Focus on the molecular mechanisms of cisplatin resistance based on multi-omics approaches
Ping
Yue
ab,
Bingjie
Han
 *a and
Yi
Zhao
*a
*a and
Yi
Zhao
*a
aDepartment of Translational Medical Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450052, China. E-mail: hanbj@mail2.sysu.edu.cn; zhaoyi0910@163.com
bAcademy of Medical Science, Henan Medical College of Zhengzhou University, Zhengzhou, Henan 450052, China
First published on 6th January 2023
Abstract
Cisplatin is commonly used in combination with other cytotoxic agents as a standard treatment regimen for a variety of solid tumors, such as lung, ovarian, testicular, and head and neck cancers. However, the effectiveness of cisplatin is accompanied by toxic side effects, for instance, nephrotoxicity and neurotoxicity. The response of tumors to cisplatin treatment involves multiple physiological processes, and the efficacy of chemotherapy is limited by the intrinsic and acquired resistance of tumor cells. Although enormous efforts have been made toward molecular mechanisms of cisplatin resistance, the development of omics provides new insights into the understanding of cisplatin resistance at genome, transcriptome, proteome, metabolome and epigenome levels. Mechanism studies using different omics approaches revealed the necessity of multi-omics applications, which provide information at different cellular function levels and expand our recognition of the peculiar genetic and phenotypic heterogeneity of cancer. The present work systematically describes the underlying mechanisms of cisplatin resistance in different tumor types using multi-omics approaches. In addition to the classical mechanisms such as enhanced drug efflux, increased DNA damage repair and changes in the cell cycle and apoptotic pathways, other changes like increased protein damage clearance, increased protein glycosylation, enhanced glycolytic process, dysregulation of the oxidative phosphorylation pathway, ferroptosis suppression and mRNA m6A methylation modification can also induce cisplatin resistance. Therefore, utilizing the integrated omics to identify key signaling pathways, target genes and biomarkers that regulate chemoresistance are essential for the development of new drugs or strategies to restore tumor sensitivity to cisplatin.
Introduction
Malignant tumors have become a considerable threat to human health as they have high morbidity and mortality rates.1 The development of cancer is a complex process with remarkable biological features, such as abnormal cell proliferation and differentiation, heterogeneity, and epithelial-mesenchymal transformation (EMT).2,3 Since cisplatin was approved by the US Food and Drug Administration (FDA) in 1978, platinum-based combined chemotherapy has become the first-line treatment for solid tumors, including breast cancer, lung cancer, bladder cancer, ovarian, head and neck squamous cell cancer, stomach cancer, etc.4,5 The clinical efficacy of cisplatin is significantly reduced due to acquired or inherent drug resistance in the treatment, which results in poor prognosis. Therefore, exploring the underlying mechanisms of cisplatin resistance has great significance to improving chemotherapy effects and overcoming chemoresistance, ultimately prolonging the survival time of patients.Studies have shown that cisplatin resistance is caused by many factors and the proposed mechanisms are multiple, i.e., decreased accumulation of cisplatin in cells due to active efflux, detoxification by GSH conjugates, inactivation of cisplatin by metallothioneins and other antioxidants, increased DNA damage repair including nucleotide excision repair and mismatch repair, activation of the anti-apoptotic pathway and dysfunction of the apoptotic signaling pathway, etc.4–6 In recent years, most studies have focused on the interaction of cisplatin with individual protein targets in the signaling pathway to identify mechanisms of cisplatin susceptibility regulation. On the other hand, there is a lack of research on the global changes in biological processes caused by cisplatin. Next-generation technologies focus on studying biological parameters (gene variation, alteration of protein concentration, epigenetic modification and metabolite abundance) from multiple dimensions. With the continuous development of omics, i.e., mass spectrometry and sequencing, the factors affecting the physiological and pathological processes will be further revealed. In addition, combined with bioinformatics, the gene expression, protein regulation and metabolic disorders in organisms exposed to the long-term stimulation of cisplatin can be comprehensively revealed.
The overall goal of this review is to summarize how to identify the gene or protein targets and signaling pathways regulated by cisplatin through omics technologies, including genomics, transcriptomics, proteomics, metabolomics, epigenetics, and single-cell transcriptomics. It will provide new ideas for explaining the molecular mechanism of cisplatin resistance in tumors, and further provide new treatment strategies for solving chemotherapy resistance in the clinic.
Molecular mechanism of cisplatin resistance based on omics
Origin and development of omics
Omics is the technique used to analyze the genes, proteins, or metabolites in biological systems.7 Genomics, transcriptomics, epigenetics and single-cell transcriptome are based on high-throughput sequencing technology, and proteomics and metabolomics mainly depend on mass spectrometry technology.8,9 Since the first generation of sequencing technology (Sanger sequencing) was developed by Frederick Sanger and Coulson in 1977, it has developed rapidly. At present, third-generation sequencing technology has progressed, which can detect the nucleic acid sequences containing tens of billions of bases.10,11 Genomics came into being in the 1980s, and can characterize and quantify all genes in organisms. Furthermore, the human genome project opened a new avenue for genomics research.12 Transcriptomics is the use of high-throughput sequencing technology to study gene expression at the RNA level, so as to provide gene transcription and regulation rules at the overall level.13 In addition, the single-cell transcriptome, also based on high-throughput sequencing technology, can detect mRNA in a single cell, which characterizes the single-cell or cell group.14 Epigenetics was first developed by Waddington in 1942, and the consensus on its definition was achieved at the Cold Spring Harbor Conference in 2008, which specifically refers to the stable and heritable phenotypes caused by chromosome changes without changing the DNA sequence. Common epigenetics modification mechanisms include DNA/RNA methylation, chromatin remodeling, histone modification, etc.15–17 Moreover, next-generation sequencing technology also promotes the research on epigenetics, such as the whole genome bisulfite sequencing (WGBS), methylated RNA immunoprecipitation sequencing (MeRIP-Seq), and chromatin immunoprecipitation sequencing (ChIP-Seq).18 High throughput sequencing technology creates favorable conditions for exploring the sequences and structures in biological systems. Combined with bioinformatics, omics technology shows broad application prospects in life science fields.Proteomics and metabolomics are mainly based on high-resolution biological mass spectrometry technologies. With the improvement of the sensitivity and resolution of the mass spectrometer, the scanning speed and recognition accuracy for samples have also been significantly improved.19,20 At present, the commonly used mass spectrometers include gas chromatography-mass spectrometry (GC-MS), liquid chromatography-mass spectrometry (LC-MS), and matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF/TOF).21 The concept of proteomics was first proposed by Peter James in 1997, and International Human Protein Organization established in 2001 further promotes the development of proteomics.22 Proteomics is used to qualitatively and quantitatively analyze all proteins in cells, tissues or organisms, and to reveal the protein interaction network and dynamic regulation process in complex biological systems at the protein level.23 In addition to relying on mass spectrometry, the research of protein also depends on the protein database based on gene sequence. Metabolomics was developed in the 1990s, and is used to analyze small molecular metabolites (MW < 1000 Da) to explore the biomarkers related to physiology and pathology.24,25 We summarize the origin and development of omics technologies and present them in the chronology (Fig. 1).
