Open Access Article
Open Access ArticleA regio- and stereoselective Heck–Matsuda process for construction of γ-aryl allylsulfonyl fluorides†
Hao-Yong Qin,
Houying Gui,
Zai-Wei Zhang,
Tao Shu* and
Hua-Li Qin *
*
School of Chemistry, Chemical Engineering and Life Sciences, Wuhan University of Technology, Wuhan 430070, China. E-mail: tao.shu@whut.edu.cn; qinhuali@whut.edu.cn
First published on 4th July 2022
Abstract
A highly efficient regio- and stereoselective Heck–Matsuda method was developed employing aryl diazoniums and allylsulfonyl fluorides for the construction of a class of novel γ-aryl allylsulfonyl fluorides in the presence of Pd(OAc)2 and PPh3. The method features excellent regio- and stereoselectivity (up to 100% E-selectivity), broad substrate scope and mild reaction conditions. Further application of γ-aryl allylsulfonyl fluoride in SuFEx reactions was achieved to provide their corresponding sulfonates and sulfonamides in excellent yields.
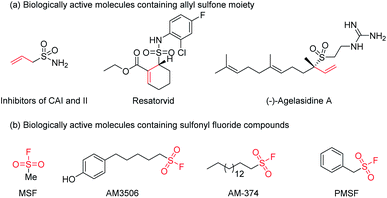
Sulfur(VI) fluoride exchange (SuFEx), firstly developed by K. B. Sharpless and co-workers in 2014,1 has gained burgeoning attention in the fields of drug discovery,2 protein target identification,3 bioconjugation,4 surface chemistry,5 and polymer chemistry.6 The sulfonyl fluoride moiety as a vital functionality of SuFEx chemistry is widely applied in many fields based on the unique balance between aqueous stability and chemical reactivity of the SV–F bond.7 Aliphatic sulfonyl fluorides, as a class of representative sulfonyl fluoride molecules have been utilized as privileged “warheads” in enzyme inhibitors for decades,8 involving MSF,9 AM3506,10 AM-374,11 and PMSF12 (Fig. 1). Allylic sulfones also have emerged as important building blocks in organic synthesis,13 which widely present in a large variety of biologically active compounds (Fig. 1a).14
Considering the increasing importance of allyl sulfone moiety and sulfonyl fluoride functionality in various fields, the simultaneous introduction of these two moieties into one molecule is highly desirable. However, due to the lack of efficient methods, access to γ-substituted allyl sulfonyl fluoride derivatives still remains less explored. Hence, it is of great significance to design and synthesize various sulfonyl fluoride compounds for the further SuFEx reactions.
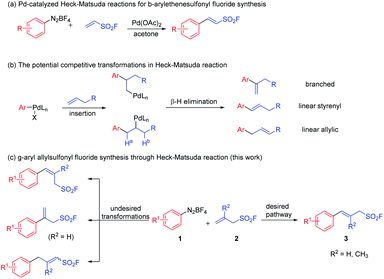
The Heck–Matsuda reaction has been recognized as one of the most versatile and powerful synthetic tools for the construction of C–C bond since its discovery by Matsuda.15 Palladium-catalyzed Heck–Matsuda reaction for the synthesis of β-arylethenesulfonyl fluorides from aryl diazonium tetrafluoroborates with exclusive regio- and stereoselectivity has been well studied (Scheme 1a).16 Inspired by this seminal work, we assumed that the Heck–Matsuda reaction would also offer a feasible approach to construct unprecedented γ-aryl allylsulfonyl fluoride molecules. However, controlling regioselectivity and stereoselectiveity of Heck–Matsuda reaction with allylsulfonyl fluoride is still challenging.17 The low selectivity mainly results from the uncontrollable migratory insertion into the olefins (branched vs. linear olefin products) and indiscriminate β-hydride elimination with either Ha or Hb (styrenyl vs. allylic linear olefin products) (Scheme 1b).18 Herein, we report our recent progress of palladium-catalyzed highly regio- and stereoselective Heck–Matsuda reaction for the construction of γ-aryl allylsulfonyl fluorides (3) (Scheme 1c).
