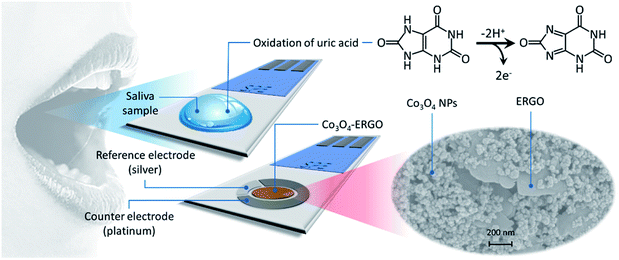
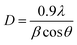An electrochemical sensor based on a Co3O4–ERGO nanocomposite modified screen-printed electrode for detection of uric acid in artificial saliva†
Gizem
Turkkan
a,
Salih Zeki
Bas
 *a,
Keziban
Atacan
*a,
Keziban
Atacan
 b and
Mustafa
Ozmen
b and
Mustafa
Ozmen
 *a
*a
aDepartment of Chemistry, Selcuk University, 42250, Konya, Turkey. E-mail: musozmen@gmail.com; salihzekibas@gmail.com
bBiomedical, Magnetic and Semiconductor Materials Application and Research Center (BIMAS-RC), Sakarya University, 54187, Sakarya, Turkey
First published on 25th November 2021
Abstract
In this study, we report the fabrication of a nanocomposite consisting of Co3O4 nanoparticles (Co3O4 NPs) and electrochemically reduced graphene oxide (ERGO) on a screen-printed electrode (SPE) and its sensing performance in the electrochemical detection of uric acid (UA). The surface modification of the electrode was confirmed by using a variety of characterization techniques (FE-SEM, XRD, AFM, EDX, WCA, FTIR, and Raman spectroscopy). In addition, the surface modification was electrochemically characterized step by step through CV, EIS and DPV techniques, and the results showed that the Co3O4–ERGO nanocomposite exhibited highly sensitive and selective sensing performance towards the oxidation of UA in 0.1 M (pH 7.0) phosphate buffer solution (PBS). The sensor (Co3O4–ERGO/SPE) signals were observed to be linear to the UA concentration in the range of 5 μM to 500 μM (R2 = 0.9985). After revealing its other performance characteristics, such as repeatability, reproducibility, stability, sensitivity, and selectivity, the sensor was successfully applied to the analysis of UA in artificial saliva samples.
1. Introduction
Human saliva is basically a fluid secreted from the salivary glands, keeping the oral cavity moist and lining the teeth together with mucosa.1 Although human saliva is composed of 98% water, the remaining 2% is made up of many important substances such as electrolytes (sodium, potassium, magnesium, calcium, chloride, bicarbonate, phosphate, and iodine), mucus, antibacterial compounds (thiocyanate, hydrogen peroxide, and IgA), and various enzymes (lysozyme, α-amylase, lingual lipase, and lactoferrin).2,3 In addition, saliva, which correlates strongly with blood components, provides an alternative to direct blood analysis.4,5 One of these components is uric acid (UA), which is found in a lower concentration in saliva than in blood serum. UA is the end product of the metabolic degradation of purines in the human body. In healthy individuals, the normal concentration of UA in the blood is in the range of 240–520 μM.6 The abnormal concentration of UA in a human body may cause several diseases, such as gout, hyperuricemia, and Lesch–Nyhan syndrome,7 and poses a risk for obesity,8 cardiovascular disease,9 and kidney disease.10 Therefore, it is very important to determine UA, which is a critical biomarker in biological fluids. In addition to various conventional methods,11–16 the electrochemical techniques17,18 are also used for the determination of UA. Among these methods, electrochemical sensing systems exhibit an effective approach to target analyte detection due to their advantages such as fast response, high sensitivity, and easy applicability to the sample.19,20 Also, different sensing platforms applied to saliva samples have been reported for the detection of UA.6,21 However, the development of electrochemical (bio)sensors applicable to biological fluids for UA detection with high sensitivity and selectivity is still ongoing.In this work, we aimed to design a new electrochemical sensor to electrochemically detect UA in saliva samples using a screen-printed electrode (SPE) that allows detection of the analyte in a few drops of samples as well as point-of-care testing. The SPE was modified with ERGO and Co3O4 NPs to improve its sensing performance towards the electrooxidation of UA. After the structural and the morphological characterization of Co3O4–ERGO, its current response was examined in detail utilizing EIS, CV, and DPV techniques. As a form of graphene oxide (GO), reduced graphene oxide (rGO) is processed using chemical,19 electrochemical, thermal, and other methods to reduce oxygen-containing functional groups in the structure of GO. Recently, electrochemical reduction of GO to produce graphene has shown great promise since it can be performed without any hazardous reagents.22,23 In addition, the electrochemical reduction method is more environmentally friendly and cost-effective, and more appropriate for making rGO sheets with fewer defects.23
A wide variety of nanomaterials are used as modifiers in the design of an electrochemical sensor. For example, metal nanoparticles,24,25 various metal oxide nanoparticles,26 carbonaceous nanomaterials,27,28 and nanocomposites29,30 provide many advantages such as high surface-to-volume ratio and strong catalytic properties to the electrochemical sensor. Co3O4 as a convenient material has also received significant attention in sensors, batteries, water splitting, catalysis and energy storage31 due to its remarkable oxidation ability, nontoxicity, chemical stability, and low cost.32,33 The composites based on Co3O4 can also be formed with various supports such as graphene,31,34 TiO2,35 g-C3N4,36 and MoS2 (ref. 37) to improve the activity and stability of Co3O4. For example, Ezhil Vilian and coworkers34 reported MOF derived RGO–Co3O4 to construct a non-enzymatic glucose biosensor. Herein, Co3O4–ERGO was utilized as a modification material for the electrochemical determination of UA. Co3O4 NPs can easily catalyze the oxidation reaction of UA, thereby amplifying the electrochemical signal of the sensor for UA detection. UA molecules are catalytically oxidized by CoOOH as a catalyst to dehydrourate (1H-purine-2,6,8(3H)-trione) molecules.38
| Co3O4 + OH− + H2O → 3CoOOH + e− |
By using reduced graphene oxide, an efficient electrical network can be provided through Co3O4 anchored directly on the surface of reduced graphene oxide. This can increase the electron transfer rate and accordingly improve the sensitivity of the sensor.39 In the light of these literature reports, the Co3O4–ERGO modified SPE (Co3O4–ERGO/SPE) shows a linearity in the concentration range of 5 μM to 500 μM with a detection limit of 1.5 μM. Furthermore, the electrochemical results reveal that Co3O4–ERGO/SPE can be practically applied for the determination of UA in artificial saliva samples. As far as we can ascertain, no study has been found on the use of Co3O4–ERGO for the electrochemical determination of UA. Compared to similar sensing studies, this study presents some new approaches for UA detection in terms of both the modification step and the use of a new generation combined electrode that allows the use of a small amount of sample.
