Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Al2O3 co-doped with Cr3+ and Mn4+, a dual-emitter probe for multimodal non-contact luminescence thermometry
V. B.
Mykhaylyk
 *a,
H.
Kraus
b,
L.-I.
Bulyk
c,
I.
Lutsyuk
d,
V.
Hreb
*a,
H.
Kraus
b,
L.-I.
Bulyk
c,
I.
Lutsyuk
d,
V.
Hreb
 d,
L.
Vasylechko
d,
L.
Vasylechko
 d,
Y.
Zhydachevskyy
cd,
A.
Wagner
a and
A.
Suchocki
d,
Y.
Zhydachevskyy
cd,
A.
Wagner
a and
A.
Suchocki
 c
c
aDiamond Light Source, Harwell Campus, Didcot, OX11 0DE, UK. E-mail: vitaliy.mykhaylyk@diamond.ac.uk
bUniversity of Oxford, Department of Physics, Denys Wilkinson Building, Keble Road, Oxford, OX1 3RH, UK
cInstitute of Physics, Polish Academy of Sciences, Al. Lotnikow 32/46, Warsaw 02-668, Poland
dLviv Polytechnic National University, 12 Bandera, Lviv 79013, Ukraine
First published on 1st October 2021
Abstract
Luminescence probes that facilitate multimodal non-contact measurements of temperature are of particular interest due to the possibility of cross-referencing results across different readout techniques. This intrinsic referencing is an essential addition that enhances accuracy and reliability of the technique. A further enhancement of sensor performance can be achieved by using two luminescent ions acting as independent emitters, thereby adding in-built redundancy to non-contact temperature sensing, using a single readout technique. In this study we combine both approaches by engineering a material with two luminescent ions that can be independently probed through different readout modes of non-contact temperature sensing. The approach was tested using Al2O3 co-doped with Cr3+ and Mn4+, exhibiting sharp emission lines due to 2E → 4A2 transitions. The temperature sensing performance was examined by measuring three characteristics: temperature-induced changes of the intensity ratio of the emission lines, their spectral position, and the luminescence decay time constant. The processes responsible for the changes with temperature of the measured luminescence characteristics are discussed in terms of relevant models. By comparing temperature resolutions achievable by different modes of temperature sensing it is established that in Al2O3-Cr,Mn spectroscopic methods provide the best measurement accuracy over a broad temperature range. A temperature resolution better than ±2.8 K can be achieved by monitoring the luminescence intensity ratio (40–145 K) and the spectral shift of the R-line of Mn4+ (145–300 K range).
1. Introduction
When reliable temperature measurements are required in challenging applications, such as in harsh environments or with restricted access to an object of interest, non-contact probes with all their advantages are the temperature sensors of choice.1–3 Examples include very hot or cold apparatus, aggressive chemicals, and strong electromagnetic and radiation fields where the necessity of isolation, shielding, or motion of the object would add complications. Many areas of science and technology benefit from using this method, such as aerospace,4 catalysis,5 life science,6 medicine7 and biology.8,9 Importantly, it is one of the few methods capable of delivering temperature mapping with submicron spatial resolution.10,11 Thus, the field of luminescence thermometry develops rapidly, as demonstrated by a steady stream of publications and reviews.12–17Temperature changes affect a range of luminescence characteristics, such as shapes of emission spectra, intensities, or luminescence decay time constants. Either of these observables can be used to monitor temperature.16,18 The choice of detection scheme is often governed by the sensitivity to temperature changes that a sensor's specific luminescence characteristics exhibits. Most common are luminescence decay time and intensity ratio of two peaks.14 The intensity ratio method compares the emission intensities observed from two thermally coupled levels, obeying Boltzmann statistics. The energy difference between the two levels defines the range of temperatures where the method exhibits highest sensitivity.12,19 The key advantage of this method is the ability to derive absolute temperature, without requiring sensor calibration.20 In general, in luminescence decay time thermometry, it is the decay time of the excited state that is used for temperature monitoring. Depopulation of the excited level can proceed via the radiative and the non-radiative path for which the rate can be a strong function of temperature. Thus, a value for temperature can be derived by measuring the rate of photons emitted via the radiative process. The thermal depopulation of the excited state through a non-radiative decay channel is the dominant process that determines the sensitivity of a probe over a broad temperature range.14,21,22 Given that both techniques rely on a change in population of the excited state, they are complementary by nature and can be adopted within the same material. This idea gave rise to the concept of dual-mode sensing that is gaining in popularity.23–28 Dual-mode sensing benefits from the possibility of cross-referencing sensor readings by using two methods, thereby improving reliability and accuracy of measurements. This concept received a fresh impetus recently when the feasibility of multimodal temperature sensing using three different techniques, i.e. intensity ratio, decay time and peak shift, was demonstrated in Cr-doped oxides.29 Materials doped with transition metal ions are well suited for multimodal sensing owing to a noticeable shift of the luminescence peaks with temperature, caused by electron–phonon interaction of impurity ions with the host lattice.30–32
2. Concept of the multimodal dual-emitter sensor
A further performance enhancement of luminescence probes can be achieved by using a pair of emitters.33 Combinations of different rare earth ions34–37 as well as pairing rare-earth with transition metal ions38–42 have been explored extensively and it has been shown that having two emitters in the same crystal enables a sensitivity enhancement and permits operation of the sensor over an extended temperature range. It is therefore important to assess this enhancement that a material with dual-emitters, multimodal sensor of temperature can bring, the topic of this feasibility study.To employ all three schemes of temperature monitoring, including the shift of emission peaks as a function of temperature, hosts doped with two d3-type transition metal ions are deemed to be most suitable because of the narrow band emission (2E → 4A2) which depends on the strength of the crystal field. The application of single ions of Cr3+ or Mn4+ for triple temperature readout has been recently demonstrated.29,32 Here we test the multimodal dual-emitter approach by co-doping Al2O3 with both these ions. Aluminium oxide is a stable material with simple structure and is comprehensively characterised as temperature sensor based on luminescence (see ref. 29 and reference herein). Moreover, Al2O3 is capable of accommodating transition metal ions in different valence states.43–45 The sharp emission peaks of chromium and manganese ions observed at around 700 nm are strongly influenced by the crystal field environment and sensitive to variation of temperature. Thus, Al2O3 co-doped with Cr3+ and Mn4+ (Al2O3-Cr,Mn) represents an attractive material for these investigations. Here we will demonstrate how these two ions can be harnessed to enhance the performance of Al2O3 in temperature sensing applications.