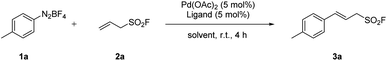
Our initial investigation started with the reaction of 4-methylbenzenediazonium tetrafluoroborate (1a, 0.2 mmol) and allylsulfonyl fluoride (2a, 0.4 mmol) in the presence of a catalytic amount of Pd(OAc)2 and PPh3 ligand at 25 °C in DMF (Table 1, entry 1).
| Entry | Ligand | 2a (x equiv.) | Solvent | Yield (%) |
|---|---|---|---|---|
a Reaction conditions: aryldiazonium tetrafluoroborate (1a, 0.2 mmol), Pd(OAc)2 (5 mol%) and PPh3 (5 mol%) were dissolved in solvent (0.2 M, 2.0 mL) before the subsequent addition of allylsulfonyl fluoride (0.4 mmol, 2.0 eq.). Then the resulting mixture was stirred at room temperature for 4 h.b The yields were determined by HPLC using pure 3a as the external standard (tR = 6.1 min, λmax = 258.3 nm, acetonitrile/water = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v)).c 2 mol% Pd(OAc)2, 2 mol% PPh3.d 3 mol% Pd(OAc)2, 3 mol% PPh3. 20 (v/v)).c 2 mol% Pd(OAc)2, 2 mol% PPh3.d 3 mol% Pd(OAc)2, 3 mol% PPh3. |
||||
| 1 | PPh3 | 2.0 | DMF | 66 |
| 2 | Dppb | 2.0 | DMF | 36 |
| 3 | Dppf | 2.0 | DMF | Trace |
| 4 | PPh3 | 2.0 | DMA | 51 |
| 5 | PPh3 | 2.0 | MeOH | 71 |
| 6 | PPh3 | 2.0 | Acetone | 16 |
| 7c | PPh3 | 2.0 | MeOH | 61 |
| 8d | PPh3 | 2.0 | MeOH | 72 |
| 9d | PPh3 | 1.1 | MeOH | 78 |
| 10d | PPh3 | 1.2 | MeOH | 78 |
| 11d | PPh3 | 1.5 | MeOH | 76 |
To our delight, the 1H NMR of crude product indicated that the (E)-styrenyl product 3a was formed exclusively, while the possible isomeric products were not observed. Subsequently, a variety of ligands such as triphenylphosphine, 1,4-bis(diphenylphosphino) butane (dppb), 1,1′-bis(diphenylphosphino)ferrocene (dppf) were screened, and triphenylphosphine was found to be the optimal ligand for this process (Table 1, entry 1–3). The screening of the solvent indicated that methanol was the best choice (Table 1, entry 4–6). Subsequent examination on the loading of catalyst and ligand revealed that 3 mol% Pd(OAc)2 and 3 mol% PPh3 were essential (Table 1, entry 7–8). Reducing the amount of allylsulfonyl fluoride from 2.0 equivalents to 1.1 equivalents led to nearly identical yields of desired product 3a (Table 1, entry 9–11). Therefore, Pd(OAc)2 (3 mol%), PPh3 (3 mol%), allylsulfonyl fluoride (1.1 equiv.) in MeOH (0.1 M) was eventually selected as the optimized conditions for the process.