2. Experimental
2.1. Chemicals and apparatus
Chemicals and apparatus are detailed in the ESI.†2.2. Synthesis of Co3O4 NPs
Co3O4 NPs were prepared according to the procedure of Shao et al.36 2.8 g of cobalt nitrate hexahydrate was dissolved in 30 mL water under stirring at 200 rpm. Then, 60 mL of NaOH (0.33 M) and 8 mL of ammonia solution were added. The solution was placed in a stainless steel autoclave and heated at 120 °C for 12 h. Subsequently, the mixture was centrifuged, washed, and dried at 70 °C. The dried Co3O4 NPs were annealed at 450 °C for 3 h.2.3. Preparation of Co3O4–ERGO/SPE
Graphene oxide (GO) was prepared by oxidizing graphite according to the synthesis steps reported by Marcano et al.40 Then, GO was dispersed in 0.1 M PBS with the aid of ultrasonic treatment to prepare a final concentration of 1 mg mL−1. After a 150 μL volume of the GO dispersion was dropped onto the SPE surface, reduction was performed using the CV technique with a scanning rate of 50 mV s−1 in the potential range of −1.5 V to 0 V.41 After the electrochemical reduction step, the GO drop on the surface of the SPE was intently removed, and the ERGO modified SPE was quickly cleaned with distilled water. To obtain Co3O4–ERGO/SPE, a dispersed mixture of Co3O4 (1 mg mL−1) and GO (1 mg mL−1) was used following the same route. Scheme 1 shows a schematic illustration of the modified SPE used in the electrochemical studies and the oxidation mechanism of UA42 on the electrode surface.3. Results and discussion
3.1. Characterization
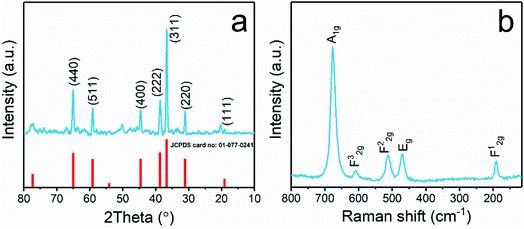
Fig. 1a shows the XRD patterns of the synthesized Co3O4 nanoparticles, and the characteristic diffraction lines of the cubic spinel Co3O4 phase (JCPDS card no. 01-077-0241) were observed. The sharp diffraction peaks indicated that the Co3O4 nanoparticles possessed good crystallinity. The strong reflections at 2θ values of 19.2, 31.1, 36.7, 38.6, 44.6, 59.2, and 65.1 degrees were attributed to the typical diffraction peaks of spinel Co3O4.43 Raman spectra were also recorded to confirm the structure of the Co3O4 nanoparticles and to gain more information about the oxide lattice distortion. As seen in Fig. 1b, five bands were found corresponding to the A1g, 3 F2g, and Eg Raman-active modes of the Co3O4 spinel structure.44 Of these, approximately 190 and 680 cm−1 can be attributed to the tetrahedral and octahedral Co regions, respectively.45The average crystal size of Co3O4 NPs was calculated using the Scherrer equation:46,47
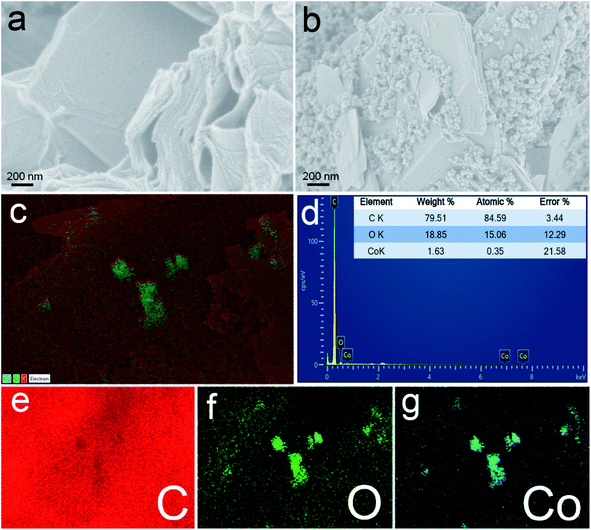
FE-SEM analysis was performed to study the morphology of the prepared surfaces. The FE-SEM image of ERGO (Fig. 2a) shows the sheet like structure. On the other hand, Co3O4 nanoparticles are seen well deposited and distributed between the ERGO sheets (Fig. 2b). The distribution of elements present on the Co3O4–ERGO/SPE surface was examined by energy-dispersive X-ray spectroscopy (EDX) elemental mapping analysis. C (red color), O (green color), and Co (blue color) elements were observed as shown in the EDX mapping profile of the Co3O4–ERGO/SPE surface (Fig. 2c). The EDX spectrum of the Co3O4–ERGO/SPE surface (Fig. 2d) shows elemental C, O, and Co signatures, which confirms the material on the electrode surface. In addition, the independent elemental C, O, and Co distributions are shown in Fig. 2e, f and g.48
The surface morphologies of the prepared ERGO/SPE and Co3O4–ERGO/SPE were also examined via AFM. The 2D and 3D AFM images of the ERGO/SPE (Fig. 3a and b) exhibit a typical sheet-like morphology of the reduced graphene and the average roughness (Ra) and root mean square (rms) values are 144.66 nm and 189.168 nm, respectively. After deposition of Co3O4, the AFM images of the Co3O4–ERGO/SPE (Fig. 3c and d) show the decrease in roughness (Ra = 64.85 nm, rms = 80.54 nm) over the ERGO sheets due to the presence of smooth and nanostructured Co3O4 nanoparticles. It can be clearly seen that the intermediate spaces are covered by the Co3O4 nanoparticles compared with ERGO/SPE.