 | ||
| Fig. 1 The origin and development of omics technologies (genomics, transcriptomics, proteomics, metabolomics, epigenetics and single-cell transcriptomics). | ||
Genomics
Genomics can be used to detect the structure, function and location of all genes in organisms, and comprehensively characterize and compare the gene expression in different systems.26 What's more, the differential genes in cisplatin sensitive or resistant cells, tissues and organisms are analyzed by genomics, which further identifies the molecular mechanism of cisplatin resistance. Recent studies have indicated that there is a significant correlation between the expression levels of HNRNPU and MSH2 and the sensitivity of bladder cancer cells to cisplatin, which is based on the CRISPR-Cas9 whole-genome analysis. Combined with RNA-Seq, it is confirmed that knockdown of HNRNPU enhances the sensitivity of cells to cisplatin by promoting cell apoptosis and regulating DNA damage repair genes. Meanwhile, the inhibition of MSH2 reduces the apoptosis rate, resulting in cisplatin resistance.27,28 What's more, according to the functional genomics screening of high-grade serous ovarian cancer (HGSOC), the overexpression of anti-apoptotic proteins BCL-XL, BCL-w, MCL-1, and BCL-2 can reduce cell apoptosis and increase resistance to cisplatin.29 The whole-genome analysis of chromosome 9q32-q33.1 in nude mice transplanted with non-seminal testicular germ cell tumor (TGCT) showed that the gene regulation in this region affects the resistance of cells to cisplatin, and the inhibition of glucosylceramide synthase (GCS) increases the apoptosis rate and further reverses the resistance to cisplatin.30Full exon sequencing of tumor, normal tissue and blood samples from 39 patients with esophageal squamous cell carcinoma (ESCC) showed that knockout of BRCA1/2 could regulate DNA damage repair and significantly increase the sensitivity of ESCC cells to cisplatin.31 Recent studies have indicated that it could reduce the clearance rate of protein damage and improve the sensitivity to cisplatin by targeting the ubiquitin proteasome system (UPS) through genome-wide RNAi library screening and RNA-Seq studies in human breast cancer cells. Meanwhile, downregulated genes encoding the mitochondrial respiratory complex could induce cisplatin resistance.32 When using integrated functional genomics to study the molecular mechanism of cisplatin reaction differences among different subtypes of triple-negative breast cancer (TNBC), it is found that multiple genes, such as ABCC2, AKT1, BCL2L1, CASP8 and MSH2, participate in the transport of cisplatin, detoxification and EMT, which enhances the tolerance of cells to cisplatin.33 These studies suggest that genomics can identify the genes related to cisplatin resistance in various types of tumors and then determine their location and function. At present, the physiological processes involved in gene mutation or expression difference in cisplatin-resistant cells mainly include cell apoptosis, DNA damage repair, protein damage clearance, mitochondrial respiration, activity change of drug transporter and EMT signaling transduction (Fig. 2A).
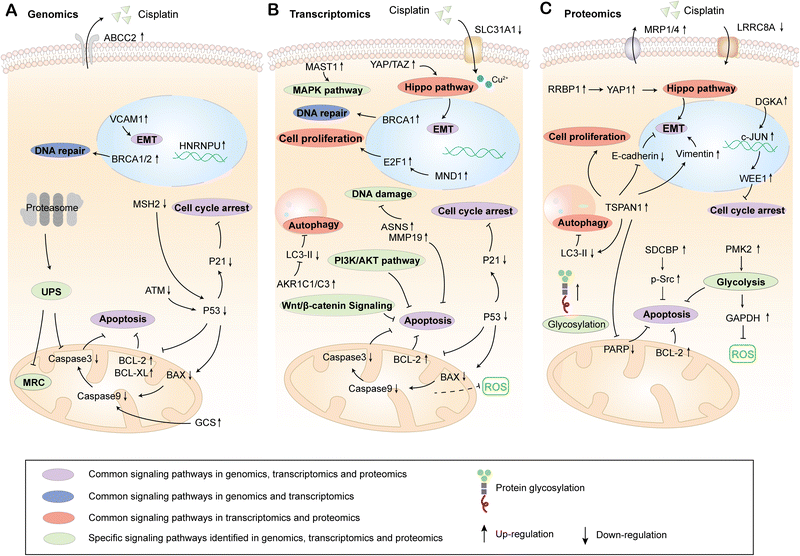 | ||
| Fig. 2 The reported up/down-regulated genes, proteins and signaling pathways related to cisplatin resistance identified by genomics (A), transcriptomics (B) and proteomics (C). | ||
Transcriptomics and single-cell transcriptomics
The transcriptome is a collection of all coding and non-coding RNAs of a tissue or cell at a certain developmental stage or physiological condition, which mainly includes microarray technology and RNA sequencing technology. Microarray technology has the advantages of high throughput and relatively low cost, whereas it is more dependent on existing genome sequences, with large background noise and a limited dynamic range of detection. Whereas RNA-Seq can directly sequence the cDNA chain.34 With the development of hybridization technology and new generation sequencing technology, RNA-Seq based on deep sequencing has many advantages, such as high sequencing flux, reduced sequencing time and cost. It also has an extremely low background noise and needs low total RNA samples.35 Transcriptomics can be used to analyze the differential expression of genes in chemosensitive and chemoresistant cells, tissues or organisms, so as to screen the target genes related to drug resistance, which may provide new targets for the clinical treatment of cisplatin resistant tumors.According to the transcriptome screening of TCGA and GEO database, it is found that MND1 was significantly up-regulated in lung adenocarcinoma (LUAD). Furthermore, it plays its proliferative function by alleviating the E2F1 transcriptional inhibition induced by KLF6 and inducing cisplatin resistance in LUAD.36 In addition, long non-coding RNA (lncRNA) plays a key role in the occurrence and progression of cancer. Microarray analysis indicated that the expression level of lncRNA UCA1 was significantly up-regulated in A549/DDP cells and the inhibition of lncRNA AK126698 could negatively regulate the Wnt/β-Catenin signaling pathway, which significantly reduced the apoptosis rate of LUAD cells and led to cisplatin resistance. On the other hand, the lncRNA NLUCAT1 knockout significantly induced cell oxidative stress, increased apoptosis and sensitivity to cisplatin.37–39 When analyzing the mechanism of cisplatin resistance in cervical cancer by transcriptome, it is found that the activation of the MAPK pathway caused by the PI3K/AKT pathway and the up-regulation of serine/threonine kinase 1 (MAST1) can induce cisplatin resistance.40,41 What's more, it is also found that the lncRNA-AC010198.2/miR-34b-3p/STC2 and lncRNA NCK1-AS1/miR-134-5p/MSH2 may be the key pathways of cisplatin resistance in cervical cancer cells.42,43 Meanwhile, the transcription factors EZH2 and SREBP2 play a key role in maintaining the chemotherapy resistance of ovarian cancer (OV), and their target genes are mainly enriched in cell cycle, cell apoptosis, cholesterol metabolism and other signaling pathways.44 In addition to the long non-coding RNAs, such as lncRNA ZEB1-AS1, which improves the sensitivity of ovarian cancer cells to cisplatin by regulating the expression of matrix metallopeptidase 19 (MMP19), microRNAs (miRNA), such as miR-142-5p and miR-137, can enhance the susceptibility of cells to cisplatin by regulating target genes such as MCL1, BCL2 and XIAP.45–48
RNA-Seq shows that the differentially expressed genes AKR1C1 and AKR1C3 between signet ring cell gastric cancer (SRCGC) parents and drug-resistant strains mediate cisplatin resistance by regulating redox-dependent autophagy.49 The transcriptomics results of head and neck squamous cell carcinoma (HNSC) showed that inhibition of FoxM1 downregulated NBS1, which further inhibited DNA damage repair mediated by MRN-ATM axis and improved the sensitivity of nasopharyngeal carcinoma cells to cisplatin.50 Meanwhile, asparagine synthetase (ASNs) and MMP19 promoted the sensitivity of nasopharyngeal carcinoma cells to cisplatin by enhancing DNA damage and apoptosis.51 Genomics and transcriptomics analysis in SMARCA4 deletion triple-negative breast cancer cells (TNBC) showed that the activation of the EMT pathway mediated by the Hippo-YAP/TAZ target gene induced the resistance to cisplatin.52 The analysis of the TCGA data set combined with transcriptomics indicated that the expression of copper transporter SLC31A1 is upregulated by PTBP1 inhibition, which enhances the chemosensitivity of osteosarcoma cells to cisplatin.53 In addition, the miRNA193 is highly expressed in cisplatin resistant esophageal cancer cells, which silences the TFAP2C transcription factor and mediates drug resistance of esophageal cancer cells by regulating the cell cycle and apoptosis.54 Therefore, cisplatin resistance is common in various tumors, and the study on the mechanisms of cisplatin resistance using transcriptomics is more extensive than genomics. In general, the sensitivity of tumors to cisplatin could be regulated by affecting key targets in the signaling pathways, such as cell proliferation, cell cycle, apoptosis, autophagy, oxidative stress, DNA damage, PI3K/AKT, MAPK, EMT and copper ion transport (Fig. 2B).
