Evolution of a solid electrolyte interphase enabled by FeNX/C catalysts for sodium-ion storage†
Abstract
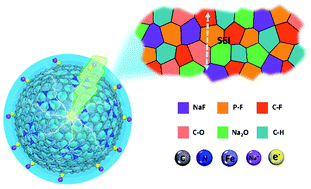
The structure and chemical engineering of a solid electrolyte interphase (SEI) play a vital role in rechargeable batteries. The underlying correlation between the properties of the enhanced sodium (Na) ion storage and SEIs in metal–nitrogen (MNX) platform electrodes was not revealed during charging and discharging cycles. Herein, the capacity enhancement of FeNX/C anodes was first clarified by employing in situ temperature-dependent Nyquist plots and ex situ X-ray photoelectron spectroscopy. It was evidenced that physiochemical evolution of the SEI and surface carbonaceous materials made a significant contribution to the improved sodium storage performance through the following three mechanisms: (1) FeNX catalyzed the reversible conversion of SEIs, beneficial to the storage and release of extra Na ions, (2) a large number of spin-polarized charges were stored on the surface of the reduced Fe species, and (3) the carbon delivered additional capacity through the surface-capacitive effects. As a result, the FeNX/C anode provided a high capacity of 217 mA h g−1 after 1000 cycles at 2000 mA g−1. Therefore, the FeNX species catalyzed the reversible conversion reaction of SEIs, which contributed novel avenues to the design of conversion-type electrode materials.


 Please wait while we load your content...
Please wait while we load your content...