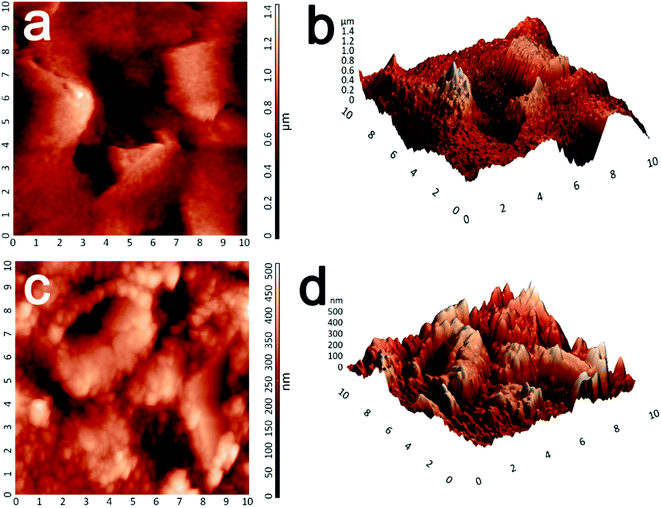 | ||
| Fig. 3 Two- and three-dimensional AFM images of ERGO/SPE (a and b) and Co3O4–ERGO/SPE (c and d) surfaces. | ||
The investigations on the presence of functional groups in ERGO/SPE and Co3O4–ERGO/SPE were done by FTIR analysis and are shown in Fig. S1.† In the FTIR spectrum of ERGO/SPE, the peak at around 3300 cm−1 belonging to the hydroxyl functionality has completely disappeared. The peaks at around 1030, 1380, 1450, and 1730 cm−1 correspond to the alkoxy (–C–O), alkenyl (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), carboxyl (–COOH), and ketone (–C
C), carboxyl (–COOH), and ketone (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups, respectively. In the case of ERGO, either the intensity of these peaks is lower or these peaks disappear.49 This shows that the electrochemical reduction process has been carried out successfully. Furthermore, the Co3O4–ERGO/SPE surface exhibits two absorption peaks at 662 cm−1 and 582 cm−1 associated with the Co–O stretching vibrations.50
O) groups, respectively. In the case of ERGO, either the intensity of these peaks is lower or these peaks disappear.49 This shows that the electrochemical reduction process has been carried out successfully. Furthermore, the Co3O4–ERGO/SPE surface exhibits two absorption peaks at 662 cm−1 and 582 cm−1 associated with the Co–O stretching vibrations.50
The Raman spectrum of the Co3O4–ERGO/SPE surface (Fig. S2†) displays two prominent peaks, a G-peak at 1578 cm−1 along with a D-peak at 1311 cm−1 that belongs to ERGO. The ratio of ID/IG is found to be 1.106. The peaks corresponding to Co3O4 (192, 472, 516, 613, and 685 cm−1) are also present in the prepared sensor, which is evidence for existence of Co3O4 in ERGO.51,52
To prove the formation of films on the SPE, the change of wettability characteristics on the surface can be observed by measuring the water contact angle (WCA).53 The wetting characteristics of bare SPE, GO/SPE, ERGO/SPE, and Co3O4–ERGO/SPE surfaces are illustrated in Fig. S3.† The water contact angles were measured to be 127°, 37°, 125°, and 122° for bare SPE, GO/SPE, ERGO/SPE, and Co3O4–ERGO/SPE, respectively.