Single-cell RNA sequencing (scRNA-Seq) is a technology for full transcriptome amplification and high-throughput sequencing at the single-cell level. It provides the biological information of a single cell and the changes in cell heterogeneity and microenvironment.55–57 The heterogeneity of tumor cells, especially the information of transcriptomics, including the limitations of time and space, may be constantly changing.58 Therefore, exploring dynamic tumor cell heterogeneity may better explain the problem of tumor drug resistance. Using single-cell sequencing technology to analyze cisplatin resistance can explore information at the gene level in detail, and further explore the mechanisms of cisplatin resistance in tumor cells, in order to provide a theoretical basis for the prediction and treatment of cisplatin resistant tumors.
scRNA-Seq and cluster differentiation are conducted on ovarian cancer, normal ovary and embryonic tissues. It is found that PEG10 in malignant epithelial clusters is downregulated, which inhibits the self-renewal signaling transduction of tumor stem cells (CSCs) through the Notch signaling pathway and increases the apoptosis induced by cisplatin. Therefore, the sensitivity of OV to cisplatin increased.59 The results of scRNA-Seq combined with TCGA and GSEA data sets for OV show that the down-regulation of interferon α-inducible protein 6 (IFI6) may be involved in the regulation of the NF-κB pathway, which inhibits the proliferation of OV cells and enhances the sensitivity to cisplatin.60 Single-cell transcriptome analysis combined with cluster analysis reveal that the clonal heterogeneity of 13dR-H460 cells with short-term drug resistance was not increased. Instead, the epigenetics mechanisms have been suggested to be responsible for resistance to cisplatin.61 The scRNA-Seq for urothelial carcinoma cell lines 5637 and 5637PR cells shows that the 12 genes (COX7B, MT1E, LGALS1, KRT17, EIF3E, TMA7, ARL6IP1, HES1, UQCR10, MORF4L1, CDKN3, PSMD10) are consistently downregulated in resistant cells, and knockdown of COX7B decreased the cisplatin sensitivity.62
Compared to bulk transcriptomics, single-cell transcriptomics is more focused on the interpretation of the cell atlas. scRNA-Seq is commonly used to search for cell subclasses in which key genes are located and significant differences in target genes are found among cell subgroups. At the level of cisplatin resistance in tumor cells, for instance, the marker gene regulating cisplatin susceptibility in ovarian cancer by scRNA-Seq was found to be PEG10, located in malignant epithelial clusters, whereas the bulk RNA-Seq focused on identifying key genes such as EZH2 and SREBP2, which had significant effects on cisplatin sensitivity. In conclusion, the gene expression levels of the system can be quantified with the single-cell transcriptomics combined with RNA-Seq to further explore the cell subsets containing altered genes involved in drug resistance and analyze the key sites of the cellular interaction network in the drug-resistant tumor microenvironment, ultimately clarifying the molecular mechanism of cisplatin resistance.
Proteomics
Proteomics can identify and quantify proteins expressed by specific cells, tissues, or organisms at a certain stage.63 With the development of mass spectrometry technology, the expression of proteins and their changes under biological interference such as disease or treatment can be comprehensively evaluated in a very short time.64,65 Meanwhile, the post-translational modifications of proteins, such as phosphorylation, glycosylation, acetylation and ubiquitination, provide the theory for exploring the mechanism of protein regulation. To explore the molecular mechanism of cisplatin resistance, proteomics can be used to compare the protein expression differences between cisplatin resistant cells and sensitive cells, which may declare the key protein molecules and signaling pathways involved in the regulation of cisplatin sensitivity.The results of proteomics on cisplatin resistant lung cancer showed that muscle pyruvate kinase isoenzyme 2 (PKM2) was closely related to cisplatin susceptibility and PKM2 promoted the cell glycolysis to eliminate ROS produced by cisplatin, which inhibited cell apoptosis and enhanced the proliferation of NSCLC cells.66 At the same time, the dual inhibition of apurinic/apyrimidinic endonuclease 1 (APE1) and autophagy promotes cell apoptosis and increases the sensitivity of KRASG125-mutant A549 cells to cisplatin.67 In addition, the results of N-glycoproteomics show that the increase of protein glycosylation, the down-regulation of drug uptake channel protein LRRC8A and the up-regulation of drug efflux pump proteins MRP1 and MRP4 in cisplatin resistant lung cancer cells decrease the accumulation of cisplatin in cells, which leads to cisplatin resistance.68 In ovarian cancer, proteome and genome analysis indicate that diacylglycerol kinase α (DGKA) and phosphatidic acid (PA) bind to JUK, which activates the transcription factor c-JUN to promote the expression of mitotic inhibitor protein kinase (WEE1), to avoid cell cycle arrest induced by cisplatin and promote the proliferation of cisplatin resistant cells.69 Proteome study on cisplatin-resistant head and neck squamous cell carcinoma shows that TSPAN1 and SDCBP are involved in the EMT pathway of tumor cells. They induce cisplatin resistance by activating autophagy and apoptosis inhibition mediated by p-SRC.70,71 Proteomics and genomics analysis of oral squamous cell carcinoma with different levels of cisplatin resistance suggest that the ribosomal binding protein 1 (RRBP1) induces the resistance by activating Yes-related protein 1 (YAP1) through the Hippo signaling pathway.72 To sum up, proteomics results show that multiple signaling pathways, such as cell cycle, autophagy, apoptosis, drug efflux, glycolysis and Hippo, are involved in the regulation of cisplatin sensitivity of tumor cells. In addition, the tumor resistance to cisplatin is improved by regulating protein glycosylation modification (Fig. 2C).
