and T. Fukushima, PeerJ, 2019, 7, 7915 CrossRef PubMed.
- J. David, Z. Steinmetz, J. Kucerik and G. E. Schaumann, Anal. Chem., 2018, 90, 8793–8799 CrossRef CAS PubMed.
- R. R. Hurley, A. L. Lusher, M. Olsen and L. Nizzetto, Environ. Sci. Technol., 2018, 52, 7409–7417 CrossRef CAS PubMed.
- Z. Steinmetz, A. Kintzi, K. Muñoz and G. E. Schaumann, J. Anal. Appl. Pyrolysis, 2020, 147, 104803 CrossRef CAS.
- Z. Wang, S. E. Taylor, P. Sharma and M. Flury, PLoS One, 2018, 13, 0208009 Search PubMed.
- A. L. Lusher, N. A. Welden, P. Sobral and M. Cole, Anal. Methods, 2017, 9, 1346–1360 RSC.
- M. B. Phillips and T. H. Bonner, Mar. Pollut. Bull., 2015, 100, 264–269 CrossRef CAS PubMed.
- M.-T. Nuelle, J. H. Dekiff, D. Remy and E. Fries, Environ. Pollut., 2014, 184, 161–169 CrossRef CAS PubMed.
- J. Bianchi, T. Valente, U. Scacco, R. Cimmaruta, A. Sbrana, C. Silvestri and M. Matiddi, Mar. Pollut. Bull., 2020, 154, 111050 CrossRef CAS PubMed.
- G. F. Schirinzi, C. Pedà, P. Battaglia, F. Laface, M. Galli, M. Baini, P. Consoli, G. Scotti, V. Esposito, C. Faggio, M. Farré, D. Barceló, M. C. Fossi, F. Andaloro and T. Romeo, J. Hazard. Mater., 2020, 397, 122794 CrossRef CAS PubMed.
- A. J. Underwood, M. G. Chapman and M. A. Browne, Anal. Methods, 2017, 9, 1332–1345 RSC.
- P. Hanachi, S. Karbalaei, T. R. Walker, M. Cole and S. V. Hosseini, Environ. Sci. Pollut. Res., 2019, 26, 23777–23787 CrossRef CAS PubMed.
- S. Karbalaei, A. Golieskardi, D. U. Watt, M. Boiret, P. Hanachi, T. R. Walker and A. Karami, Mar. Pollut. Bull., 2020, 150, 110687 CrossRef CAS PubMed.
- C. J. Thiele, M. D. Hudson, A. E. Russell, M. Saluveer and G. Sidaoui-Haddad, Sci. Rep., 2021, 11, 2045 CrossRef CAS PubMed.
- S. Gündoğdu, O. T. Eroldoğan, E. Evliyaoğlu, G. M. Turchini and X. G. Wu, Aquaculture, 2021, 534, 736316 CrossRef.
- A. Salvaggio, F. Marino, M. Albano, R. Pecoraro, G. Camiolo, D. Tibullo, V. Bramanti, B. M. Lombardo, S. Saccone, V. Mazzei and M. V. Brundo, Front. Physiol., 2016, 7, 153 Search PubMed.
- A. Karami, A. Golieskardi, C. K. Choo, N. Romano, Y. Bin Ho and B. Salamatinia, Sci. Total Environ., 2017, 578, 485–494 CrossRef CAS PubMed.
- Fisher Scientific, Fisher scientific [internet], 2020, Available from: https://www.fishersci.co.uk/gb/en/home.html, (accessed 8th December, 2020) Search PubMed.
- A. Stolte, S. Forster, G. Gerdts and H. Schubert, Mar. Pollut. Bull., 2015, 99, 216–229 CrossRef CAS PubMed.
- B. Quinn, F. Murphy and C. Ewins, Anal. Methods, 2017, 9, 1491–1498 RSC.
- M. Liu, Y. Song, S. Lu, R. Qiu, J. Hu, X. Li, M. Bigalke, H. Shi and D. He, Sci. Total Environ., 2019, 691, 341–347 CrossRef CAS PubMed.
- R. L. Coppock, M. Cole, P. K. Lindeque, A. M. Queirós and T. S. Galloway, Environ. Pollut., 2017, 230, 829–837 CrossRef CAS PubMed.
- M. Kedzierski, V. Le Tilly, G. César, O. Sire and S. Bruzaud, Mar. Pollut. Bull., 2017, 115, 120–129 CrossRef CAS PubMed.
