A lysosomal targeted fluorescent probe based on coumarin for monitoring hydrazine in living cells with high performance†
Jin-Hua
Jiang
*a,
Zhi-Hao
Zhang
b,
Jianbo
Qu
b and
Jian-Yong
Wang
 *b
*b
aSchool of Aeronautics, Shandong Jiaotong University, Jinan, Shandong 250357, P. R. China. E-mail: jiangjinhua@sdjtu.edu.cn
bSchool of Light Industry and Engineering, Qilu University of Technology (Shandong Academy of Sciences), Jinan, 250353, P. R. China. E-mail: wjy@qlu.edu.cn
First published on 23rd November 2021
Abstract
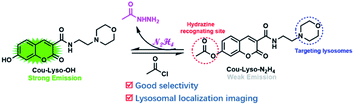
A lysosomal targeted fluorescent probe based on coumarin for monitoring hydrazine (N2H4) in living cells was designed and synthesised. The novel fluorescent probe Cou-Lyso-N2H4, in response to N2H4, exhibited good selectivity, low cytotoxicity, and lysosomal localization, which could be suitable for studying the harmfulness of N2H4 in subcellular organelles during various physiological processes.
Hydrazine (N2H4) possesses strong reducibility and highly reactive alkalinity due to the α-effect,1 and is widely used as missile and rocket fuel,2 reducing agent,3 polymer cross-linking agent,4 and raw material in medicine.5 On the other hand, hydrazine also shows good water solubility and high toxicity, however, excessive inhalation would bring a serious threat to human health and the environment. According to previous literatures, when people are often exposed to N2H4, it results in some diseases, such as liver injury,6 DNA damage,7 and even cancer.8 Furthermore, US-EPA and IARC defined the low threshold limit value (TLV) of N2H4 to be 10 ppb, that is, 0.31 μM.9 Therefore, seeking a simple detection method for real-time monitoring of hydrazine, especially in living cells, is important and practically significant for understanding the threat of hydrazine to people's health.
Until now, there have been many efficient methods for the determination of N2H4, such as electrochemical method10 and ultra-high-performance liquid chromatography-tandem mass spectrometry.11 However, these above methods require tedious operation and expensive instruments, and is difficult to achieve real-time detection. On the contrary, fluorescence imaging method is popular due to some merits, including low cost, simple operation, high sensitivity, short detection time, and in situ imaging.12–15 In the past few years, many fluorescent probes based on different recognizing sites toward N2H4 have been reported. The previous probes were mainly divided into the following categories: N2H4-triggered cleavage of an acetyl group,16–18 N2H4-triggered cleavage of a 4-bromobutyrate moiety,19–22 N2H4-triggered elimination of an acrylonitrile group,23–26 N2H4-triggered Schiff-base reaction,27–29 N2H4-triggered cyclization reaction,30 N2H4-triggered elimination of 2-benzothiazole acetonitrile,31,32 and others.33,34 Nevertheless, a few of these probes possessed poor selectivity, and they also detected other ions at the same time. So, developing a novel fluorescent probe with good selectivity for monitoring hydrazine is very interesting.
It is a pity that only few organelle-targeted fluorescent hydrazine-probes have been reported, and they are mainly located in the mitochondria.35–39 As far as we know, studies on how hydrazine is distributed in living cells are not complete; therefore, developing a novel fluorescent probe, which could monitor the level of hydrazine in organelles would be helpful to deeply understand the biomechanism of hydrazine poisoning to further guide the diagnosis and treatment of hydrazine poisoning. Among all organelles, lysosomes play critical roles in some physical activities, not only digesting most of biomolecules and cellular debris, but also regulating growth factors and repairing plasma membrane.40,41 Moreover, the redox homeostasis in the lysosomes can be regulated by antioxidants, such as hydrazine from drugs. In other words, developing a lysosomal targeted fluorescent probe for monitoring hydrazine to understand the chemical and biological properties of hydrazine in lysosomes would be significant for guiding the treatment of hydrazine poisoning.