Metabolomics
Metabolomics is a comprehensive and rapid analysis method for small molecule metabolites by nuclear magnetic resonance (NMR) spectroscopy or mass spectrometry (usually combined with chromatographic separation), which provides high-throughput analysis at a low cost.73 It evaluates the abundance of different metabolites in biological samples to explore the metabolic processes in the body.74 At present, studies have shown that the metabolic disorder of cells is related to cell resistance, and the drug resistance may be reversed by targeting key metabolic enzymes.75It is reported that the metabolism of glutathione (GSH) is closely related to cisplatin resistance, which clears ROS produced by cisplatin in cells and improves the tolerance of cells to cisplatin.76–78 Ovarian cancer is the most lethal malignant tumor of the female reproductive system. The research on its pathogenesis by metabolomics also provides insights into the diagnosis and treatment of ovarian cancer. For example, it is found that the expression and enzyme activity of glucose-6-phosphate dehydrogenase (G6PDH) in the pentose phosphate pathway (PPP) is increased in cisplatin resistant ovarian cancer cell lines, which produces NADPH to maintain the GSH level and redox balance in drug-resistant cells.79 Meanwhile, in cisplatin resistant A2780 cells, the levels of glutamine, glutamic acid and glutathione are increased to induce cisplatin resistance, and the activity of glutamine synthetase (GS) is inhibited, which negatively regulates the production of glutamine. What's more, supplementation of glutamine reduces the deficiency of nutrition in cisplatin resistant cells by acting on the nucleotide biosynthesis pathway.80–82 In addition, in chemoresistant ovarian cancer cells, cysteine synthesis and lipid metabolism are increased, and methionine metabolism is abnormal.81,83,84 The result of integrated metabolomics for cisplatin resistant xenografts from patients with epithelial ovarian cancer shows that the dysregulation of glycolysis, tricarboxylic acid cycle and urea cycle, and inhibition of oxidative phosphorylation may be an effective means to overcome cisplatin resistance.85 The metabolomics combined with proteomics results for the cisplatin resistant group and sensitive group in brain metastases (BM) of lung adenocarcinoma indicates that the metabolites related to oxidative stress steady-state pathways, such as PPP and GSH metabolism, are up-regulated in the drug-resistant group. And the GPX4 is activated by the Wnt/NR2F2 signaling axis, which promotes acquired drug resistance through high consumption of GSH and inhibition of iron death.86 It can be seen from the above studies that the identification of biomarkers by metabolomics provides a theoretical basis for the diagnosis and prediction of clinical diseases. Furthermore, the dysregulated metabolic pathway can be used as a potential tumor treatment target to delay or inhibit the production of cisplatin resistance (Fig. 3).
 | ||
| Fig. 3 The representative metabolic pathways and key proteins/enzymes regulating cisplatin resistance in tumor cells. | ||
Epigenetics
Epigenetics is a phenomenon in which a DNA base sequence remains unchanged, while the gene expression and phenotype can be genetically changed.87 Epigenetic modifications of chromatin mainly include DNA methylations, histone post-translational modifications (i.e., acetylation, methylation, phosphorylation), non-coding RNA regulation, and RNA modifications.88–90 In recent years, with the emergence of epigenetics, it is further confirmed that there are changes in genetic effects and epigenetic modifications in the development of diseases. The use of epigenetics modification mechanisms to treat tumors and reverse drug resistance has promising applications (Fig. 4). | ||
| Fig. 4 The representative epigenetic mechanisms related to cisplatin resistance, including histone post-transcriptional modification and mRNA m6A methylation modification. | ||
The methylation of DNA can regulate the expression of target genes by changing the chromatin structure, DNA conformation and stability, and DNA–protein interaction.91 Studies have shown that the high methylation of spermidine/spermine N1 acetyltransferase (SAT1) and arginine succinate synthase 1 (ASS1) in bladder cancer cells leads to the down-regulated expression, which induces cisplatin resistance.92 The genome-wide analysis of hypermethylated CpG islands in cisplatin resistant ovarian cancer cells shows that the hypermethylation of TRIB2 results in its significant down-regulation at the mRNA level. And the hypermethylated TRIB2 further regulates cisplatin-dependent cell cycle arrest and apoptosis, leading to cisplatin resistance.93 Meanwhile, the methyltransferase DOT1L is activated by transcription factor C/EBPβ, which methylates the resistance gene H3K79 and keeps the chromatin open, resulting in cisplatin resistance of serous ovarian cancer cells.94
Histone post-translational modification regulates many biological processes such as transcription, replication and DNA repair in cells. For example, the histone demethylase JHDM2A in epithelial ovarian cancer is recruited into promoter SLC31A1 by ZNF711, and the H3K9me2 level is reduced, which leads to the activation of SLC31A1 transcription and uptake enhancement for cisplatin.95 The accumulation of acetyl coenzyme A (Acetyl-CoA) in nasopharyngeal carcinoma cells (NPC) promotes the acetylation of histone H3K27, and further promotes cell proliferation, metastasis and cisplatin resistance of NPC by regulating the PADI1-MAPK-MMP2/9 pathway.96 In lung adenocarcinoma cells, the overexpression of ubiquitin specific peptidase 22 (USP22) promotes the phosphorylation of histone H2AX through deubiquitination of histone H2A to enhance DNA damage repair and reduces the acetylation of Ku70 by stabilizing SIRT1 to inhibit Bax-mediated apoptosis and finally induce cisplatin resistance.97
In addition, many studies have shown that RNA methylation modification affects the primary and acquired drug resistance of tumors, among which the m6A modifications are the most common. The post-transcriptional modifications of mRNA are dynamic and reversible biological processes that affect mRNA shearing, enucleation, degradation, mRNA stability and translation efficiency.98 Studies also indicate that inhibition of m6A “reader” YTHDF1 mediates cisplatin resistance in non-small cell lung cancer via the KEAP1/NRF2/AKR1C1 axis.99 Furthermore, methyltransferase METTL3 regulates the expression of target gene TRIM11 in an m6A-dependent manner to induce drug resistance in NPC.100 At present, the mechanisms of m5C modification in cisplatin resistance of tumors has not been reported. Therefore, the relationship between RNA methylation modifications and cisplatin resistance still needs further study to find the key drivers.
Conclusions
In conclusion, cisplatin, the first metallodrug approved by FDA to treat solid tumors, plays a milestone role in the course of anti-cancer treatments. It inhibits the proliferation of tumor cells by destroying the DNA structure after being hydrolyzed and activated. However, tumor cells are prone to develop resistance toward cisplatin. Cisplatin resistance is a complex physiological process involving multiple factors and genes, such as DNA damage repair, drug efflux, and glutathione system. The sensitivity of tumors to cisplatin can be improved by acting on a single target or signaling pathway, but it is difficult to fundamentally solve the problem. Through multi-omics approaches, the molecular regulatory networks can be constructed at different levels, including genes, mRNAs, proteins, metabolites and epigenetic modifications, in order to integrate the complex molecular mechanisms in the development of cisplatin resistance. It is inevitable that the multi-omics approaches also have their limitations. First, the complex network of genes, proteins and metabolites makes the association strength between omics data susceptible to experimental design and conditions. In addition, the processing of multi-omics data is highly dependent on data integration and interpretation tools and methods, with a certain instability. Although the multi-omics approaches provide more comprehensive disease-related information, mining information of interest from large and complex data and integrating omics to form a system-level understanding is a major challenge. However, it is undeniable that the multi-omics approaches are still the most promising methods to reveal the comprehensive molecular characteristics of tumor drug resistance.The heterogeneity and high dimensionality of multi-omics datasets drive the need to choose an appropriate data integration strategy.101 A metabolomics-centric approach in combination with other omics may be more suitable for a comprehensive description of cisplatin resistance. In general, genomics, transcriptomics, and proteomics are considered to provide molecular markers at different levels. The combined analysis of the metabolome and transcriptome or metabolome and proteome can reveal significantly differential metabolites, as well as DEGs or differential proteins associated with the biosynthesis of metabolites, leading to the identification of biomarkers and key regulators related to drug resistance. In addition to genetic molecules, multiple other factors such as the environment play a key role in tumor heterogeneity.102 The linkage between metabolomics and epigenetics has been demonstrated, e.g., the level of intracellular acetyl coenzyme A influences histone acetylation and further regulates the process of cisplatin resistance.96 Linking regulated genes and proteins, centered on key metabolites or metabolic pathways, contributes to interconnecting the full picture of the resistance hotspots.
