Strain-release arylations for the bis-functionalization of azetidines†
Abstract
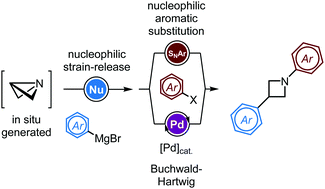
The addition of nucleophilic organometallic species onto in situ generated azabicyclobutanes enables the selective formation of 3-arylated azetidine intermediates through strain-release. Single pot strategies were further developed for the N-arylation of resulting azetidines, employing either SNAr reactions or Buchwald–Hartwig couplings.
- This article is part of the themed collection: 2022 Pioneering Investigators


 Please wait while we load your content...
Please wait while we load your content...