3.2. Electrochemical characterization
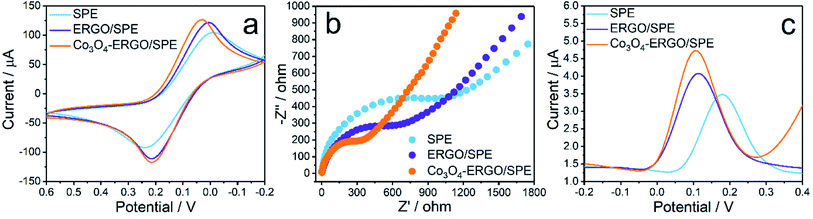
Each modification step in the preparation of the proposed sensor was characterized by CV, DPV, and EIS techniques, respectively. Fig. 4a shows the CVs of SPE, ERGO/SPE, and Co3O4–ERGO/SPE in 5.0 mM K3Fe(CN)6/K2Fe(CN)6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution with 0.1 M KCl as a supporting electrolyte. The two well-defined redox peaks with ΔEp of 251 mV, ΔEp of 210 mV, and ΔEp of 184 mV were obtained for SPE, ERGO/SPE, and Co3O4–ERGO/SPE, respectively. Co3O4–ERGO/SPE showed a smaller peak-to-peak separation than both SPE and ERGO/SPE. Additionally, Co3O4–ERGO/SPE exhibited a maximum enhanced peak current compared to ERGO/SPE and Co3O4–ERGO/SPE, which can be attributed to an easier electron transfer process between the electrode and Fe(CN)63−/4−.54 The electrochemical surface areas of SPE, ERGO/SPE, and Co3O4–ERGO/SPE were calculated by the use of the Randles–Sevcik formula.55 The effective surface areas of SPE, ERGO/SPE, and Co3O4–ERGO/SPE were found to be 0.080, 0.129 and 0.145 cm2, respectively. According to the results, the effective surface area of Co3O4–ERGO/SPE is maximum as compared with that of other electrodes, which indicates that Co3O4–ERGO/SPE provides an effective sensor performance towards the electrooxidation of UA. In order to investigate the electron transfer ability of the modified SPEs, the Nyquist plots (Fig. 4b) of bare SPE, ERGO modified SPE, and Co3O4–ERGO modified SPE were obtained in a 0.1 M KCl solution in the presence of 5.0 mM K3Fe(CN)6/K2Fe(CN)6 (1
1) solution with 0.1 M KCl as a supporting electrolyte. The two well-defined redox peaks with ΔEp of 251 mV, ΔEp of 210 mV, and ΔEp of 184 mV were obtained for SPE, ERGO/SPE, and Co3O4–ERGO/SPE, respectively. Co3O4–ERGO/SPE showed a smaller peak-to-peak separation than both SPE and ERGO/SPE. Additionally, Co3O4–ERGO/SPE exhibited a maximum enhanced peak current compared to ERGO/SPE and Co3O4–ERGO/SPE, which can be attributed to an easier electron transfer process between the electrode and Fe(CN)63−/4−.54 The electrochemical surface areas of SPE, ERGO/SPE, and Co3O4–ERGO/SPE were calculated by the use of the Randles–Sevcik formula.55 The effective surface areas of SPE, ERGO/SPE, and Co3O4–ERGO/SPE were found to be 0.080, 0.129 and 0.145 cm2, respectively. According to the results, the effective surface area of Co3O4–ERGO/SPE is maximum as compared with that of other electrodes, which indicates that Co3O4–ERGO/SPE provides an effective sensor performance towards the electrooxidation of UA. In order to investigate the electron transfer ability of the modified SPEs, the Nyquist plots (Fig. 4b) of bare SPE, ERGO modified SPE, and Co3O4–ERGO modified SPE were obtained in a 0.1 M KCl solution in the presence of 5.0 mM K3Fe(CN)6/K2Fe(CN)6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The electron transfer resistance (Rct) values which are correlated with the diameter of the semicircles can be found to be 500 Ω for Co3O4–ERGO/SPE, 700 Ω for ERGO/SPE and 1250 Ω for the SPE, respectively. The smaller Rct means that the Co3O4–ERGO nanocomposite exhibits a lower electron transfer resistance and a higher electron transfer ability.56Fig. 4c shows the DPV peaks of bare SPE, ERGO/SPE, and Co3O4–ERGO/SPE in 0.1 M PBS (pH 7.0) in the presence of 90 μM UA. The peak potential obtained at the Co3O4–ERGO modified SPE is 0.108 V, which is a more negative potential than those of other electrodes (0.112 V for the ERGO/SPE and 0.180 V for the SPE). Also, Co3O4–ERGO/SPE has a higher peak response than both bare SPE and ERGO modified SPE, which is due to the enhanced electron transfer rate.
1). The electron transfer resistance (Rct) values which are correlated with the diameter of the semicircles can be found to be 500 Ω for Co3O4–ERGO/SPE, 700 Ω for ERGO/SPE and 1250 Ω for the SPE, respectively. The smaller Rct means that the Co3O4–ERGO nanocomposite exhibits a lower electron transfer resistance and a higher electron transfer ability.56Fig. 4c shows the DPV peaks of bare SPE, ERGO/SPE, and Co3O4–ERGO/SPE in 0.1 M PBS (pH 7.0) in the presence of 90 μM UA. The peak potential obtained at the Co3O4–ERGO modified SPE is 0.108 V, which is a more negative potential than those of other electrodes (0.112 V for the ERGO/SPE and 0.180 V for the SPE). Also, Co3O4–ERGO/SPE has a higher peak response than both bare SPE and ERGO modified SPE, which is due to the enhanced electron transfer rate.
3.3. Optimization study
Several important parameters such as pH of the buffer solution, Co3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ERGO ratio, and Co3O4–ERGO composite thickness were optimized to obtain more selective and sensitive detection of UA. First, the effect of the Co3O4
ERGO ratio, and Co3O4–ERGO composite thickness were optimized to obtain more selective and sensitive detection of UA. First, the effect of the Co3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ERGO ratio on the current signal of 60 μM UA at the proposed sensor was examined using various electrodes fabricated with various ratios (0.5
ERGO ratio on the current signal of 60 μM UA at the proposed sensor was examined using various electrodes fabricated with various ratios (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 3
1, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of Co3O4
1) of Co3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ERGO. As shown in Fig. 5a, Co3O4–ERGO/SPE fabricated at a ratio of 1
ERGO. As shown in Fig. 5a, Co3O4–ERGO/SPE fabricated at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 possessess the highest DPV signal. In the following ratios, the peak currents decreased, which indicates that the decrease in the amount of ERGO in the composite composition decreases the sensitivity of Co3O4–ERGO/SPE. As another parameter, the effect of the Co3O4–ERGO composite thickness on the current response of the proposed sensor was examined with various electrodes prepared by changing the number of scan cycles (between 5 and 35) in the potential range of −1.5 to 0 (Fig. 5b). The peak current of Co3O4–ERGO/SPE began to decrease after 20 cycles. The findings can be attributed to the slower electron transfer process between UA and Co3O4–ERGO/SPE as a consequence of more cycles due to the formation of a thicker film on the surface of the SPE.57 In subsequent experiments, the modification of the SPE with the Co3O4–ERGO composite was carried out between −1.5 V and 0 V for 20 cycles at a scan rate of 50 mV s−1. To decide on the optimal pH value of the buffer solution, the DPV signals of 60 μM UA at the Co3O4–ERGO/SPE was monitored in 0.1 M PBS at different pH values in the range of 5.0–9.0 (Fig. 5c). The DPV current signal obtained at pH 6.5 is slightly larger than that obtained at pH 7. However, the pH of the electrolyte was chosen as 7.0 for all subsequent measurements. Because the applicability of Co3O4–ERGO/SPE to human saliva for UA detection has been aimed at in this study, the pH value of the electrolyte has been chosen in the pH region (6.8–7.8) suitable for healthy human saliva.58
1 possessess the highest DPV signal. In the following ratios, the peak currents decreased, which indicates that the decrease in the amount of ERGO in the composite composition decreases the sensitivity of Co3O4–ERGO/SPE. As another parameter, the effect of the Co3O4–ERGO composite thickness on the current response of the proposed sensor was examined with various electrodes prepared by changing the number of scan cycles (between 5 and 35) in the potential range of −1.5 to 0 (Fig. 5b). The peak current of Co3O4–ERGO/SPE began to decrease after 20 cycles. The findings can be attributed to the slower electron transfer process between UA and Co3O4–ERGO/SPE as a consequence of more cycles due to the formation of a thicker film on the surface of the SPE.57 In subsequent experiments, the modification of the SPE with the Co3O4–ERGO composite was carried out between −1.5 V and 0 V for 20 cycles at a scan rate of 50 mV s−1. To decide on the optimal pH value of the buffer solution, the DPV signals of 60 μM UA at the Co3O4–ERGO/SPE was monitored in 0.1 M PBS at different pH values in the range of 5.0–9.0 (Fig. 5c). The DPV current signal obtained at pH 6.5 is slightly larger than that obtained at pH 7. However, the pH of the electrolyte was chosen as 7.0 for all subsequent measurements. Because the applicability of Co3O4–ERGO/SPE to human saliva for UA detection has been aimed at in this study, the pH value of the electrolyte has been chosen in the pH region (6.8–7.8) suitable for healthy human saliva.58
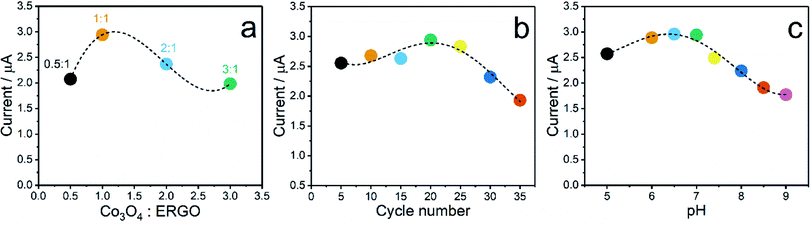 | ||
Fig. 5 Effect of the (a) Co3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ERGO ratio, (b) Co3O4–ERGO composite thickness, and (c) pH of the buffer solution on the current response of 60 μM UA at Co3O4–ERGO/SPE. ERGO ratio, (b) Co3O4–ERGO composite thickness, and (c) pH of the buffer solution on the current response of 60 μM UA at Co3O4–ERGO/SPE. | ||
3.4. Electrochemical detection of UA
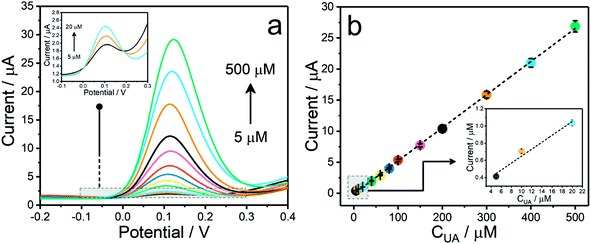
In order to find out the sensitivity of the present sensor towards the electrooxidation reaction of UA under the optimal conditions, the electrochemical signals of Co3O4–ERGO/SPE were monitored by the DPV technique in the presence of increasing concentrations of UA (Fig. 6a). Fig. 6b shows that the current signals are linear with UA concentrations ranging from 5 μM to 500 μM. The linear regression of the calibration curve yielded the equation IUA (μA) = 0.0465 + 0.0517CUA (μM) with a linear correlation coefficient of 0.9985. The detection limit was calculated to be 1.5 μM, as estimated using the equation of 3SD/m.59 Based on the slope of the calibration curve, the analytical sensitivity of Co3O4–ERGO/SPE was obtained to be 0.0465 μA μM−1. The analytical characteristics of Co3O4–ERGO/SPE were also compared with those of several sensors for the detection of salivary UA in the literature, and these characteristics are summarized in Table 1. By comparison, accordingly, Co3O4–ERGO/SPE shows either better or comparable analytical performance. This sensing performance of Co3O4–ERGO/SPE may be due to the fact that ERGO increases the electron flow rate in the oxidation reaction of UA60 and Co3O4 NPs exhibit high catalytic activity as well as its large surface area and highly conductive nature.61| Sensor | Linear range (μM) | LOD (μM) | Sample | Reference |
|---|---|---|---|---|
| Uricase/MWCNTs/SPE | 5–1000 | 0.33 | Artificial saliva | 21 |
| α-Ni0.75Zn0.25(OH)2-modified FTO | 100–1400 | 0.023 | Saliva and artificial saliva | 62 |