This review summarizes recent studies on the mechanisms of cisplatin resistance in diverse tumor cell types by omics (Table 1) and illustrates the different gene targets or signaling pathways identified by comparing different omics methods. Meanwhile, biomarkers and metabolic pathways affecting cisplatin resistance are verified by metabolomics. In addition, epigenetics and single-cell transcriptomics, which function as emerging hot subjects, have revealed the molecular mechanisms of cisplatin resistance at the level of heritable phenotypic changes and cell subsets, respectively. Therefore, the integrated analysis of the gene–protein–metabolites interaction network induced by cisplatin is of great significance for identifying new therapeutic targets, developing novel anti-tumor drugs and improving the clinical therapeutic effects of cisplatin.
| Multi-omics approaches | Study subjects | Types of tumorsRef. |
|---|---|---|
| Abbreviations: bladder cancer (BLCA); ovarian cancer (OV); non-seminal testicular germ cell tumor (TGCT); esophageal squamous cell carcinoma (ESCC); breast cancer (BRCA); triple-negative breast cancer (TNBC); lung adenocarcinoma (LUAD); cervical cancer (CESC); signet ring cell gastric cancer (SRCGC); head and neck squamous cell carcinoma (HNSC); osteosarcoma (OS); esophageal cancer (ESCA); non-small cell lung cancer (NSCLC); urothelial carcinoma (UC); oral squamous cell carcinoma (OSCC); brain metastases (BM); lung adenocarcinoma (LUAD); nasopharyngeal carcinoma cells (NPC). | ||
| Genomics | DNA | BLCA,27,28 OV,29 TGCT,30 ESCC,31 BRCA,32 TNBC33 |
| Transcriptomics | mRNA (biological samples) | LUAD,36–39 CESC,40–43 OV,44–48 SRCGC,49 HNSC,50,51 TNBC,52 OS,53 ESCA54 |
| Single-cell transcriptomics | mRNA (single-cell) | OV,59,60 NSCLC,61 UC62 |
| Proteomics | Proteins | LUNG,66–68 OV,69 HNSC,70,71 OSCC72 |
| Metabolomics | Metabolites | OV,79–85 BM of LUAD86 |
| Epigenetics | Epigenetic modifications | BLCA,92 OV,93–95 NPC,96,100 LUAD,97 NSCLC99 |
Author contributions
Conceptualization, Bingjie Han; writing – original draft preparation, Ping Yue; writing – review and editing, Yi Zhao and Bingjie Han; visualization, Ping Yue and Bingjie Han; supervision, Yi Zhao and Bingjie Han; funding acquisition, Yi Zhao and Bingjie Han. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors would like to thank everyone who provided productive suggestions. This work was funded by the National Natural Science Foundation of China (22007085 & 81903448), the Postdoctoral Science Foundation of China (2020M682366), the Henan Medical Science and Technology Joint Building Program (LHGJ20220417) and the starting fund for postdoctoral research of the First Affiliated Hospital of Zhengzhou University.References
- H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram and A. Jemal, et al., Global cancer statistics 2020: globocan stimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA. Cancer J. Clin., 2021, 71(3), 209–249 CrossRef PubMed.
- I. Dagogo-Jack and A. T. Shaw, Tumour heterogeneity and resistance to cancer therapies, Nat. Rev. Clin. Oncol., 2018, 15(2), 81–94 CrossRef CAS PubMed.
- M. Saitoh, Involvement of partial EMT in cancer progression, J. Biochem., 2018, 164(4), 257–264 CrossRef CAS PubMed.
- L. Kelland, The resurgence of platinum-based cancer chemotherapy, Nat. Rev. Cancer, 2007, 7(8), 573–584 CrossRef CAS PubMed.
- D. W. Shen, L. M. Pouliot, M. D. Hall and M. M. Gottesman, Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes, Pharmacol. Rev., 2012, 64(3), 706–721 CrossRef CAS PubMed.
- S. H. Chen and J. Y. Chang, New insights into mechanisms of cisplatin resistance: from tumor cell to microenvironment, Int. J. Mol. Sci., 2019, 20(17), 4136–4157 CrossRef PubMed.
- Y. J. Heo, C. Hwa, G. H. Lee, J. M. Park and J. Y. An, Integrative multi-omics approaches in cancer research: from biological networks to clinical subtypes, Mol. Cells, 2021, 44(7), 433–443 CrossRef CAS PubMed.
- W. W. Soon, M. Hariharan and M. P. Snyder, High-throughput sequencing for biology and medicine, Mol. Syst. Biol., 2013, 9, 640–654 CrossRef PubMed.
- S. Rai, U. Raj and P. K. Varadwaj, Systems biology: a powerful tool for drug development, Curr. Top. Med. Chem., 2018, 18(20), 1745–1754 CrossRef CAS PubMed.
- B. E. Slatko, A. F. Gardner and F. M. Ausubel, Overview of next-generation sequencing technologies, Curr. Protoc. Mol. Biol., 2018, 122(1), e59–e70 Search PubMed.
- E. R. Mardis, DNA sequencing technologies: 2006-2016, Nat. Protoc., 2017, 12(2), 213–218 CrossRef CAS PubMed.
- C. Lee, S. E. Antonarakis, A. Hamosh and J. Burn, Three decades of the human genome organization, Am. J. Med. Genet., Part A, 2021, 185(11), 3314–3321 CrossRef PubMed.
- P. A. McGettigan, Transcriptomics in the RNA-seq era, Curr. Opin. Chem. Biol., 2013, 17(1), 4–11 CrossRef CAS PubMed.
- C. Paolillo, E. Londin and P. Fortina, Single-cell genomics, Clin. Chem., 2019, 65(8), 972–985 CrossRef CAS PubMed.
- C. H. Waddington, The epigenotype, Int. J. Epidemiol., 2012, 41(1), 10–13 CrossRef CAS PubMed.
- A. D. Goldberg, C. D. Allis and E. Bernstein, Epigenetics: a landscape takes shape, Cell, 2007, 128(4), 635–638 CrossRef CAS PubMed.
- P. Peixoto, P. F. Cartron, A. A. Serandour and E. Hervouet, From 1957 to nowadays: a brief history of epigenetics, Int. J. Mol. Sci., 2020, 21(20), 7571–7589 CrossRef CAS PubMed.
- P. J. Park, Epigenetics meets next-generation sequencing, Epigenetics, 2008, 3(6), 318–321 CrossRef PubMed.
- R. G. Cooks, K. L. Busch and G. L. Glish, Mass spectrometry: analytical capabilities and potentials, Science, 1983, 222(4621), 273–291 CrossRef CAS PubMed.
- B. Domon and R. Aebersold, Mass spectrometry and protein analysis, Science, 2006, 312(5771), 212–217 CrossRef CAS PubMed.
- X. M. Han, A. Aslanian and J. R. Yates, Mass spectrometry for proteomics, Curr. Opin. Chem. Biol., 2008, 12(5), 483–490 CrossRef CAS PubMed.
- N. Li, Z. Xu, L. Zhai, Y. Li, F. Fan and J. Zheng, et al., Rapid development of proteomics in China: from the perspective of the human liver proteome project and technology development, Sci. China: Life Sci., 2014, 57(12), 1162–1171 CrossRef CAS PubMed.