It is pertinent to highlight that the dual-emitter approach in combination with the multimodal readout may offer an easy way of cross-referencing the selected temperature sensing method by a simple switch between the emission peaks of the activators. This offers an innovative solution to the cross-referencing and has as further advantage in-built redundancy of temperature sensing. The latter could provide a vital cross-check in critical applications due to being able to derive temperature from two emitters simultaneously without having to change the method of detection. Thus, this study is an important step towards the development of effective multifunctional thermometers with intrinsic redundancy of temperature sensing.
3. Experimental
3.1. Sample preparation
In this study we used powder samples of Al2O3 with nominal concentration of Mn = 0.02 at% and Cr = 0.005 at%, prepared via the sol–gel citrate route. These concentrations were selected based on our prior knowledge of the luminescence properties of the material, aiming to ensure that no energy transfer between the impurity ions occurs. As the ionic radius of Mn4+ (0.54 Å, CN = 6) is almost identical to that of Al3+ (0.53 Å, CN = 6) when doped into α-Al2O3 it can replace the Al3+ ions in the AlO6 octahedra but requires charge compensation. This is achieved by the admixture of other ions in a 2+ valence state (typically Mg2+).46 Therefore Mg was added to the mixture in a concentration equal to that of Mn (0.02 at%). Water solutions of corresponding metal nitrates, taken in stoichiometric ratio according to nominal composition, were mixed together on a magnetic stirring device for 30 min. After that, the water solution of citric acid was added drop by drop to the reaction mixture, ensuring a molar ratio of metals to citric acid equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The solution prepared in this way was evaporated at a temperature of 80 °C and dried at 100 °C to form a polymer gel. The reaction occurs with intense release of NO2 gas. The polymer gel was heat treated in several stages: at temperatures of 300 and 450 °C for 30 min to remove the organic component and at temperatures of 700, 800, 900 and 1000 °C with exposure at maximum temperature for 2 h. The final heat treatment of the product was performed at a temperature of 1200 °C for 4 h.
2. The solution prepared in this way was evaporated at a temperature of 80 °C and dried at 100 °C to form a polymer gel. The reaction occurs with intense release of NO2 gas. The polymer gel was heat treated in several stages: at temperatures of 300 and 450 °C for 30 min to remove the organic component and at temperatures of 700, 800, 900 and 1000 °C with exposure at maximum temperature for 2 h. The final heat treatment of the product was performed at a temperature of 1200 °C for 4 h.
3.2. Luminescence characterisation
Photoluminescence characterization was performed using a Horiba/Jobin–Yvon Fluorolog-3 spectrofluorometer with a 450 W continuous spectrum xenon lamp for excitation. The emission was detected using a Hamamatsu R928P photomultiplier operating in photon counting mode. The luminescence decay kinetics were measured using the same Fluorolog-3 spectrofluorometer with the excitation light modulated by a mechanical modulator (chopper). The spectroscopic measurements in the temperature range between 4.4 and 330 K were carried out in a Janis continuous-flow liquid-helium cryostat using a Lake Shore 331 temperature controller. The spectra presented here are corrected for the instrumental response of the detection system.4. Results
4.1. X-ray diffraction and structure determination
Heat treatment of sol–gel derived precursors at 700 °C for 2 h leads to the formation of an X-ray amorphous product. Crystallization of the nanocrystalline γ-Al2O3 phase occurs after additional heating to 800 °C and this structure is preserved after further treatment at 900 °C. A well-crystallized α-Al2O3 corundum phase appears after further annealing of the material at 1000 °C for 2 h. Thus, the material heated to 1000 °C consists of two alumina polymorphs – nanocrystalline γ-Al2O3 and rhombohedral (sub)microcrystalline α-Al2O3 (Fig. 1). A practically pure corundum α-Al2O3 phase was obtained after additional heat treatment of the powder at 1200 °C for 4 h (see (Fig. 2)). Only few extra peaks with relative intensity less than 1% could be detected in the XRD pattern (i.e. at 27.6, 30.43, 31.88 and 44.65 deg. 2 theta), which, however, could not be identified among the phases containing Al–O–Mn–Mg. | ||
| Fig. 1 X-ray diffraction patterns of sol–gel derived powders of Al2O3, subsequently heat treated in air at 800 °C, 900 °C and 1000 °C for 2 h. | ||
The precise structural parameters of Al2O3 annealed at 1200 °C were derived by full profile Rietveld refinement from experimental XRD data collected over a wide 2Θ range, spanning 10 to 155° using an XPERT-PRO diffractometer. Unit cell dimensions, coordinates and isotropic displacement parameters of atoms in the rhombohedral Al2O3 structure were refined together with profile parameters, applying corrections for absorption and instrumental sample shift. As the final stage of the refinement procedure, anisotropic displacement parameters of Al and O species were refined. As a result, an excellent fit between calculated and experimental profiles was achieved (Fig. 2). Refined structural parameters of the final product and corresponding residuals are presented in Table 1.