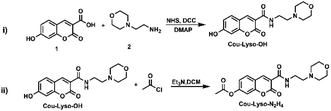
In this study, a novel lysosome-targeted fluorescent probe Cou-Lyso-N2H4 with good selectivity for imaging N2H4 was designed and constructed (Scheme 2), and the characterization of the probe Cou-Lyso-N2H4 is depicted in the ESI† by 1H NMR, 13C NMR and HRMS. As shown in Scheme 1, we used the morpholine unit as an effective lysosome-targeted group due to its alkaline nature, which guided the probe to lysosomes. Furthermore, coumarin was used as a fluorophore, which possesses a high fluorescence quantum yield due to an intramolecular charge transfer (ICT) mechanism, and an ethoxycarbonyl group as the recognition site to N2H4, which could be specifically hydrolyzed by hydrazine. This turn-on fluorescent probe Cou-Lyso-N2H4 exhibited advantages of good selectivity and low cytotoxicity, and was applied to monitor hydrazine in lysosomes successfully with a high Pearson's coefficient of 0.8674, which might be a useful method to monitor the functions of N2H4 in various physiological processes.
The photophysical properties of probe Cou-Lyso-N2H4 were studied first. As shown in Fig. S1,† the absorption band of Cou-Lyso-N2H4 was at around 335 nm; when the different concentrations of N2H4 were added to the reaction system, the initial maximum absorption peak at 335 nm decreased gradually, and a new absorption peak appeared at 410 nm at the same time, which indicated the generation of Cou-Lyso-OH as hydrazine deprotected the acetyl group. In addition, Fig. 1 shows that Cou-Lyso-N2H4 had almost no fluorescence at 450 nm in PBS buffer (pH = 7.4) and DMSO (v/v = 4/1) in the absence of N2H4, but fluorescence emission at 450 nm (λex = 410 nm) increased significantly with the addition of N2H4. Moreover, the fluorescence intensity of probe Cou-Lyso-N2H4 at 450 nm showed a good linear relationship with the concentration of N2H4 from 20 to 70 μM (Fig. S3†). The corresponding detection limit was calculated to be 3.93 μM with the equation of 3σ/k, where k is the slope and σ is the standard deviation of the blank sample.
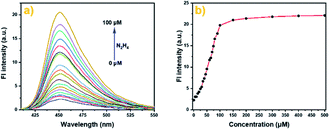 | ||
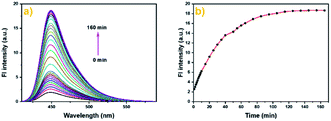
| Fig. 1 The fluorescence spectra (a) and fluorescence intensity (b) of Cou-Lyso-N2H4 (10 μM) in pH 7.4 PBS buffer (containing 20% DMSO) in the absence or presence of N2H4 (0–50 equiv.). | ||
Furthermore, we also studied the time-dependent fluorescence response. As illustrated in Fig. 2a and b, the fluorescence intensity at 450 nm exhibited a significant enhancement after the addition of N2H4 (10.0 equiv.) within 60 min, then remained almost steady after about 100 min, and the maximum increase was about 9-fold. The result suggested that Cou-Lyso-N2H4 could detect hydrazine with high sensitivity.
In addition, the possible sensing mechanism was studied by means of mass spectrometry. When the probe Cou-Lyso-N2H4 (10 μM) was dissolved in PBS buffer (pH = 7.4) and DMSO (v/v = 4/1), an excess of N2H4 were added to previous solvent. The spectra showed that there was a clear peak at m/z 319.1295 corresponding to the Cou-Lyso-N2H4-adduct, which was Cou-Lyso-OH (Fig. S10†). In addition, the absorption spectra and fluorescence spectra (Fig. S2†) of the dye Cou-Lyso-OH and the probe Cou-Lyso-N2H4 before and after adding N2H4 were obtained. The result was that the peak of the dye Cou-Lyso-OH was consistent with the newly appeared peak, which was from the probe Cou-Lyso-N2H4 after addition of N2H4, indicating that the proposed mechanism is correct (Scheme 1).
Another important factor, the pH value of PBS buffer, was investigated, which might have significant impact on the response between Cou-Lyso-N2H4 and N2H4. As shown in Fig. 3, we measured the fluorescence spectra of probe Cou-Lyso-N2H4 (10 μM) in the absence and presence of N2H4 (10.0 equiv.) in the range from 3.5 to 11.0. Obviously, the probe Cou-Lyso-N2H4 itself was stable, and there was almost no change in a wide pH range from 3.5 to 11.0 in the absence of N2H4. However, the fluorescence intensity of probe Cou-Lyso-N2H4 increased significantly in pH ranges from 3.5 to 4.5, the fluorescence intensity increased sharply from 4.5 to 7.5, and remained steady from 8.5 to 11.0 upon addition of hydrazine, which indicated that the probe Cou-Lyso-N2H4 was suitable for detecting hydrazine in acidic subcellular organelle lysosomes so as to study the harmfulness of N2H4 in subcellular organelles during various physiological processes, and the probe Cou-Lyso-N2H4 was potential for monitoring hydrazine in a relatively wide pH range especially in a weak alkaline physiological environment.