- A. F. Altelaar, J. Munoz and A. J. Heck, Next-generation proteomics: towards an integrative view of proteome dynamics, Nat. Rev. Genet., 2013, 14(1), 35–48 CrossRef CAS PubMed.
- C. H. Johnson, J. Ivanisevic and G. Siuzdak, Metabolomics: beyond biomarkers and towards mechanisms, Nat. Rev. Mol. Cell Biol., 2016, 17(7), 451–459 CrossRef CAS PubMed.
- O. Fiehn, Metabolomics--the link between genotypes and phenotypes, Plant Mol. Biol., 2002, 48(1–2), 155–171 CrossRef CAS PubMed.
- R. Kramer and D. Cohen, Functional genomics to new drug targets, Nat. Rev. Drug Discovery, 2004, 3(11), 965–972 CrossRef CAS PubMed.
- Z. D. Shi, L. Hao, X. X. Han, Z. X. Wu, K. Pang and Y. Dong, et al., Targeting HNRNPU to overcome cisplatin resistance in bladder cancer, Mol. Cancer, 2022, 21(1), 37–53 CrossRef CAS PubMed.
- A. Goodspeed, A. Jean and J. C. Costello, A whole-genome CRISPR screen identifies a role of MSH2 in cisplatin-mediated cell death in muscle-invasive bladder cancer, Eur. Urol., 2019, 75(2), 242–250 CrossRef CAS PubMed.
- E. H. Stover, M. B. Baco, O. Cohen, Y. Y. Li, E. L. Christie and M. Bagul, et al., Pooled genomic screens identify anti-apoptotic genes as targetable mediators of chemotherapy resistance in ovarian cancer, Mol. Cancer Res., 2019, 17(11), 2281–2293 CrossRef CAS PubMed.
- J. M. Piulats, A. Vidal, F. J. Garcia-Rodriguez, C. Munoz, M. Nadal and C. Moutinho, et al., Orthoxenografts of testicular germ cell tumors demonstrate genomic changes associated with cisplatin resistance and identify PDMP as a resensitizing agent, Clin. Cancer Res., 2018, 24(15), 3755–3766 CrossRef CAS PubMed.
- T. Yan, H. Cui, Y. Zhou, B. Yang, P. Kong and Y. Zhang, et al., Multi-region sequencing unveils novel actionable targets and spatial heterogeneity in esophageal squamous cell carcinoma, Nat. Commun., 2019, 10, 1670–1685 CrossRef PubMed.
- F. Shao, X. Lyu, K. Miao, L. Xie, H. Wang and H. Xiao, et al., Enhanced protein damage clearance induces broad drug resistance in multitype of cancers revealed by an evolution drug-resistant model and genome-wide siRNA screening, Adv. Sci., 2020, 7(23), 2001914 CrossRef CAS PubMed.
- D. P. Hill, A. Harper, J. Malcolm, M. S. McAndrews, S. M. Mockus and S. E. Patterson, et al., Cisplatin-resistant triple-negative breast cancer subtypes: multiple mechanisms of resistance, BMC Cancer, 2019, 19(1), 1039–1052 CrossRef PubMed.
- T. E. Royce, J. S. Rozowsky and M. B. Gerstein, Toward a universal microarray: prediction of gene expression through nearest-neighbor probe sequence identification, Nucleic Acids Res., 2007, 35(15), e99–e109 CrossRef PubMed.
- Z. Wang, M. Gerstein and M. Snyder, RNA-Seq: a revolutionary tool for transcriptomics, Nat. Rev. Genet., 2009, 10(1), 57–63 CrossRef CAS PubMed.
- Q. L. Zhang, R. Shi, Y. K. Bai, L. J. Meng, J. W. Hu and H. Y. Zhu, et al., Meiotic nuclear divisions 1 (MND1) fuels cell cycle progression by activating a KLF6/E2F1 positive feedback loop in lung adenocarcinoma, Cancer Commun., 2021, 41(6), 492–510 CrossRef PubMed.
- L. Hu, J. Chen, F. Zhang, J. Wang, J. Pan and J. Chen, et al., Aberrant long noncoding RNAs expression profiles affect cisplatin resistance in lung adenocarcinoma, Biomed Res. Int., 2017, 2017, 7498151 Search PubMed.
- Y. Yang, H. Li, S. Hou, B. Hu, J. Liu and J. Wang, The noncoding RNA expression profile and the effect of lncRNA AK126698 on cisplatin resistance in non-small-cell lung cancer cell, PLoS One, 2013, 8(5), e65309–e65321 CrossRef CAS PubMed.
- L. M. Leon, M. Gautier, R. Allan, M. Ilie, N. Nottet and N. Pons, et al., The nuclear hypoxia-regulated NLUCAT1 long non-coding RNA contributes to an aggressive phenotype in lung adenocarcinoma through regulation of oxidative stress, Oncogene, 2019, 38(46), 7146–7165 CrossRef PubMed.
- Y. M. Wang, L. N. Liu and Z. Chen, Transcriptome profiling of cervical cancer cells acquired resistance to cisplatin by deep sequencing, Artif. Cells, Nanomed., Biotechnol., 2019, 47(1), 2820–2829 CrossRef CAS PubMed.
- C. Pan, J. Kang, J. S. Hwang, J. Li, A. C. Boese and X. Wang, et al., Cisplatin-mediated activation of glucocorticoid receptor induces platinum resistance via MAST1, Nat. Commun., 2021, 12(1), 4960–4975 CrossRef CAS PubMed.
- H. M. Lv, S. S. Jin, B. B. Zou, Y. X. Liang, J. Xie and S. H. Wu, Analyzing the whole-transcriptome profiles of ncRNAs and predicting the competing endogenous RNA networks in cervical cancer cell lines with cisplatin resistance, Cancer Cell Int., 2021, 21(1), 532–545 CrossRef CAS PubMed.
- W. Y. Zhang, Y. J. Liu, Y. He and P. Chen, Suppression of long noncoding RNA NCK1-AS1 increases chemosensitivity to cisplatin in cervical cancer, J. Cell. Physiol., 2019, 234(4), 4302–4313 CrossRef CAS PubMed.
- X. Xu, Z. Zheng, L. Jia, S. Suo, B. Liu and T. Shao, et al., Overexpression of SMARCA2 or CAMK2D is associated with cisplatin resistance in human epithelial ovarian cancer, Oncol. Lett., 2018, 16(3), 3796–3804 Search PubMed.
- C. C. Dai, P. F. Xu, S. Y. Liu, S. J. Xu, J. Xu and Z. Y. Fu, et al., Long noncoding RNA ZEB1-AS1 affects paclitaxel and cisplatin resistance by regulating MMP19 in epithelial ovarian cancer cells, Arch. Gynecol. Obstet., 2021, 303(5), 1271–1281 CrossRef CAS PubMed.
- D. P. Bartel, MicroRNAs: genomics, biogenesis, mechanism, and function, Cell, 2004, 116(2), 281–297 CrossRef CAS PubMed.
- X. D. Li, W. Chen, Y. H. Jin, R. X. Xue, J. C. Su and Z. K. Mu, et al., miR-142-5p enhances cisplatin-induced apoptosis in ovarian cancer cells by targeting multiple anti-apoptotic genes, Biochem. Pharmacol., 2019, 161, 98–112 CrossRef CAS PubMed.
- W. Chen, J. Du, X. Li, Z. Zhi and S. Jiang, microRNA-137 downregulates MCL1 in ovarian cancer cells and mediates cisplatin-induced apoptosis, Pharmacogenomics, 2020, 21(3), 195–207 CrossRef CAS PubMed.