| SPE-AuNPs | 20–200 | 14.64 | Artificial saliva | 6 |
| PEDOT–GO/ITO | 2–1000 | 0.75 | Artificial saliva | 63 |
| UOx–GO/PANI/Nf–Gr/Pt | 3–300 | 3 | Saliva | 64 |
| β-CD/CPE | 10–170 | 4.6 | Saliva | 65 |
| Co3O4–ERGO/SPE | 5–500 | 1.5 | Artificial saliva | Present work |
3.5. Repeatability, reproducibility, stability and interference
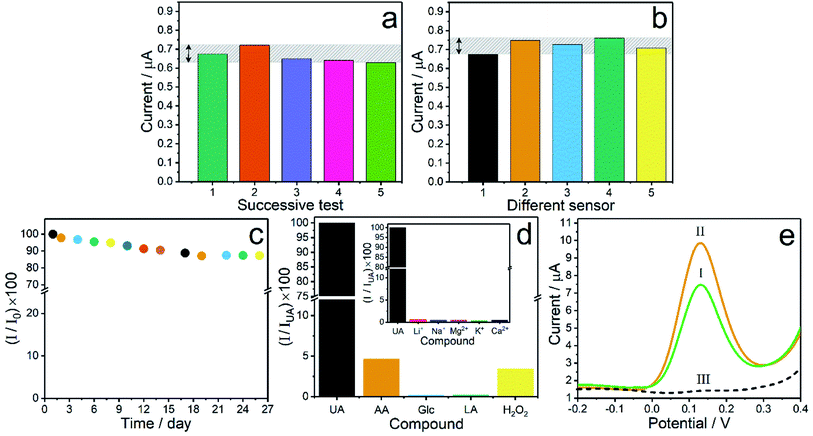
To prove the precision of Co3O4–ERGO/SPE, the repeatability and the reproducibility of the present sensor were examined by recording the DPV responses in 0.1 M PBS (pH 7.0) containing 10 μM UA. The repeatability was analyzed by monitoring five successive current responses using the same sensor for UA detection, and the current responses illustrated in Fig. 7a provided an RSD value of 3.62%. The reproducibility was evaluated by preparing five identical Co3O4–ERGO/SPEs designed using the similar protocol for UA detection, and the RSD value of the current responses was found to be 3.38%, as presented in Fig. 7b. The long-term stability of Co3O4–ERGO/SPE was examined by comparing the DPV responses obtained for 20 μM UA over 26 days. When the sensor was not used, it was stored in a desiccator under N2 at RT. The proposed sensor retained 95% of its initial response after 8 days. At the end of 26 days of storage, the peak current was approximately 90% of its initial response, indicating that the proposed sensor has good stability (Fig. 7c). To determine the selectivity of the prepared sensor towards UA, the effect of various possible interfering species (AA, LA, Glc, H2O2, Li+, Na+, Mg2+, K+, and Ca2+) was studied by recording the peak responses of 30 μM of UA and each compound in 0.1 M PBS (pH 7.0). Fig. 7d shows a comparison of the current signals of the interfering species in the peak potential of UA. The RSD values of the current responses for the interfering species were found in the range of 2.71–3.35%. According to the DPV signals of all species recorded on Co3O4–ERGO/SPE, it is clear that the proposed sensor shows an acceptable selectivity for the detection of UA.3.6. Sample analysis
To assess the applicability of the proposed sensor in practical analysis, the electrochemical response of Co3O4–ERGO/SPE was examined in artificial saliva which has a similar electrolyte concentration to human saliva.4 The artificial saliva was prepared by dissolving 5 mM NaCl, 1 mM CaCl2, 15 mM KCl, 1.1 mM KSCN, 4 mM NH4Cl, and 1 mM citric acid in ultrapure water, and the pH of the solution was adjusted to 6.7.21 Prior to the electrochemical detection of UA, the saliva samples containing UA were prepared in an artificial saliva solution. Then, the saliva samples and 0.1 M PBS were mixed in the same volume ratio. The concentrations of UA in the final mixture were chosen in keeping with the level of UA (100–250 μM) in healthy human saliva.62 UA concentrations corresponding to the peak signals (peak I and II) recorded for the artificial saliva samples were found by means of the calibration plot of the proposed sensor (Fig. 7e). Peak III corresponds to the DPV peak of 0.1 M PBS + artificial saliva without UA in the same volume ratio. This peak also proves that none of the species in the artificial saliva samples showed an interference effect for the detection of UA at the proposed sensor. The recoveries summarized in Table 2 are in the range of 98.20–98.78%, which confirms the reliability of Co3O4–ERGO/SPE for the determination of salivary UA.| Sample no. | UA in the sample (μM) | Found (μM) | Recovery (%) |
|---|---|---|---|
| I | 100 | 98.20 | 98.20 |
| II | 150 | 148.17 | 98.78 |
4. Conclusions
We presented in this study a new electrochemical sensor prepared using Co3O4–ERGO as a sensing material. After detailed optimization and characterization studies, it has been proven that the fabricated sensor can be utilized for the detection of UA. The sensor demonstrated better or comparable analytical performance (linear range: 5–500 μM, detection limit: 1.5 μM, and sensitivity: 0.0465 μA μM−1) as compared with the sensing characteristics of the electrodes given in Table 1. Moreover, the Co3O4–ERGO film was found to have a good selectivity for UA against various interfering molecules, such as ascorbic acid, lactic acid, glucose, hydrogen peroxide and some ions. With the aim of examining the applicability of Co3O4–ERGO/SPE in practical analysis, the current signals were monitored for UA detection in artificial saliva samples, and satisfactory recovery results were obtained. Eventually, the selectivity and sensitivity of Co3O4–ERGO towards the electrooxidation of UA could provide a new platform for electrochemical sensing applications.Conflicts of interest
All authors declare that they have no conflict of interest.Acknowledgements
The authors greatly thank the Research Foundation of Selcuk University (BAP Project No. 20201062) for the financial support.References
- S. Gupta and N. Ahuja, Histology, IntechOpen, London, United Kingdom, 2019, pp. 63–76 Search PubMed.
- A. Ilea, V. Andrei, C. N. Feurdean, A.-M. Băbţan, N. B. Petrescu, R. S. Câmpian, A. B. Boşca, B. Ciui, M. Tertiş and R. Săndulescu, Biosensors, 2019, 9, 27 CrossRef CAS PubMed.
- R. Pink, J. Simek, J. Vondrakova, E. Faber, P. Michl, J. Pazdera and K. Indrak, Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub., 2009, 153, 103–110 CrossRef CAS PubMed.
- J. Kim, S. Imani, W. R. de Araujo, J. Warchall, G. Valdés-Ramírez, T. R. Paixão, P. P. Mercier and J. Wang, Biosens. Bioelectron., 2015, 74, 1061–1068 CrossRef CAS PubMed.
- K. Ngamchuea, C. Batchelor-McAuley and R. G. Compton, Sens. Actuators, B, 2018, 262, 404–410 CrossRef CAS.
- J. Piedras, R. B. Dominguez and J. M. Gutiérrez, Chemosensors, 2021, 9, 73 CrossRef CAS.
- L. M. Niu, N. B. Li and W. J. Kang, Microchim. Acta, 2007, 159, 57–63 CrossRef CAS.
- J. G. Puig and M. A. Martinez, Curr. Opin. Rheumatol., 2008, 20, 187–191 CrossRef CAS PubMed.
- C.-C. Chang, C.-H. Wu, L.-K. Liu, R.-H. Chou, C.-S. Kuo, P.-H. Huang, L.-K. Chen and S.-J. Lin, Sci. Rep., 2018, 8, 1–6 Search PubMed.
- T. Miyaoka, T. Mochizuki, T. Takei, K. Tsuchiya and K. Nitta, Heart Vessels, 2014, 29, 504–512 CrossRef PubMed.
- A. Fang, Q. Wu, Q. Lu, H. Chen, H. Li, M. Liu, Y. Zhang and S. Yao, Biosens. Bioelectron., 2016, 86, 664–670 CrossRef CAS PubMed.
- Q. Long, A. Fang, Y. Wen, H. Li, Y. Zhang and S. Yao, Biosens. Bioelectron., 2016, 86, 109–114 CrossRef CAS PubMed.
- A. Vernerová, L. K. Krčmová, O. Heneberk, V. Radochová, O. Strouhal, A. Kašparovský, B. Melichar and F. Švec, Talanta, 2021, 233, 122598 CrossRef PubMed.
- H. Wang, Q. Lu, Y. Hou, Y. Liu and Y. Zhang, Talanta, 2016, 155, 62–69 CrossRef CAS PubMed.
- C. Westley, Y. Xu, B. Thilaganathan, A. J. Carnell, N. J. Turner and R. Goodacre, Anal. Chem., 2017, 89, 2472–2477 CrossRef CAS PubMed.
- G. Yue, S. Li, W. Liu, F. Ding, P. Zou, X. Wang, Q. Zhao and H. Rao, Sens. Actuators, B, 2019, 287, 408–415 CrossRef CAS.
- M. Liu, Q. Chen, C. Lai, Y. Zhang, J. Deng, H. Li and S. Yao, Biosens. Bioelectron., 2013, 48, 75–81 CrossRef CAS PubMed.
- L. Yang, N. Huang, Q. Lu, M. Liu, H. Li, Y. Zhang and S. Yao, Anal. Chim. Acta, 2016, 903, 69–80 CrossRef CAS PubMed.
- N. Demir, K. Atacan, M. Ozmen and S. Z. Bas, New J. Chem., 2020, 44, 11759–11767 RSC.
- L. Jothi, S. Neogi, S. K. Jaganathan and G. Nageswaran, Biosens. Bioelectron., 2018, 105, 236–242 CrossRef PubMed.
- W. Shi, J. Li, J. Wu, Q. Wei, C. Chen, N. Bao, C. Yu and H. Gu, Anal. Bioanal. Chem., 2020, 412, 7275–7283 CrossRef CAS PubMed.
- Y. Harima, S. Setodoi, I. Imae, K. Komaguchi, Y. Ooyama, J. Ohshita, H. Mizota and J. Yano, Electrochim. Acta, 2011, 56, 5363–5368 CrossRef CAS.
- X. Zhao, J. Zuo, S. Qiu, W. Hu, Y. Wang and J. Zhang, Food Anal. Methods, 2017, 10, 3329–3337 CrossRef.
- S. K. Ponnaiah and P. Periakaruppan, Microchim. Acta, 2018, 185, 1–7 CrossRef CAS PubMed.
- B. Vellaichamy, S. K. Ponniah and P. Prakash, Sens. Actuators, B, 2017, 253, 392–399 CrossRef CAS.
- R. Liu, M. Lv, Q. Wang, H. Li, P. Guo and X. Zhao, J. Magn. Magn. Mater., 2017, 424, 155–160 CrossRef CAS.
- Q. Lu, J. Deng, Y. Hou, H. Wang, H. Li and Y. Zhang, Chem. Commun., 2015, 51, 12251–12253 RSC.
- S. K. Ponnaiah and P. Prakash, Mater. Sci. Eng., C, 2020, 113, 111010 CrossRef CAS PubMed.
- S. K. Ponnaiah, P. Prakash and J. Balasubramanian, Chemosphere, 2021, 271, 129533 CrossRef CAS PubMed.
- S. K. Ponnaiah, P. Prakash and S. Muthupandian, Ultrason. Sonochem., 2019, 58, 104629 CrossRef CAS PubMed.
- K. Pourzare, S. Farhadi and Y. Mansourpanah, Acta Chim. Slov., 2017, 64, 945–958 CrossRef CAS PubMed.
- H. Li, C. Sun, Y. Zhao, X. Xu and H. Yu, J. Nanopart. Res., 2018, 20, 1–13 CrossRef.
- B. Wang, X.-Y. Lu and Y. Tang, J. Mater. Chem. A, 2015, 3, 9689–9699 RSC.
- A. E. Vilian, B. Dinesh, M. Rethinasabapathy, S.-K. Hwang, C.-S. Jin, Y. S. Huh and Y.-K. Han, J. Mater. Chem. A, 2018, 6, 14367–14379 RSC.
- J. Liu, J. Ke, Y. Li, B. Liu, L. Wang, H. Xiao and S. Wang, Appl. Catal., B, 2018, 236, 396–403 CrossRef CAS.
- H. Shao, X. Zhao, Y. Wang, R. Mao, Y. Wang, M. Qiao, S. Zhao and Y. Zhu, Appl. Catal., B, 2017, 218, 810–818 CrossRef CAS.
- J. Liu, J. Wang, B. Zhang, Y. Ruan, H. Wan, X. Ji, K. Xu, D. Zha, L. Miao and J. Jiang, J. Mater. Chem. A, 2018, 6, 2067–2072 RSC.
- M. A. Zahed, S. C. Barman, R. Toyabur, M. Sharifuzzaman, X. Xuan, J. Nah and J. Y. Park, J. Electrochem. Soc., 2019, 166, B304 CrossRef CAS.
- S.-J. Li, J.-M. Du, J. Chen, N.-N. Mao, M.-J. Zhang and H. Pang, J. Solid State Electrochem., 2014, 18, 1049–1056 CrossRef CAS.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806 CrossRef CAS PubMed.
- A. Gevaerd, E. Watanabe, K. Fernandes, M. Papi, C. Banks, M. Bergamini and L. Marcolino-Junior, Electroanalysis, 2020, 32, 1689–1695 CrossRef CAS.
- F. Mazzara, B. Patella, G. Aiello, A. O'Riordan, C. Torino, A. Vilasi and R. Inguanta, Electrochim. Acta, 2021, 388, 138652 CrossRef CAS.
- W. Yue and W. Zhou, J. Mater. Chem., 2007, 17, 4947–4952 RSC.
- W. Tang, J. Weng, X. Lu, L. Wen, A. Suburamanian, C.-Y. Nam and P.-X. Gao, Appl. Catal., B, 2019, 256, 117859 CrossRef CAS.
- S. Mo, Q. Zhang, Y. Sun, M. Zhang, J. Li, Q. Ren, M. Fu, J. Wu, L. Chen and D. Ye, J. Mater. Chem. A, 2019, 7, 16197–16210 RSC.
- K. Atacan, M. Özacar and M. Özacar, Int. J. Biol. Macromol., 2018, 109, 720–731 CrossRef CAS PubMed.
- M. A. Haija, A. F. Abu-Hani, N. Hamdan, S. Stephen and A. I. Ayesh, J. Alloys Compd., 2017, 690, 461–468 CrossRef.
- M. M. Shahid, P. Rameshkumar, A. Pandikumar, H. N. Lim, Y. H. Ng and N. M. Huang, J. Mater. Chem. A, 2015, 3, 14458–14468 RSC.
- J. Yu and T. H. Kim, J. Electrochem. Sci. Technol., 2017, 8, 274–281 CrossRef CAS.
- Y. Xu, F. Zhang, T. Sheng, T. Ye, D. Yi, Y. Yang, S. Liu, X. Wang and J. Yao, J. Mater. Chem. A, 2019, 7, 23191–23198 RSC.
- Q. Feng, Y. Zeng, P. Xu, S. Lin, C. Feng, X. Li and J. Wang, J. Mater. Chem. A, 2019, 7, 27522–27534 RSC.
- G. Vinodhkumar, R. Ramya, I. V. Potheher, M. Vimalan and A. C. Peter, Res. Chem. Intermed., 2019, 45, 3033–3051 CrossRef CAS.
- G. Ibáñez-Redín, D. Wilson, D. Gonçalves and O. N. Oliveira Jr, J. Colloid Interface Sci., 2018, 515, 101–108 CrossRef PubMed.
- X. Niu, W. Yang, G. Wang, J. Ren, H. Guo and J. Gao, Electrochim. Acta, 2013, 98, 167–175 CrossRef CAS.
- S. K. Ponnaiah, P. Periakaruppan and B. Vellaichamy, J. Phys. Chem. B, 2018, 122, 3037–3046 CrossRef CAS PubMed.
- Q. Wang, J. Zhang, Y. Xu, Y. Wang, L. Wu, X. Weng, C. You and J. Feng, Anal. Methods, 2021, 13, 56–63 RSC.
- M. H. Ghanbari, Z. Norouzi and M. M. Ghanbari, Microchem. J., 2020, 156, 104994 CrossRef CAS.
- C. Zuliani, G. Matzeu and D. Diamond, Electrochim. Acta, 2014, 132, 292–296 CrossRef CAS.
- R. Villalonga, P. Diez, M. Eguilaz, P. Martinez and J. M. Pingarron, ACS Appl. Mater. Interfaces, 2012, 4, 4312–4319 CrossRef CAS PubMed.
- U. Solaem Akond, K. Barman, A. Mahanta and S. Jasimuddin, Electroanalysis, 2021, 33, 900–908 CrossRef CAS.
- N. H. Khand, I. M. Palabiyik, J. A. Buledi, S. Ameen, A. F. Memon, T. Ghumro and A. R. Solangi, J. Nanostruct. Chem., 2021, 1–14 Search PubMed.
- N. F. Azeredo, J. M. Gonçalves, P. O. Rossini, K. Araki, J. Wang and L. Angnes, Microchim. Acta, 2020, 187, 1–11 CrossRef PubMed.
- X. Huang, W. Shi, J. Li, N. Bao, C. Yu and H. Gu, Anal. Chim. Acta, 2020, 1103, 75–83 CrossRef CAS PubMed.
- C. Liao, C. Mak, M. Zhang, H. L. Chan and F. Yan, Adv. Mater., 2015, 27, 676–681 CrossRef CAS PubMed.
- M. Ramírez-Berriozabal, L. Galicia, S. Gutiérrez-Granados, J. S. Cortes and P. Herrasti, Electroanalysis, 2008, 20, 1678–1683 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Chemicals and apparatus, FTIR analysis, Raman analysis, and contact angle measurements. See DOI: 10.1039/d1ay01744f |
| This journal is © The Royal Society of Chemistry 2022 |