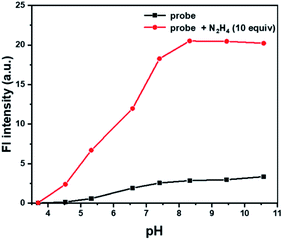 | ||
| Fig. 3 The pH effects on the fluorescence spectra of Cou-Lyso-N2H4 (10 μM) at different pH values of PBS buffer (containing 20% DMSO) in the absence (■) or presence (●) of N2H4 (10 equiv.). | ||
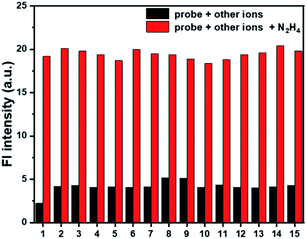
To evaluate whether probe Cou-Lyso-N2H4 could effectively work in a complex environment, the selectivity of the probe was investigated. Various analytes including metal ions (Al3+, Ca2+, K+, Mg2+, Cu2+, Na+), anions (Cl−, CO32−, I−, SO32−), nucleophilic biological species (Cys, GSH, Ala), and esterase were measured. As listed in Fig. 4, the fluorescence intensity was basically unchanged with the addition of other test species. On the contrary, with the addition of N2H4, there was a significant fluorescence change, which indicated that the probe possessed good selectivity. In addition, we performed the selectivity test together with competitive ions to further evaluate the anti-interference characteristics of the probe Cou-Lyso-N2H4. As shown in Fig. 4, when hydrazine and other analytes co-existed, all of the selected species had no interference in the detection of hydrazine, and the fluorescence intensity of the probe still increased significantly, which indicated that the probe possessed a strong anti-interference ability. These results showed that the Cou-Lyso-N2H4 probe could be applied to monitor N2H4 with good selectivity even in the presence of other species.
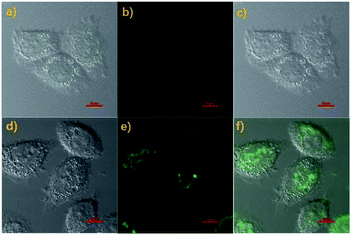
With the above good properties of probe Cou-Lyso-N2H4 in hands, we thought that the probe Cou-Lyso-N2H4 could be suitable for detecting N2H4 in a living biosystem as it is highly sensitive and selective. Firstly, the MTT assay of Cou-Lyso-N2H4 was carried out. As illustrated in Fig. S3,† the HeLa cell survival rate remained more than 85% after treating with Cou-Lyso-N2H4 for 24 h even at high concentrations (30.0 μM), which indicated that Cou-Lyso-N2H4 possessed low cytotoxicity to living cells, and could be suitable for monitoring N2H4 in living cells. Then, the capability of Cou-Lyso-N2H4 for imaging N2H4 in HeLa cells was investigated. As depicted in Fig. 5, the living HeLa cells were incubated with 10 μM Cou-Lyso-N2H4 for 30 min at 37 °C, and washed three times by PBS buffer for removing the excess of probe Cou-Lyso-N2H4. As shown in Fig. 5b, the HeLa cells incubated only with Cou-Lyso-N2H4 exhibited almost no fluorescence in the green channel. However, after further treating with N2H4 (100 μM) for another 30 min, a strong green fluorescence signal emerged obviously (Fig. 5e). Therefore, the probe Cou-Lyso-N2H4 exhibited good membrane permeability and possessed ability to image exogenous N2H4 in living HeLa cells.