- N. L. L. Phoo, P. Dejkriengkraikul, P. Khaw-On and S. Yodkeeree, Transcriptomic profiling reveals AKR1C1 and AKR1C3 mediate cisplatin resistance in signet ring cell gastric carcinoma via autophagic cell death, Int. J. Mol. Sci., 2021, 22(22), 12512–12531 CrossRef CAS PubMed.
- D. Li, L. Ye, Y. Lei, J. Wan and H. Chen, Downregulation of FoxM1 sensitizes nasopharyngeal carcinoma cells to cisplatin via inhibition of MRN-ATM-mediated DNA repair, BMB Rep., 2019, 52(3), 208–213 CrossRef CAS PubMed.
- R. Y. Liu, Z. Dong, J. Liu, L. Zhou, W. Huang and S. K. Khoo, et al., Overexpression of asparagine synthetase and matrix metalloproteinase 19 confers cisplatin sensitivity in nasopharyngeal carcinoma cells, Mol. Cancer Ther., 2013, 12(10), 2157–2166 CrossRef CAS PubMed.
- J. Kim, G. Jang, S. H. Sim, I. H. Park, K. Kim and C. Park, SMARCA4 depletion induces cisplatin resistance by activating YAP1-mediated epithelial-to-mesenchymal transition in triple-negative breast cancer, Cancers, 2021, 13(21), 5474–5493 CrossRef CAS PubMed.
- C. Cheng, Q. Y. Ding, Z. C. Zhang, S. Y. Wang, B. L. Zhong and X. Huang, et al., PTBP1 modulates osteosarcoma chemoresistance to cisplatin by regulating the expression of the copper transporter SLC31A1, J. Cell. Mol. Med., 2020, 24(9), 5274–5289 CrossRef CAS PubMed.
- S. F. Shi, X. Huang, X. Ma, X. Y. Zhu and Q. X. Zhang, Research of the mechanism on miRNA193 in exosomes promotes cisplatin resistance in esophageal cancer cells, PLoS One, 2020, 15(5), e0225290 CrossRef CAS PubMed.
- J. Peng, B. F. Sun, C. Y. Chen, J. Y. Zhou, Y. S. Chen and H. Chen, et al., Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma, Cell Res., 2019, 29(9), 725–738 CrossRef CAS PubMed.
- L. Ma, M. O. Hernandez, Y. Zhao, M. Mehta, B. Tran and M. Kelly, et al., Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer, Cancer Cell, 2019, 36(4), 418–430 CrossRef CAS PubMed e416.
- A. P. Patel, I. Tirosh, J. J. Trombetta, A. K. Shalek, S. M. Gillespie and H. Wakimoto, et al., Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma, Science, 2014, 344(6190), 1396–1401 CrossRef CAS PubMed.
- S. V. Puram, I. Tirosh, A. S. Parikh, A. P. Patel, K. Yizhak and S. Gillespie, et al., Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer, Cell, 2017, 171(7), 1611–1624 CrossRef CAS PubMed e1624.
- H. Zhao, Y. Gao, J. Miao, S. Chen, J. Li and Z. Li, et al., Single-cell RNA-seq highlights a specific carcinoembryonic cluster in ovarian cancer, Cell Death Dis., 2021, 12(11), 1082–1093 CrossRef CAS PubMed.
- H. Zhao, Z. Li, Y. Gao, J. Li, X. Zhao and W. Yue, Single-cell RNA-sequencing portraying functional diversity and clinical implications of IFI6 in ovarian cancer, Front. Cell Dev. Biol., 2021, 9, 677697 CrossRef PubMed.
- C. Liu, Y. Zhang, X. Li and D. Wang, Ovarian cancer-specific dysregulated genes with prognostic significance: scRNA-Seq with bulk RNA-Seq data and experimental validation, Ann. N. Y. Acad. Sci., 2022, 1512, 154–173 CrossRef CAS PubMed.
- N. Tanaka, S. Katayama, A. Reddy, K. Nishimura, N. Niwa and H. Hongo, et al., Single-cell RNA-seq analysis reveals the platinum resistance gene COX7B and the surrogate marker CD63, Cancer Med., 2018, 7(12), 6193–6204 CrossRef CAS PubMed.
- Z. Noor, S. B. Ahn, M. S. Baker, S. Ranganathan and A. Mohamedali, Mass spectrometry-based protein identification in proteomics-a review, Briefings Bioinf., 2021, 22(2), 1620–1638 CrossRef CAS PubMed.
- N. M. Riley, A. S. Hebert and J. J. Coon, Proteomics moves into the fast lane, Cell Syst., 2016, 2(3), 142–143 CrossRef CAS PubMed.
- N. L. Anderson and N. G. Anderson, Proteome and proteomics: new technologies, new concepts, and new words, Electrophoresis, 1998, 19(11), 1853–1861 CrossRef CAS PubMed.
- D. Wang, C. Zhao, F. Xu, A. Zhang, M. Jin and K. Zhang, et al., Cisplatin-resistant NSCLC cells induced by hypoxia transmit resistance to sensitive cells through exosomal PKM2, Theranostics, 2021, 11(6), 2860–2875 CrossRef CAS PubMed.
- S. T. Pan, J. Zhou, F. Yang, S. F. Zhou and T. Ren, Proteomics reveals a therapeutic vulnerability via the combined blockade of APE1 and autophagy in lung cancer A549 cells, BMC Cancer, 2020, 20(1), 634–645 CrossRef CAS PubMed.
- W. Zeng, S. Zheng, Y. Mao, S. Wang, Y. Zhong and W. Cao, et al., Elevated N-glycosylation contributes to the cisplatin resistance of non-small cell lung cancer cells revealed by membrane proteomic and glycoproteomic analysis, Front. Pharmacol., 2021, 12, 805499 CrossRef CAS PubMed.
- J. Li, C. Pan, A. C. Boese, J. Kang, A. D. Umano and K. R. Magliocca, et al., DGKA provides platinum resistance in ovarian cancer through activation of c-JUN-WEE1 signaling, Clin. Cancer Res., 2020, 26(14), 3843–3855 CrossRef CAS PubMed.
- Y. Garcia-Mayea, C. Mir, L. Carballo, J. Castellvi, J. Temprana-Salvador and J. Lorente, et al., TSPAN1: a novel protein involved in head and neck squamous cell carcinoma chemoresistance, Cancers, 2020, 12(11), 3269–3289 CrossRef CAS PubMed.
- C. Mir, Y. Garcia-Mayea, L. Garcia, P. Herrero, N. Canela and R. Tabernero, et al., SDCBP modulates stemness and chemoresistance in head and neck squamous cell carcinoma through Src activation, Cancers, 2021, 13(19), 4952–4970 CrossRef CAS PubMed.
- O. Shriwas, R. Arya, S. Mohanty, P. Mohapatra, S. Kumar and R. Rath, et al., RRBP1 rewires cisplatin resistance in oral squamous cell carcinoma by regulating Hippo pathway, Br. J. Cancer, 2021, 124(12), 2004–2016 CrossRef CAS PubMed.
- F. De Castro, M. Benedetti, L. Del Coco and F. P. Fanizzi, NMR-based metabolomics in metal-based drug research, Molecules, 2019, 24(12), 2240–2254 CrossRef CAS PubMed.
- K. A. Vermeersch and M. P. Styczynski, Applications of metabolomics in cancer research, J. Carcinog., 2013, 12, 9–18 CrossRef PubMed.