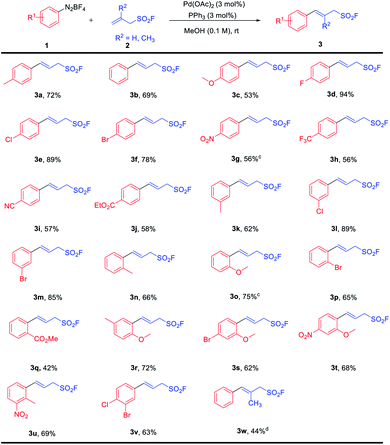
With the optimized reaction conditions in hand, we next evaluated the substrate scope and functional group tolerance of this protocol using various aryldiazonium tetrafluoroborates (1) and allylsulfonyl fluorides (2). It turned out to be that our protocol was compatible with a broad range of substrates, delivering the desired products with excellent selectivity in most cases. Besides, the substrates bearing whether electron-donating groups (1a, 1c, 1k, 1n, 1o, and 1r) or electron-withdrawing groups (1d–1j, 1l, 1m, 1q, and 1v) all worked well. In addition, ortho-, meta-, or para-mono-substituted and multi-substituted diazonium (1r–1v) salts all afforded their corresponding products in moderate to good yields. It is worth noting that the halogen atom on the aromatic rings of aryldiazonium tetrafluoroborates (1d–1f, 1l, 1m, 1p, and 1v) tolerated well and delivered their corresponding products in acceptable yields, while the C (sp2)–X bond remained untouched during the transformations. Nitro-substituted (1g) diazonium salt was also successfully converted into the corresponding allyl sulfonyl fluoride with moderate yield (3g, 56% yield) (Table 2). Furthermore, the allylsulfonyl fluoride with a methyl substituent on the vinyl skeleton (2b) was also compatible with the reaction system, albeit a moderate yield of the product (3w) was achieved. And the E configuration of compound 3w was confirmed by NOE analysis (see the ESI† for details).
a Reaction conditions: aryldiazonium tetrafluoroborate (1, 1.0 mmol), Pd(OAc)2 (3 mol%) and PPh3 (3 mol%) were dissolved in MeOH (0.1 M), before the subsequent addition of allylsulfonyl fluoride (1.1 mmol, 1.1 eq.). The resulting mixture was stirred at room temperature under air. The selectivity for (E)-styrene was >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 unless otherwise noted. The selectivity is (E)-styrene: (all other isomers), as determined by 1H NMR spectroscopy.b Isolated yields.c The selectivity for (E)-styrene was >10 1 unless otherwise noted. The selectivity is (E)-styrene: (all other isomers), as determined by 1H NMR spectroscopy.b Isolated yields.c The selectivity for (E)-styrene was >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.d The selectivity for (E)-styrene was = 9 1.d The selectivity for (E)-styrene was = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. |
|---|
 |
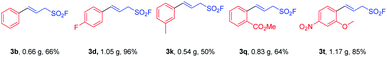
To demonstrate the practicality of this method, a series of gram-scale reactions were performed under the optimal reaction conditions (Scheme 2). And the research results revealed that these reactions worked well to transform the corresponding aryldiazonium tetrafluoroborates (1b, 1d, 1k, 1q, and 1t) into their corresponding products (3b, 3d, 3k, 3q, and 3t) in moderate to excellent yields (50–96%).
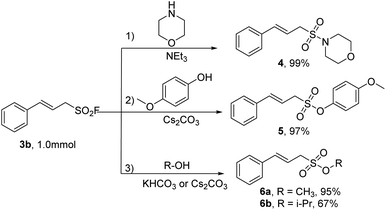
Some extended work focusing on the chemical transformations of this class of novel sulfonyl fluoride molecules was carried out as described in the Scheme 3. (E)-3-Phenylprop-2-ene-1-sulfonyl fluoride (3b) was successfully converted into sulfonamide (4) in nearly quantitative yield (99%) when coupled with morpholine in the presence of Et3N. Furthermore, the SuFEx reactions of (E)-3-phenylprop-2-ene-1-sulfonyl fluoride (3b) with phenol or alcohols also proceeded smoothly, delivering the sulfonates (5, 6a, and 6b) in good to excellent yields (67–97%).
Conclusions
In conclusion, we have successfully developed a mild, efficient, and robust Heck–Matsuda reaction for highly regio- and stereoselective construction of γ-aryl allylsulfonyl fluorides. The application of these novel molecules in SuFEx chemistry was also demonstrated. Further studies on the biological activity of these novel sulfonyl fluorides and chemical transformations of allylsulfonyl fluorides reagent are undergoing in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the National Natural Science Foundation of China (Grant No. 22071190 and 21772150), the National Key Research and Development Program of China (2018YFA0702001), Fundamental Research Funds for the Central Universities (WUT: 2021IVA072) and Wuhan University of Technology for the financial support.Notes and references
- J. Dong, L. Krasnova, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2014, 53, 9430 CrossRef CAS PubMed.
- (a) S. Kitamura, Q. Zheng, J. L. Woehl, A. Solania, E. Chen, N. Dillon, M. V. Hull, M. Kotaniguchi, J. R. Cappiello, S. Kitamura, V. Nizet, K. B. Sharpless and D. W. Wolan, J. Am. Chem. Soc., 2020, 142, 10899 CrossRef CAS PubMed; (b) F. C. Sousa e Silva, K. Doktor and Q. Michaudel, Org. Lett., 2021, 23, 5271 CrossRef CAS PubMed; (c) J.-X. Zhou, D.-Y. Zhu, J. Chen, X.-J. Zhang, M. Yan and A. S. C. Chan, Tetrahedron Lett., 2021, 65, 152792 CrossRef CAS; (d) A. S. Barrow, C. J. Smedley, Q. Zheng, S. Li, J. Dong and J. E. Moses, Chem. Soc. Rev., 2019, 48, 4731 RSC; (e) C. J. Smedley, Q. Zheng, B. Gao, S. Li, A. Molino, H. M. Duivenvoorden, B. S. Parker, D. J. D. Wilson, K. B. Sharpless and J. E. Moses, Angew. Chem., Int. Ed., 2019, 58, 4552 CrossRef CAS PubMed.
- (a) Q. Zheng, H. Xu, H. Wang, W.-G. H. Du, N. Wang, H. Xiong, Y. Gu, L. Noodleman, K. B. Sharpless, G. Yang and P. Wu, J. Am. Chem. Soc., 2021, 143, 3753 CrossRef CAS PubMed; (b) D. E. Mortenson, G. J. Brighty, L. Plate, G. Bare, W. Chen, S. Li, H. Wang, B. F. Cravatt, S. Forli, E. T. Powers, K. B. Sharpless, I. A. Wilson and J. W. Kelly, J. Am. Chem. Soc., 2018, 140, 200 CrossRef CAS PubMed.
- A. Marra, J. Dong, T. Ma, S. Giuntini, E. Crescenzo, L. Cerofolini, M. Martinucci, C. Luchinat, M. Fragai, C. Nativi and A. Dondoni, Chem. – Eur. J., 2018, 24, 18981 CrossRef CAS PubMed.
- J. D. Randall, D. J. Eyckens, F. Stojcevski, P. S. Francis, E. H. Doeven, A. J. Barlow, A. S. Barrow, C. L. Arnold, J. E. Moses and L. C. Henderson, ChemPhysChem, 2018, 19, 3176 CrossRef CAS PubMed.
- (a) B. Gao, L. Zhang, Q. Zheng, F. Zhou, L. M. Klivansky, J. Lu, Y. Liu, J. Dong, P. Wu and K. B. Sharpless, Nat. Chem., 2017, 9, 1083 CrossRef CAS PubMed; (b) H. Wang, F. Zhou, G. Ren, Q. Zheng, H. Chen, B. Gao, L. Klivansky, Y. Liu, B. Wu, Q. Xu, J. Lu, K. B. Sharpless and P. Wu, Angew. Chem., Int. Ed., 2017, 56, 11203 CrossRef CAS PubMed; (c) C. Yang, J. P. Flynn and J. Niu, Angew. Chem., Int. Ed., 2018, 57, 16194 CrossRef CAS PubMed; (d) Z. Li, X. Ren, P. Sun, H. Ding, S. Li, Y. Zhao and K. Zhang, ACS Macro Lett., 2021, 10, 223 CrossRef CAS PubMed.
- (a) T. Abdul Fattah, A. Saeed and F. Albericio, J. Fluorine Chem., 2018, 213, 87 CrossRef CAS; (b) Y.-P. Meng, S.-M. Wang, W.-Y. Fang, Z.-Z. Xie, J. Leng, H. Alsulami and H.-L. Qin, Synthesis, 2020, 52, 673 CrossRef CAS.
- (a) C. Dubiella, H. Cui, M. Gersch, A. J. Brouwer, S. A. Sieber, A. Krüger, R. M. J. Liskamp and M. Groll, Angew. Chem., Int. Ed., 2014, 53, 11969 CrossRef CAS PubMed; (b) R. Artschwager, D. J. Ward, S. Gannon, A. J. Brouwer, H. van de Langemheen, H. Kowalski and R. M. J. Liskamp, J. Med. Chem., 2018, 61, 5395 CrossRef CAS PubMed; (c) R. Xu, T. Xu, M. Yang, T. Cao and S. Liao, Nat. Commun., 2019, 10, 3752 CrossRef PubMed.
- (a) C. V. Borlongan, I. C. Sumaya and D. E. Moss, Brain Res., 2005, 1038, 50 CrossRef CAS PubMed; (b) T. Imanishi, M. M. Hossain, T. Suzuki, P. Xu, I. Sato and H. Kobayashi, Adv. Pharmacol. Sci., 2012, 2012, 708178 Search PubMed; (c) D. E. Moss, R. G. Fariello, J. Sahlmann, I. Sumaya, F. Pericle and E. Braglia, Br. J. Clin. Pharmacol., 2013, 75, 1231 CrossRef CAS PubMed; (d) D. E. Moss, R. G. Perez and H. Kobayashi, J. Alzheimer's Dis., 2017, 55, 1285 CAS.
- D. A. Karanian, Q. B. Brown, A. Makriyannis, T. A. Kosten and B. A. Bahr, J. Neurosci., 2005, 25, 7813 CrossRef CAS PubMed.
- S. O. Alapafuja, S. P. Nikas, I. T. Bharathan, V. G. Shukla, M. L. Nasr, A. L. Bowman, N. Zvonok, J. Li, X. Shi, J. R. Engen and A. Makriyannis, J. Med. Chem., 2012, 55, 10074 CrossRef CAS PubMed.
- (a) J. C. Powers, J. L. Asgian, Ö. D. Ekici and K. E. James, Chem. Rev., 2002, 102, 4639 CrossRef CAS PubMed; (b) A. Narayanan and L. H. Jones, Chem. Sci., 2015, 6, 2650 RSC.
- (a) A. El-Awa, M. N. Noshi, X. M. du Jourdin and P. L. Fuchs, Chem. Rev., 2009, 109, 2315 CrossRef CAS PubMed; (b) A.-N. R. Alba, X. Companyó and R. Rios, Chem. Soc. Rev., 2010, 39, 2018 RSC.
- (a) C. Chazalette, M. Rivière-Baudet, C. T. Supuran and A. Scozzafava, J. Enzyme Inhib., 2001, 16, 475 CrossRef CAS PubMed; (b) F. Reck, F. Zhou, M. Girardot, G. Kern, C. J. Eyermann, N. J. Hales, R. R. Ramsay and M. B. Gravestock, J. Med. Chem., 2005, 48, 499 CrossRef CAS PubMed; (c) E. P. Stout, L. C. Yu and T. F. Molinski, Eur. J. Org. Chem., 2012, 2012, 5131 CrossRef CAS PubMed.
- (a) F. Otte and B. Schmidt, J. Org. Chem., 2019, 84, 14816 CrossRef CAS PubMed; (b) N. Riemer, M. Shipman, P. Wessig and B. Schmidt, J. Org. Chem., 2019, 84, 5732 CrossRef CAS PubMed; (c) J. Le Bras and J. Muzart, Chem. Rev., 2011, 111, 1170 CrossRef CAS PubMed.
- H.-L. Qin, Q. Zheng, G. A. L. Bare, P. Wu and K. B. Sharpless, Angew. Chem., Int. Ed., 2016, 55, 14155 CrossRef CAS PubMed.
- E. W. Werner and M. S. Sigman, J. Am. Chem. Soc., 2011, 133, 9692 CrossRef CAS PubMed.
- (a) C. Tang, R. Zhang, B. Zhu, J. Fu, Y. Deng, L. Tian, W. Guan and X. Bi, J. Am. Chem. Soc., 2018, 140, 16929 CrossRef CAS PubMed; (b) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra03733e |
| This journal is © The Royal Society of Chemistry 2022 |