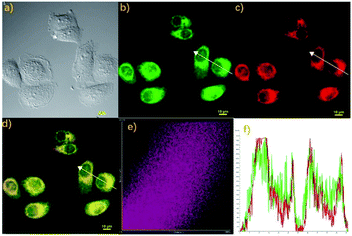
Finally, we also studied the localization property of the probe Cou-Lyso-N2H4 due to the above ideal imaging capability to N2H4. The feasibility of Cou-Lyso-N2H4 to target lysosomes was investigated in living HeLa cells. As illustrated in Fig. 6, the living HeLa cells were stained with probe Cou-Lyso-N2H4 (10 μM) and N2H4 for 30 min at 37 °C initially, and were further co-stained with the commercially available Lysosome-Tracker (2.0 mM) for another 30 min. As shown in Fig. 6b and c, the HeLa cells treated with Cou-Lyso-N2H4 and N2H4 showed a strong green fluorescence signal, and after further incubation with Lysosome-Tracker, the cells exhibited red fluorescence in red channel. Furthermore, the merged images indicated that red fluorescence overlapped well with the green fluorescence. Moreover, the intensity distribution of the linear regions of interest in HeLa cells stained with Lysosome-Tracker and Cou-Lyso-N2H4 was almost the same (Fig. 6f). Notably, the Pearson's coefficient was 0.8674 in HeLa cells, suggesting that Cou-Lyso-N2H4 was located at the lysosomes. With the above excellent feature of lysosome targeting in hands, this novel probe Cou-Lyso-N2H4 possessed a huge potential for imaging N2H4 in living HeLa cells.
In summary, a lysosomal targeted organic fluorescent probe Cou-Lyso-N2H4 for the detection of N2H4 was constructed. The ideal probe Cou-Lyso-N2H4 exhibited good properties, including good selectivity and low cytotoxicity. In addition, the probe Cou-Lyso-N2H4 was used for monitoring N2H4 in lysosomes successfully. Hence, the lysosomal targeted probe Cou-Lyso-N2H4 might be applied to other functional bio-probes for the recognition of different analytes and to investigate biological and pathological connection of N2H4 in living bio-samples.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21801145), the Shandong Provincial Key R&D Program/Major Science and Technology Innovation Project of Shandong Province (2019JZZY020230), the project ZR2017BB012 supported by Shandong Provincial Natural Science Foundation and the Doctoral Scientific Research Foundation of Shandong Jiaotong University (BS2018019).Notes and references
- Y. Ren and H. Yamataka, J. Org. Chem., 2007, 72, 5660–5667 CrossRef CAS PubMed.
- Y. Kao, C. Chong, W. Ng and D. Lim, Occup. Med., 2007, 57, 535–537 CrossRef PubMed.
- N. Nobari, M. Behboudnia and R. Maleki, Appl. Surf. Sci., 2016, 385, 9–17 CrossRef CAS.
- T. Campbell, V. Foldi and J. Farago, J. Appl. Polym. Sci., 1959, 2, 155–162 CrossRef CAS.
- H. Ioannidou and P. Koutentis, Tetrahedron, 2009, 65, 7023–7037 CrossRef CAS.
- T. Leakakos and R. Shank, Toxicol. Appl. Pharmacol., 1994, 126, 295–300 CrossRef CAS PubMed.
- M. Runge-Morris, N. Wu and R. Novak, Toxicol. Appl. Pharmacol., 1994, 125, 123–132 CrossRef CAS PubMed.
- P. Vivekanandan, K. Gobianand, S. Priya, P. Vijayalakshmi and S. Karthikeyan, Drug Chem. Toxicol., 2007, 30, 241–252 CrossRef CAS PubMed.
- K. Nguyen, Y. Hao, W. Chen, Y. Zhang, M. Xu, M. Yang and Y. Liu, Luminescence, 2018, 33, 816–836 CrossRef PubMed.
- S. Sharma, S. Ganeshan, P. Pattnaik, S. Kanungo and K. Chappanda, Mater. Lett., 2020, 262, 127150 CrossRef CAS.
- J. Oh and H. Shin, J. Chromatogr. A, 2015, 1395, 73–78 CrossRef CAS PubMed.
- F. Cai, B. Hou, S. Zhang, H. Chen, S. Ji, X. Shen and H. Liang, J. Mater. Chem. B, 2019, 7, 2493–2498 RSC.
- J. Hou, B. Wang, S. Wang, Y. Wu, Y. Liao and W. Ren, Dyes Pigm., 2020, 178, 108366 CrossRef CAS.
- S. Zhang, H. Chen, L. Wang, C. Liu, L. Liu, Y. Sun and X. Shen, J. Mater. Chem. B, 2021, 9, 1089 RSC.
- S. Zhang, H. Chen, L. Wang, X. Qin, B. Jiang, S. Ji, X. Shen and H. Liang, Angew. Chem., Int. Ed., 2021 DOI:10.1002/anie.202107076.
- C. Liu, K. Liu, M. Tian and W. Lin, Spectrochim. Acta, Part A, 2019, 212, 42–47 CrossRef CAS PubMed.
- S. Guo, Z. Guo, C. Wang, Y. Shen and W. Zhu, Tetrahedron, 2019, 75, 2642–2646 CrossRef CAS.
- X. Shi, F. Huo, J. Chao, Y. Zhang and C. Yin, New J. Chem., 2019, 43, 10025–10029 RSC.
- H. Lv, H. Sun, S. Wang and F. Kong, Spectrochim. Acta, Part A, 2018, 196, 160–167 CrossRef CAS PubMed.
- Q. Wu, J. Zheng, W. Zhang, J. Wang, W. Liang and F. Stadler, Talanta, 2019, 195, 857–864 CrossRef CAS PubMed.
- M. Zhu, Y. Xu, L. Sang, Z. Zhao, L. Wang, X. Wu, F. Fan, Y. Wang and H. Li, Environ. Pollut., 2020, 256, 113427 CrossRef CAS PubMed.
- K. Wang, Y. Ye, C. Jiang, M. Guo, X. Zhang, A. Jiang, Y. Yang, Q. Jiao and H. Zhu, Dyes Pigm., 2021, 194, 109587 CrossRef CAS.
- Z. Li, M. Ren, L. Wang and W. Lin, Anal. Methods, 2018, 10, 4016–4019 RSC.
- J. Wu, J. Pan, Z. Ye, L. Zeng and D. Su, Sens. Actuators, B, 2018, 274, 274–284 CrossRef CAS.
- J. Qu, Z. Zhang, H. Zhang, Z. Weng and J. Wang, Front. Chem., 2021, 8, 1263 Search PubMed.
- S. Mu, H. Gao, C. Li, S. Li, Y. Wang, Y. Zhang, C. Ma, H. Zhang and X. Liu, Talanta, 2021, 221, 121606 CrossRef CAS PubMed.
- W. Xu, W. Liu, T. Zhou, Y. Yang and W. Li, J. Photochem. Photobiol., A, 2018, 356, 610–616 CrossRef CAS.
- Y. Jung, I. Ju, Y. Choe, Y. Kim, S. Park, Y. Hyun, M. Oh and D. Kim, ACS Sens., 2019, 4, 441–449 CrossRef CAS PubMed.
- X. Li, M. Li, Y. Chen, G. Qiao, Q. Liu, Z. Zhou, W. Liu and Q. Wang, Chem. Eng. J., 2021, 415, 128975 CrossRef CAS.
- R. Chen, G. Shi, J. Wang, H. Qin, Q. Zhang, S. Chen, Y. Wen, J. Guo, K. Wang and Z. Hu, Spectrochim. Acta, Part A, 2021, 252, 119510 CrossRef CAS PubMed.
- T. Zhang, L. Zhu and W. Lin, Dyes Pigm., 2021, 188, 109177 CrossRef CAS.
- X. Kong, M. Li, Y. Zhang, Y. Yin and W. Lin, Sens. Actuators, B, 2021, 329, 129232 CrossRef CAS.
- B. Zhu, X. Wu, J. Rodrigues, X. Hu, R. Sheng and G. Bao, Spectrochim. Acta, Part A, 2021, 246, 118953 CrossRef CAS PubMed.
- N. Rao, Y. Le, D. Li, Y. Zhang, Q. Wang, L. Liu and L. Yan, Chem. Pap., 2021, 1–9 Search PubMed.
- Y. Ran, H. Xu, K. Li, K. Yu, J. Yang and X. Yu, RSC Adv., 2016, 6, 111016–111019 RSC.
- B. Shi, Y. He, P. Zhang, Y. Wang, M. Yu, H. Zhang, L. Wei and Z. Li, Dyes Pigm., 2017, 147, 152–159 CrossRef CAS.
- Y. Ban, R. Wang, Y. Li, Z. An, M. Yu, C. Fang, L. Wei and Z. Li, New J. Chem., 2018, 42, 2030–2035 RSC.
- X. Kong, B. Dong, C. Wang, N. Zhang, W. Song and W. Lin, J. Photochem. Photobiol., A, 2018, 356, 321–328 CrossRef CAS.
- B. Wang, R. Yang and W. Zhao, J. Hazard. Mater., 2021, 406, 124598 Search PubMed.
- C. Renfrew and A. Hubbard, J. Biol. Chem., 1991, 266, 21265–21273 CrossRef CAS.
- M. Marks, P. Roche, E. Donselaar, L. Woodruff, P. Peters and J. Bonifacino, J. Cell Biol., 1995, 131, 351–369 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ay01821c |
| This journal is © The Royal Society of Chemistry 2022 |