- P. L. Corcoran, S. L. Belontz, K. Ryan and M. J. Walzak, Environ. Sci. Technol., 2020, 54, 818–825 CrossRef CAS PubMed.
- American Chemistry Council, Plastic Packaging Resin Identification Codes, https://plastics.americanchemistry.com/Plastic-Packaging-Resin-Identification-Codes/, (accessed 21st October, 2020) Search PubMed.
- British Plastics Federation, Plastipedia, https://www.bpf.co.uk/plastipedia/default.aspx, (accessed March 2020), 2020 Search PubMed.
- R. C. Thompson, Y. Olsen, R. P. Mitchell, A. Davis, S. J. Rowland, A. W. G. John, D. McGonigle and A. E. Russell, Science, 2004, 304, 838 CrossRef CAS PubMed.
- J. S. Hanvey, P. J. Lewis, J. L. Lavers, N. D. Crosbie, K. Pozo and B. O. Clarke, Anal. Methods, 2017, 9, 1369–1383 RSC.
- C. Way, M. D. Hudson, I. D. Williams and G. J. Langley, Sci. Total Environ., 2022, 805, 150227 CrossRef CAS PubMed.
- J. R. Centre, I. f. Environment and Sustainability, Marine litter: technical recommendations for the implementation of MSFD requirements, Publications Office, 2011 Search PubMed.
- H. K. Imhof, J. Schmid, R. Niessner, N. P. Ivleva and C. Laforsch, Limnol. Oceanogr. Methods, 2012, 10, 524–537 CrossRef CAS.
- M. Claessens, L. Van Cauwenberghe, M. B. Vandegehuchte and C. R. Janssen, Mar. Pollut. Bull., 2013, 70, 227–233 CrossRef CAS PubMed.
- M. Renzi, E. Grazioli and A. Blašković, Bull. Environ. Contam. Toxicol., 2019, 103, 367–373 CrossRef CAS PubMed.
- R. Sussarellu, M. Suquet, Y. Thomas, C. Lambert, C. Fabioux, M. E. J. Pernet, N. Le Goic, V. Quillien, C. Mingant, Y. Epelboin, C. Corporeau, J. Guyomarch, J. Robbens, I. Paul-Pont, P. Soudant and A. Huvet, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 2430–2435 CrossRef CAS PubMed.
- I. Salaberria, C. Nadvornik-Vincent, G. Monticelli, D. Altin and A. M. Booth, Mar. Pollut. Bull., 2020, 157, 111328 CrossRef CAS PubMed.
- R. C. Ropp, in Encyclopedia of the Alkaline Earth Compounds, ed. R. C. Ropp, Elsevier, Amsterdam, 2013, pp. 25–104, DOI: DOI:10.1016/B978-0-444-59550-8.00002-8.
- Peters Chemical Copmany, Properties of Calcium Chloride, https://www.peterschemical.com/properties-of-calcium-chloride/, (accessed 22 September, 2021) Search PubMed.
- F. Radford, L. M. Zapata-Restrepo, A. A. Horton, M. D. Hudson, P. J. Shaw and I. D. Williams, Anal. Methods, 2021, 13, 1695–1705 RSC.
- P. Vermeiren, C. Muñoz and K. Ikejima, Environ. Pollut., 2020, 262, 114298 CrossRef CAS PubMed.
- A. A. de Souza Machado, C. W. Lau, J. Till, W. Kloas, A. Lehmann, R. Becker and M. C. Rillig, Environ. Sci. Technol., 2018, 52, 9656–9665 CrossRef CAS PubMed.
- A. I. Catarino, R. Thompson, W. Sanderson and T. B. Henry, Environ. Toxicol. Chem., 2017, 36, 947–951 CrossRef CAS PubMed.
- M. G. J. Loder, H. K. Imhof, M. Ladehoff, L. A. Loschel, C. Lorenz, S. Mintenig, S. Piehl, S. Primpke, I. Schrank, C. Laforsch and G. Gerdts, Environ. Sci. Technol., 2017, 51, 14283–14292 CrossRef PubMed.
- C. J. Thiele, M. D. Hudson and A. E. Russell, Mar. Pollut. Bull., 2019, 142, 384–393 CrossRef CAS PubMed.
- H. Wang, C. Q. Wang, J. G. Fu and G. H. Gu, Waste Manag., 2014, 34, 309–315 CrossRef CAS PubMed.
- W. Pipkin, R. Belganeh, W. Robberson, H. Allen, A.-M. Cook and A. Watanabe, LCGC North Am., 2021, 39, 179–186 Search PubMed.
| This journal is © The Royal Society of Chemistry 2022 |