- Y. Zhao, E. B. Butler and M. Tan, Targeting cellular metabolism to improve cancer therapeutics, Cell Death Dis., 2013, 4, e532–e542 CrossRef CAS PubMed.
- H. H. Chen and M. T. Kuo, Role of glutathione in the regulation of cisplatin resistance in cancer chemotherapy, Met. Based Drugs, 2010, 2010, 430939 Search PubMed.
- Y. Shi, Y. Wang, W. Huang, Y. Wang, R. Wang and Y. Yuan, Integration of metabolomics and transcriptomics to reveal metabolic characteristics and key targets associated with cisplatin resistance in nonsmall cell lung cancer, J. Proteome Res., 2019, 18(9), 3259–3267 CrossRef CAS PubMed.
- M. Vermathen, H. von Tengg-Kobligk, M. N. Hungerbuhler, P. Vermathen and N. Ruprecht, 1)H HR-MAS NMR based metabolic profiling of lung cancer cells with induced and de-induced cisplatin resistance to reveal metabolic resistance adaptations, Molecules, 2021, 26(22), 6766–6781 CrossRef CAS PubMed.
- D. Catanzaro, E. Gaude, G. Orso, C. Giordano, G. Guzzo and A. Rasola, et al., Inhibition of glucose-6-phosphate dehydrogenase sensitizes cisplatin-resistant cells to death, Oncotarget, 2015, 6(30), 30102–30114 CrossRef PubMed.
- J. Guo, K. Satoh, S. Tabata, M. Mori, M. Tomita and T. Soga, Reprogramming of glutamine metabolism via glutamine synthetase silencing induces cisplatin resistance in A2780 ovarian cancer cells, BMC Cancer, 2021, 21(1), 174–185 CrossRef CAS PubMed.
- L. M. Poisson, A. Munkarah, H. Madi, I. Datta, S. Hensley-Alford and C. Tebbe, et al., A metabolomic approach to identifying platinum resistance in ovarian cancer, J. Ovarian Res., 2015, 8, 13–27 CrossRef PubMed.
- F. Obrist, J. Michels, S. Durand, A. Chery, J. Pol and S. Levesque, et al., Metabolic vulnerability of cisplatin-resistant cancers, EMBO J., 2018, 37(14), e98597–e98612 CrossRef PubMed.
- M. Y. Lee, A. Yeon, M. Shahid, E. Cho, V. Sairam and R. Figlin, et al., Reprogrammed lipid metabolism in bladder cancer with cisplatin resistance, Oncotarget, 2018, 9(17), 13231–13243 CrossRef PubMed.
- X. Chen, S. W. Chen and D. S. Yu, Metabolic reprogramming of chemoresistant cancer cells and the potential significance of metabolic regulation in the reversal of cancer chemoresistance, Metabolites, 2020, 10(7), 289–304 CrossRef CAS PubMed.
- F. Ricci, L. Brunelli, R. Affatato, R. Chila, M. Verza and S. Indraccolo, et al., Overcoming platinum-acquired resistance in ovarian cancer patient-derived xenografts, Ther. Adv. Med. Oncol., 2019, 11, 1758835919839543 CAS.
- W. Liu, Y. Zhou, W. Duan, J. Song, S. Wei and S. Xia, et al., Glutathione peroxidase 4-dependent glutathione high-consumption drives acquired platinum chemoresistance in lung cancer-derived brain metastasis, Clin. Transl. Med., 2021, 11(9), e517–e540 CAS.
- R. Holliday, The inheritance of epigenetic defects, Science, 1987, 238(4824), 163–170 CrossRef CAS PubMed.
- T. Jenuwein and C. D. Allis, Translating the histone code, Science, 2001, 293(5532), 1074–1080 CrossRef CAS PubMed.
- D. Chung, Histone modification: the 'next wave' in cancer therapeutics, Trends Mol. Med., 2002, 8(4), S10–S11 CrossRef PubMed.
- P. A. Jones and S. B. Baylin, The epigenomics of cancer, Cell, 2007, 128(4), 683–692 CrossRef CAS PubMed.
- I. Keshet, J. Lieman-Hurwitz and H. Cedar, DNA methylation affects the formation of active chromatin, Cell, 1986, 44(4), 535–543 CrossRef CAS PubMed.
- A. Yeon, S. You, M. Kim, A. Gupta, M. H. Park and D. J. Weisenberger, et al., Rewiring of cisplatin-resistant bladder cancer cells through epigenetic regulation of genes involved in amino acid metabolism, Theranostics, 2018, 8(16), 4520–4534 CrossRef CAS PubMed.
- D. Kritsch, F. Hoffmann, D. Steinbach, L. Jansen, S. Mary Photini and M. Gajda, et al., Tribbles 2 mediates cisplatin sensitivity and DNA damage response in epithelial ovarian cancer, Int. J. Cancer, 2017, 141(8), 1600–1614 CrossRef CAS PubMed.
- D. Liu, X. X. Zhang, M. C. Li, C. H. Cao, D. Y. Wan and B. X. Xi, et al., C/EBPβ enhances platinum resistance of ovarian cancer cells by reprogramming H3K79 methylation, Nat. Commun., 2018, 9, 1739–1752 CrossRef PubMed.
- G. Wu, H. Peng, M. Tang, M. Yang, J. Wang and Y. Hu, et al., ZNF711 down-regulation promotes CISPLATIN resistance in epithelial ovarian cancer via interacting with JHDM2A and suppressing SLC31A1 expression, EBioMedicine, 2021, 71, 103558 CrossRef CAS PubMed.
- Z. Q. Zheng, Z. X. Li, J. L. Guan, X. Liu, J. Y. Li and Y. Chen, et al., Long noncoding RNA TINCR-mediated regulation of acetyl-CoA metabolism promotes nasopharyngeal carcinoma progression and chemoresistance, Cancer Res., 2020, 80(23), 5174–5188 CrossRef CAS PubMed.
- A. Wang, Z. Ning, C. Lu, W. Gao, J. Liang and Q. Yan, et al., USP22 induces cisplatin resistance in lung adenocarcinoma by regulating gamma γH2AX-mediated DNA damage repair and Ku70/Bax-mediated apoptosis, Front. Pharmacol., 2017, 8, 274–285 CrossRef PubMed.
- X. Han, M. Wang, Y. L. Zhao, Y. Yang and Y. G. Yang, RNA methylations in human cancers, Semin. Cancer Biol., 2021, 75, 97–115 CrossRef CAS PubMed.
- Y. Shi, S. Fan, M. Wu, Z. Zuo, X. Li and L. Jiang, et al., YTHDF1 links hypoxia adaptation and non-small cell lung cancer progression, Nat. Commun., 2019, 10(1), 4892–4906 CrossRef PubMed.
- R. Zhang, S. W. Li, L. Liu, J. Yang, G. Huang and Y. Sang, TRIM11 facilitates chemoresistance in nasopharyngeal carcinoma by activating the beta-catenin/ABCC9 axis via p62-selective autophagic degradation of Daple, Oncogenesis, 2020, 9(5), 45–64 CrossRef CAS PubMed.
- M. A. Wörheide, J. Krumsiek, G. Kastenmüller and M. Arnold, Multi-omics integration in biomedical research – A metabolomics-centric review, Anal. Chim. Acta, 2021, 1141, 144–162 CrossRef PubMed.
- I. Subramanian, S. Verma, S. Kumar, A. Jere and K. Anamika, Multi-omics data integration, interpretation, and its application, Bioinf. Biol. Insights, 2020, 14, 1–24 Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |