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, a = 4.75931(6) Å, c = 12.9933(1) Å
c, a = 4.75931(6) Å, c = 12.9933(1) Å
| Atoms, sites | x/a | y/b | z/c |
B
iso/eq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a, Å2 a, Å2 |
B 11 | B 22 | B 33 | B 12 | B 13 | B 23 |
|---|---|---|---|---|---|---|---|---|---|---|
a
B
iso/eq = 1/3(B11(a*)2a2 + … 2B23b*c*bc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α); T = exp(−1/4(B11(a*)2h2 + … 2B23b*c*kl)). α); T = exp(−1/4(B11(a*)2h2 + … 2B23b*c*kl)).
|
||||||||||
| Al, 12c | 0 | 0 | 0.3524(1) | 0.64(3) | 0.61(3) | B 11 | 0.70(5) | 1/2B11 | 0 | 0 |
| O, 18e | 0.3080(4) | 0 | 1/4 | 0.72(8) | 0.70(8) | 0.83(11) | 0.66(10) | 1/2B22 | −0.04(5) | 2B13 |
| R I = 0.028, RP = 0.078 | ||||||||||
4.2. Luminescence and crystal field analysis
Aluminium oxide doped with Cr3+ is an extensively studied material used as solid-state laser,47 pressure sensor in diamond anvil cells48 and sensor of temperature.49,50 Therefore, the luminescence properties of this material and their temperature evolution are well documented (see e.g. ref. 29, 51, 52 and reference there). The studies of luminescence properties of Al2O3 doped with isoelectronic Mn4+ ions are, however, scarce. The low-temperature (T = 77 K) spectra due to 2E → 4A2 transitions in Al2O3-Mn4+ have been first reported by Geschwind et al.44 and later the temperature changes of the luminescence were measured over the 2.5 to 295 K range.53 It is worth commenting that the narrow band emission at 694 nm due to Cr3+ has been identified occasionally in samples of Mn4+-doped Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45,54 as such due to unintended contamination of the material.
45,54 as such due to unintended contamination of the material.
The application of Al2O3-Cr,Mn as a dual-emitter probe for temperature sensing relies on a holistic understanding of processes governing the temperature changes of luminescence characteristics of the material. Therefore, we begin the study from measurements of the temperature-dependent spectroscopic characteristics. The luminescence excitation and emission spectra of Al2O3-Cr,Mn are shown in Fig. 3 while the positions of the main excitation bands and emission peaks of Cr3+ and Mn4+ are listed in Table 2.
 | ||
| Fig. 3 Luminescence (left) and luminescence excitation spectra (right) of Al2O3-Cr,Mn measured at T = 40 K (emission) and 4.4 K (excitation). | ||
| Ion | Ē → 4A2 (R1-line), nm (cm−1) | → 4A2 (R2-line), nm (cm−1) | R1–R2 gap, meV (cm−1) | 4A2 → 4T1g nm (cm−1) | 4A2 → 4T2g, nm (cm−1) | Ref. |
|---|---|---|---|---|---|---|
| a Tentative assignment due to overlap with the charge-transfer band. | ||||||
| Mn4+ | 676.3 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 786) 786) |
672.6 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 868) 868) |
10.1 (81.5) | 390 (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 641) 641) |
472 (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 186) 186) |
This work |
676.3 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 786) 786) |
672.7 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 865) 865) |
10.2 (82.3) | 320a (31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250) 250) |
470 (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 277) 277) |
44 | |
| Cr3+ | 693.3 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 424) 424) |
691.9 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 453) 453) |
3.6 (29) | 405 (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 691) 691) |
557 (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 953) 953) |
This work |
693.3 nm/(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 424) 424) |
691.9 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 453) 453) |
3.6 (29) | 411 (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330) 330) |
568 (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 606) 606) |
29 | |
The unfilled 3d states of Mn4+ and Cr3+ are strongly influenced by the immediate environment of the ions in the crystals. The crystal field causes a splitting of the electron states into several energy levels. The luminescence excitation bands due to 4A2 → 4T2g, 4T1g transitions of Cr3+ are clearly identified at 557 and 405 nm. The luminescence excitation spectrum of Mn4+ consists of only two bands in the examined region. The band with a maximum at about 472 nm is related to the 4A2 → 4T2g transition and it is blue-shifted compared to the band associated with the same transitions for Cr3+ ions. The second band with a maximum at 390 nm is likely to be related to a mixture of 4A2 → 4T1g and charge-transfer transitions. We therefore assume that these two types of transitions spectrally overlap.
Through knowing the positions of the bands it is possible to calculate the strength of the crystal field Dq and Racah parameters B and C, using the following formulas.55
| E(4T2g) = 10Dq | (1) |
 | (2) |
 | (3) |
| ΔE = E(4T1g) − E(4T2g) | (4) |
For the calculations of crystal field parameters for Cr3+ we used band maxima positions. However, for Mn4+ reasonable values of crystal field and nephelauxetic parameter are obtained if, instead of band peaks, the expected positions of electronic levels (zero-phonon lines) are used. The overlap between the 4A2 → 4T1g and charge-transfer transitions for Mn4+ is responsible for difficulties in establishing the exact position of the 4A2 → 4T1g band. We assume that the positions of zero-phonon lines are located at the low-energy side of the appropriate bands. Parameters Dq, B, and C, calculated in this way, are listed in Table 3.
| Parameter | Value (cm−1) | |
|---|---|---|
| Cr3+ | Mn4+ | |
| D q | 1786 | 1905 |
| B | 714 | 762 |
| C | 3041 | 3038 |
| β 1 | 1.0997 | 0.9573 |
The results obtained also allow to calculate the nephelauxetic parameter β1, defined in ref. 56 as:
 | (5) |
Having established the values of crystal field and Racah parameters, it is possible to construct Tanabe–Sugano diagrams for both ions. They are presented together in one figure (Fig. 4). The analysis of excitation spectra of Mn4+ and Cr3+ emission shows that the excitation bands of the two ions are well separated. No emission of Mn4+ is detected at the excitation in the range of the 4A2 → 4T2g band of Cr3+, while the excitation spectrum of Cr3+ is similar to the one measured earlier in Al2O3-Cr29 and exhibits no changes that could be attributed to the influence of other ions. This enables us to conclude that due to low concentration there is no energy transfer between dopant ions in the material under study that can markedly alter the luminescence characteristics.41 Consequently, emission of Mn4+ and Cr3+ can be treated independently. This is an important condition that allows to analyse the changes of their luminescence characteristics with temperature within the framework of models developed for the emission of individual ions. The spectra also allowed to select a single wavelength for the excitation of both activators with the aim to achieve similar intensity of the R1-lines.
The luminescence spectrum of Al2O3-Cr,Mn features two pairs of narrow peaks that correspond to the characteristic transitions of Mn4+ and Cr3+ (see (Fig. 3)). The peaks referred to as R1 and R2 lines are due to spin-forbidden transitions from the lowest excited 2E level split into two levels (Ē and  ) to the ground state 4A2. The splitting is due to the lowering of the octahedral symmetry of the local crystal field surrounding the ions.52 The magnitude of the splitting is proportional to the strength of the crystal field. The energy of the emission lines is further influenced by the nephelauxetic effect. The covalence increases with the charge of the ions,56 resulting in an increase of the energy of the emitted photons. Consequently, the R-lines of Mn4+ are shifted to shorter wavelength with respect to the emission of Cr3+. Notably, the correlation between the calculated nephelauxetic parameters β1 and the position of the 2E levels of the two ions fits the established trend.56
) to the ground state 4A2. The splitting is due to the lowering of the octahedral symmetry of the local crystal field surrounding the ions.52 The magnitude of the splitting is proportional to the strength of the crystal field. The energy of the emission lines is further influenced by the nephelauxetic effect. The covalence increases with the charge of the ions,56 resulting in an increase of the energy of the emitted photons. Consequently, the R-lines of Mn4+ are shifted to shorter wavelength with respect to the emission of Cr3+. Notably, the correlation between the calculated nephelauxetic parameters β1 and the position of the 2E levels of the two ions fits the established trend.56
4.3. Temperature dependent luminescence characteristics
The high sensitivity of the outer 3d shells of Cr3+ and Mn4+ to changes of the crystal field environment and their coupling with lattice vibrations are the main factors that control the changes with temperature of the luminescence characteristics of ions in different hosts. Due to the very narrow emission peaks, a small change in the population of the emitting levels as wll as their energy is clearly visible in Fig. 5.These changes are exploited by two methods of luminescence thermometry based on measurements of spectroscopic characteristics. Since the population of two emitting levels obeys Boltzmann statistics, the emission spectra exhibit a rapid decrease of the intensity of the R2 lines with respect to R1 at cooling due to an increase in the population of the lower exited state. In the framework of a statistical model, the ratio of intensities F = IR2/IR1 can be derived from the population of the levels in thermal equilibrium, resulting in the following expression:
 | (6) |
 | ||
| Fig. 6 Arrhenius plot for the intensity ratio of the R2- and R1-lines of Cr3+ and Mn4+ in Al2O3-Cr,Mn and linear fits of the dependences. | ||
The temperature dependence of the spectral position of the R1 and R2-lines are displayed in Fig. 7. The thermal shift of the lines, Δν, resulting from the interaction of electronic states of impurity ions with acoustic phonons can be described by the Debye theory:57
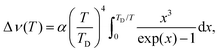 | (7) |
 | ||
| Fig. 7 Temperature shift of the R-lines of Mn4+ (top) and Cr3+ (bottom) in Al2O3-Cr,Mn measured at 505 nm excitation. The dots are the experimental data points and the solid lines show the best fit of experimental results to eqn (7), using parameters shown in Table 4. | ||
The applied fitting procedure resulted in very good fits throughout the entire measurement range. The fit parameters, summarised in Table 4, are in very good agreement with our recent results for Al2O3-Cr.29 The value of the Debye temperature derived from the wavelength shift of Mn4+ is closer to earlier results for Al2O3-Cr, TD = 750 K.57 It should be noted that the R-lines of both ions exhibit a very similar pattern regarding the spectral shift with temperature. The shift is negligible below 100 K while it can be detected at temperature above that, thus confining this temperature monitoring method to temperatures above 100 K.
| Parameter | Cr3+ in Al2O3-Cr | Cr3+ in Al2O3-Cr,Mn | Mn4+ in Al2O3-Cr,Mn |
|---|---|---|---|
| α R1, cm−1 | −518.0 | −515.2 | −1048.5 |
| α R2, cm−1 | −507.5 | −500.0 | −972.0 |
| T D, K | 867 | 856 | 736 |
| Ref. | 29 | This work | This work |
One more method of temperature monitoring that can be implemented with materials doped with transition metal ions uses the luminescence decay time of the R-lines. To evaluate the merit of the dual-emitter concept of temperature sensing further we investigated the changes of the luminescence decay time with temperature of Cr3+ and Mn4+ in Al2O3. Fig. 8 shows the decay curves recorded at 694 and 676 nm peaks and temperature dependence of respective decay time constants.
 | ||
| Fig. 8 Left – Decay curves measured at the peak of Mn4+ and Cr4+ emission at different temperatures. Right – Temperature dependence of the luminescence decay time constant of Mn4+ (top) and Cr3+ emission (bottom) in Al2O3-Cr,Mn. The solid lines show the best fit of eqn (8) to the experimental results (dots) using the parameters summarised in Table 5. | ||
Qualitatively the measured dependences are very similar for both ions. The initial increase of the decay time constant with cooling can be readily explained by the decrease of the probability for non-radiative de-excitation of the emitting level. This is a typical behaviour observed in luminescence materials.58,59 The decay time constant reaches a maximum value at ca. 100 K but below this temperature it decreases with further cooling. Such drop of τ = f(T) at very low temperatures is not very common. It has been discussed so far in few materials doped with Cr3+ (ref. 29, 60 and 61) while it is the first time that the effect is explicitly observed for Mn4+ emission. This behaviour is explained assuming different radiative transition probability from the Ē and  levels, constituting the emitting 2E state. The probability of radiative decay from the upper level
levels, constituting the emitting 2E state. The probability of radiative decay from the upper level 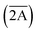 is lower in comparison with that for Ē, leading to an increase of the measured radiative decay time constant of the R-line emission. However, a decrease of temperature leads to depopulation of the upper level, thereby reducing its contribution to the emission process in favour of transitions from the low-lying level Ē with a higher radiative decay rate. This is then manifested as decrease of the measured decay time constant of the R-lines with further cooling.
is lower in comparison with that for Ē, leading to an increase of the measured radiative decay time constant of the R-line emission. However, a decrease of temperature leads to depopulation of the upper level, thereby reducing its contribution to the emission process in favour of transitions from the low-lying level Ē with a higher radiative decay rate. This is then manifested as decrease of the measured decay time constant of the R-lines with further cooling.
The observed behaviour of the decay time constant for the Cr3+ and Mn4+ emissions in Al2O3 over the examined temperature range reflects the dynamic of temperature changes in the population of the excited states and probabilities of transitions between them. Recently we demonstrated that this convoluted phenomenon can be comprehensively described by taking into account the main processes affecting the population and radiative rates of the 2E state, i.e. thermalisation, phonon-assisted interaction with lattice vibrations and thermally induced depopulation.29 Under the assumption of thermal equilibrium between the states Ē,  and 4T2 involved in the transitions and considering interactions with lattice vibrations we obtained the following expression for τ(T):
and 4T2 involved in the transitions and considering interactions with lattice vibrations we obtained the following expression for τ(T):
 | (8) |
Here 1/τi (i = 1, 2 and 3) are the radiative decay rates of the Ē, 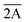 , and 4T2 levels respectively. ΔE is the energy difference between the 2E and 4T2 levels, Ep stands for “effective energy” of the phonons responsible for the exchange with the sidebands while other notations are explained above. This equation explicitly describes the thermalisation process occurring between the Ē and
, and 4T2 levels respectively. ΔE is the energy difference between the 2E and 4T2 levels, Ep stands for “effective energy” of the phonons responsible for the exchange with the sidebands while other notations are explained above. This equation explicitly describes the thermalisation process occurring between the Ē and  levels, phonon-assisted relaxation and depopulation of the levels due to thermally induced 2E → 4T2 transitions.
levels, phonon-assisted relaxation and depopulation of the levels due to thermally induced 2E → 4T2 transitions.
As shown in Fig. 8 the measured temperature dependences of the decay time constants of Cr3+ and Mn4+ perfectly fit the equation confirming agreement of experimental results with theory over the entire temperature range. The parameters of the fit for Cr3+ summarised in Table 5 show good agreement with the results published earlier while the effective energy of phonons, Ep, is comparable with the mean value of the phonon energy of Al2O3.62
| Parameter | Cr3+ in Al2O3-Cr | Cr3+ in Al2O3-Cr,Mn | Mn4+ in Al2O3-Cr,Mn |
|---|---|---|---|
| a The value of D is fixed to be equal to the energy splitting of the 2E level. | |||
| τ 1, ms | 3.98 ± 0.01 | 4.04 ± 0.01 | 0.83 ± 0.01 |
| τ 2, ms | 4.46 ± 0.01 | 5.43 ± 0.02 | 1.16 ± 0.01 |
| E p, meV | 60.9 ± 0.4 | 61.2 ± 0.1 | 55.7 ± 0.1 |
| D , meV | 3.6 | 3.6 | 10.2 |
| τ 3, μs | 33 ± 25 | 86 ± 41 | 139 ± 94 |
| ΔE, meV | 237 ± 23 | 281 ± 15 | 213 ± 25 |
| Ref. | 29 | This work | This work |
It should be noted that from the standpoint of temperature monitoring the observed change in the slope of the τ = f(T) curve is worse in comparison with temperature dependences of other luminescence characteristics that exhibit a monotonous trend. This change is caused by a lower sensitivity of the method around the peak of the curve. The consequences and implication of this feature for the dual-emitter sensing will be discussed in the next section.
4.4. Thermometric performance
To estimate the merit of a dual-emitter Al2O3-Cr,Mn probe in different modes of temperature sensing we adopted as metrics the temperature uncertainty δT. This parameter calculated by converting the uncertainty of the parameter that is measured, δQ, by dividing it by the responsivity of the sensor, |dQ/dT|.18,63,64 | (9) |
This approach allows a direct comparison of the different temperature measurement methods.29
The absolute sensitivity can be readily calculated from the measured temperature dependence of the parameter of interest. The uncertainty of the measurements of decay time and intensity ratio over the examined temperature range were estimated to be 0.5% and 2% respectively. The absolute error in determining the wavelength shift is taken as a constant value of 0.02 nm. These data were used to calculate the uncertainty of temperature measurements δT = f(T) for different modes of sensing (see Fig. 9).
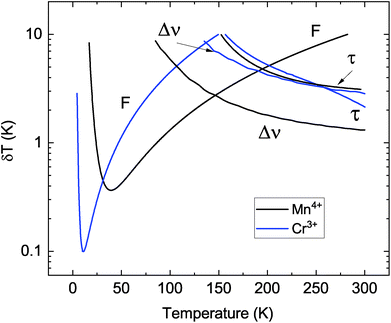 | ||
| Fig. 9 The temperature resolution of Al2O3-Cr,Mn in different modes of temperature sensing: F – luminescence intensity ratio, Δν – spectral shift and τ – decay time constant. | ||
Analysis of the plots allows to assess the potential of the multimodal dual-emitter concept as non-contact luminescence sensor of temperature. The plot clearly demonstrates the significant difference in the achievable resolution depending on the measurement technique, luminescence ion and temperature range of operation. The lowest temperature uncertainty (±0.1 K at 10 K) can be reached by using the intensity ratio of R-lines of Cr3+ while for Mn4+ emission this value is three times higher. The sensitivity of the method is proportional to the energy gap between the R-levels involved in the transitions19,20 translating to better temperature resolution achievable with Cr3+ ions in comparison with Mn4+. However, a smaller gap also implies a narrow temperature range for a ratiometric sensor.20 This shortcoming of the intensity ratio method using Cr3+ emission is clearly visible in Fig. 9. The measurement uncertainty increases very rapidly; it is more than ±1 K above 50 K. In this regard the intensity ratio of the R-lines of Mn4+ is superior in comparison with other techniques as such, permitting to achieve lowest uncertainty of temperature measurements over the 40–145 K range.
Inspecting Fig. 9 shows that the temperature monitoring techniques based on intensity ratio and wavelength shift of the Mn4+ emission exhibit the same uncertainty of ±2.8 K at about T = 145 K. This defines a cross-over point between the two methods. It is worthwhile noting that for Cr3+ emission the measurement uncertainty at the cross-over point (T = 135 K) is larger by a factor three. At higher temperatures (145–300 K) the best temperature resolution can be achieved by monitoring the wavelength shift of the R-lines of the Mn4+ luminescence. This gives more accurate readings in comparison with the shift of Cr3+ lines and methods based on the changes of decay time constants of Cr3+ or Mn4+ emission. The R-lines of Mn4+ exhibit a larger shift with temperature in comparison to Cr3+ lines (see also Fig. 5), translating into better accuracy. It is also evident that the temperature resolution of decay time thermometry in this case is inferior when compared with spectroscopic methods of temperature monitoring. Finally, it should be noted that temperature resolution obtained for Al2O3 co-doped with Cr3+ and Mn4+ is very compatible with the typical values reported for other oxides doped with transition metal ions (see Table 6).
| Material | Method | Temperature, K | Temperature resolution, K | Ref. |
|---|---|---|---|---|
| α-Al2O3-Cr | Intensity ratio | 30 | 0.45 | 63 |
| α-Ga2O3-Cr | Intensity ratio | 300 | 0.25 | 65 |
| β-Ga2O3-Cr | Intensity ratio | 300 | 0.42 | 65 |
| β-Ga2O3-Cr | Line shift | 300 | 2 | 29 |
| β-Ga2O3-Cr | Decay time | 165 | 0.3 | 29 |
| YAlO3-Cr | Intensity ratio | 20 | 0.2 | 29 |
| Bi2Al4O9-Cr | Intensity ratio | 300 | 0.23 | 66 |
| Mg2SiO4-Cr | Line shift | 310 | 7.9 | 32 |
| Mg2SiO4-Cr | Decay time | 310 | 0.2 | 32 |
| Li2TiO3-Mn | Decay time | 332 | 0.032 | 67 |
| Zn2GeO4-Mn | Decay time | 360 | 0.64 | 26 |
| MgTiO3-Mn | Intensity ratio | 93 | 0.42 | 24 |
| MgTiO3-Mn | Decay time | 278 | 0.025 | 24 |
5. Conclusion
The concept of multimodal dual-emitter luminescence sensors of temperature was put to the test using Al2O3 co-doped with transition metal ions of Cr3+ and Mn4+. To assess the feasibility of non-contact temperature measurements through monitoring the intensity ratio, wavelength shift, and decay time constant of R-lines emitted by both ions, we examined the evolution of the luminescence spectra and decay time constant of Al2O3-Cr,Mn over the 4–350 K temperature range. Detailed analysis of spectroscopic results allowed to determine the energy level diagram and establish that the crystal field acting upon each ion governs the luminescence properties of the material. The investigations were conducted by comparing experiments with relevant theoretical models. It is shown that the models provide adequate interpretation of the emission processes, evidenced by the high quality of fits obtained for the measured temperature dependences of the luminescence intensity ratio, wavelength shift and decay time constant for both emitting ions.The thermometric performance of Al2O3-Cr,Mn was assessed by analysing the temperature resolution achievable by different methods. The comparison shows that in this material the resolution obtained through measuring the luminescence decay time constant is not adequate for monitoring temperature over 4–300 K range. In contrast, the intensity ratio method is superior at very low temperature as it allows to attain a temperature uncertainty as low as ±0.1 K at 10 K by monitoring Cr3+ emission. As temperature increases, measurements of the intensity ratio of the R-lines of Mn4+ is preferable as such, giving better accuracy in comparison with that of Cr3+ emission. Though the accuracy of the method gradually decreases it remains the most accurate up to the cross-over point at 145 K when the measurement uncertainty reaches ±2.8 K. Above this point monitoring the wavelength shift of Mn4+ lines allows the temperature to be determined with better uncertainty.
The significance of these findings is two-fold. First, they demonstrated the viability of the dual-emitter multimodal concept for non-contact luminescence measurements of temperature. The luminescence characteristics of transition metal ions co-doped in Al2O3 exhibit measurable changes with temperature, laying the foundation for future designs of novel materials and read-out configurations with improved accuracy and reliability. Second, the results of this study highlighted the advantage of Mn4+ emission in aluminium oxide for thermometric application. It is very competitive in comparison with Cr-doped Al2O3 and consequently this material holds a considerable promise for the technology of non-contact sensing.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was partially supported by the Polish National Science Centre (project no. 2018/31/B/ST8/00774) and by the National Research Foundation of Ukraine (grant no. 2020.02/0373).Notes and references
- S. W. Allison and G. T. Gillies, Remote thermometry with thermographic phosphors: Instrumentation and applications, Rev. Sci. Instrum., 1997, 68, 2615–2650 CrossRef CAS
.
- N. Fuhrmann, T. Kissel, A. Dreizler and J. Brubach, Gd3Ga5O12:Cr-a phosphor for two-dimensional thermometry in internal combustion engines, Meas. Sci. Technol., 2011, 22, 045301 CrossRef
.
- V. B. Mykhaylyk, H. Kraus and A. Wagner, Non-Contact Luminescence Lifetime Microthermometry using Scintillation Sensors, Acta Phys. Pol., A, 2018, 133, 1108–1111 CrossRef CAS
.
- A. H. Khalid, K. Kontis and H. Z. Behtash, Phosphor thermometry in gas turbines: consideration factors, Proc. Inst. Mech. Eng., Part G, 2010, 224, 745–755 CrossRef
.
- T. Hartman, R. G. Geitenbeek, C. S. Wondergem, W. van der Stam and B. M. Weckhuysen, Operando Nanoscale Sensors in Catalysis: All Eyes on Catalyst Particles, ACS Nano, 2020, 14, 3725–3735 CrossRef CAS PubMed
.
- A. J. Warren, D. Axford and R. L. Owen, Direct measurement of X-ray-induced heating of microcrystals, J. Synchrotron Radiat., 2019, 26, 991–997 CrossRef CAS PubMed
.
- X. J. Zhu, W. Feng, J. Chang, Y. W. Tan, J. C. Li, M. Chen, Y. Sun and F. Y. Li, Temperature-feedback upconversion nanocomposite for accurate photothermal therapy at facile temperature, Nat. Commun., 2016, 7, 10437 CrossRef CAS PubMed
.
- K. Okabe, N. Inada, C. Gota, Y. Harada, T. Funatsu and S. Uchiyama, Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy, Nat. Commun., 2012, 3, 705 CrossRef PubMed
.
- T. Hayashi, N. Fukuda, S. Uchiyama and N. Inada, A Cell-Permeable Fluorescent Polymeric Thermometer for Intracellular Temperature Mapping in Mammalian Cell Lines, PLoS One, 2015, 10, e0117677 CrossRef PubMed
.
- C. D. S. Brites, P. P. Lima, N. J. O. Silva, A. Millan, V. S. Amaral, F. Palacio and L. D. Carlos, Thermometry at the nanoscale, Nanoscale, 2012, 4, 4799–4829 RSC
.
- T. T. Bai and N. Gu, Micro/Nanoscale Thermometry for Cellular Thermal Sensing, Small, 2016, 12, 4590–4610 CrossRef CAS PubMed
.
- X. D. Wang, O. S. Wolfbeis and R. J. Meier, Luminescent probes and sensors for temperature, Chem. Soc. Rev., 2013, 42, 7834–7869 RSC
.
- B. del Rosal, E. Ximendes, U. Rocha and D. Jaque, In Vivo Luminescence Nanothermometry: from Materials to Applications, Adv. Opt. Mater., 2017, 5, 1600508 CrossRef
.
- M. D. Dramicanin, Trends in luminescence thermometry, J. Appl. Phys., 2020, 128, 040902 CrossRef CAS
.
- J. J. Zhou, B. del Rosal, D. Jaque, S. Uchiyama and D. Y. Jin, Advances and challenges for fluorescence nanothermometry, Nat. Methods, 2020, 17, 967–980 CrossRef CAS PubMed
.
- J. D. A. Bednarkiewicz, K. Trejgis, D. Jaque, E. Ximendes and L. Marciniak, Luminescence based temperature bio-imaging: Status, challenges, and perspectives, Appl. Phys. Rev., 2021, 8, 011317 Search PubMed
.
- J. W. Kenney and J. J. Lee, Photoluminescent Metal Complexes and Materials as Temperature Sensors-An Introductory Review, Chemosensors, 2021, 9, 109 CrossRef
.
- C. D. S. Brites, S. Balabhadra and L. D. Carlos, Lanthanide-Based Thermometers: At the Cutting-Edge of Luminescence Thermometry, Adv. Opt. Mater., 2019, 7, 1801239 CrossRef
.
- S. A. Wade, S. F. Collins and G. W. Baxter, Fluorescence intensity ratio technique for optical fiber point temperature sensing, J. Appl. Phys., 2003, 94, 4743–4756 CrossRef CAS
.
- M. Suta and A. Meijerink, A Theoretical Framework for Ratiometric Single Ion Luminescent Thermometers-Thermodynamic and Kinetic Guidelines for Optimized Performance, Adv. Theory Simul., 2020, 3, 2000176 CrossRef CAS
.
- M. D. Chambers and D. R. Clarke, Doped Oxides for High-Temperature Luminescence and Lifetime Thermometry, Annu. Rev. Mater. Res., 2009, 39, 325–359 CrossRef CAS
.
- S. Yakunin, B. M. Benin, Y. Shynkarenko, O. Nazarenko, M. I. Bodnarchuk, D. N. Dirin, C. Hofer, S. Cattaneo and M. V. Kovalenko, High-resolution remote thermometry and thermography using luminescent low-dimensional tin-halide perovskites, Nat. Mater., 2019, 18, 846–852 CrossRef CAS PubMed
.
- J. S. Zhong, D. Q. Chen, Y. Z. Peng, Y. D. Lu, X. Chen, X. Y. Li and Z. G. Ji, A review on nanostructured glass ceramics for promising application in optical thermometry, J. Alloys Compd., 2018, 763, 34–48 CrossRef CAS
.
- E. Glais, V. Dordevic, J. Papan, B. Viana and M. D. Dramicanin, MgTiO3:Mn4+ a multi-reading temperature nanoprobe, RSC Adv., 2018, 8, 18341–18346 RSC
.
- S. Gharouel, L. Labrador-Paez, P. Haro-Gonzalez, K. Horchani-Naifer and M. Ferid, Fluorescence intensity ratio and lifetime thermometry of praseodymium phosphates for temperature sensing, J. Lumin., 2018, 201, 372–383 CrossRef CAS
.
- F. F. Chi, B. Jiang, Z. M. Zhao, Y. H. Chen, X. T. Wei, C. K. Duan, M. Yin and W. Xu, Multimodal temperature sensing using Zn2GeO4:Mn2+ phosphor as highly sensitive luminescent thermometer, Sens. Actuators, B, 2019, 296, 126640 CrossRef CAS
.
- L. Marciniak, K. Elzbieciak-Piecka, K. Kniec and A. Bednarkiewicz, Assessing thermometric performance of Sr2CeO4 and Sr2CeO4:Ln(3+) (Ln(3+) = Sm3+, Ho3+, Nd3+, Yb3+) nanocrystals in spectral and temporal domain, Chem. Eng. J., 2020, 388, 124347 CrossRef CAS
.
- M. Sojka, C. D. S. Brites, L. D. Carlos and E. Zych, Exploiting bandgap engineering to finely control dual-mode Lu-2(Ge,Si)O-5:Pr(3+)luminescence thermometers, J. Mater. Chem. C, 2020, 8, 10086–10097 RSC
.
- V. Mykhaylyk, H. Kraus, Y. Zhydachevskyy, V. Tsiumra, A. Luchechko, A. Wagner and A. Suchocki, Multimodal Non-Contact Luminescence Thermometry with Cr-Doped Oxides, Sensors, 2020, 20, 5259 CrossRef CAS PubMed
.
- D. Pugh-Thomas, B. M. Walsh and M. C. Gupta, Spectroscopy of BeAl2O4:Cr3+ with application to high-temperature sensing, Appl. Opt., 2010, 49, 2891–2897 CrossRef CAS PubMed
.
- S. Adachi, Review-Mn4+-Activated Red and Deep Red-Emitting Phosphors, ECS J. Solid State Sci. Technol., 2019, 9, 016001 CrossRef
.
- Z. Risti, V. Dordevic, M. Medic, S. Kuzman, M. Sekulic, Z. Antic and M. D. Dramicanin, Triple-temperature readout in luminescence thermometry with Cr3+-doped Mg2SiO4 operating from cryogenic to physiologically relevant temperatures, Meas. Sci. Technol., 2021, 32, 054004 CrossRef
.
- J. McLaurin, L. R. Bradshaw and D. R. Gamelin, Dual-Emitting Nanoscale Temperature Sensors, Chem. Mater., 2013, 25, 1285–1292 CrossRef
.
- M. K. Mahata, T. Koppe, K. Kumar, H. Hofsass and U. Vetter, Demonstration of Temperature Dependent Energy Migration in Dual-Mode YVO4: Ho3+/Yb3+ Nanocrystals for Low Temperature Thermometry, Sci. Rep., 2016, 6, 36342 CrossRef PubMed
.
- Y. Xu, Z. Sun, Y. L. Wei, M. C. Jia, B. F. Hou, X. Li, F. Lin, M. X. Zhang, H. Y. Wang and Z. L. Fu, Optical properties of the CaEuAl(3)O(7)phosphor with dual-activator Eu2+/Eu(3+)for multifunctional applications, J. Am. Ceram. Soc., 2020, 103, 5721–5730 CrossRef CAS
.
- H. L. Song, C. Wang, Q. Han, X. Y. Tang, W. C. Yan, Y. F. Chen, J. F. Jiang and T. G. Liu, Highly sensitive Tm3+/Yb3+ codoped SrWO4 for optical thermometry, Sens. Actuators, A, 2018, 271, 278–282 CrossRef CAS
.
- Z. H. Fang, L. Zhao, Q. H. Yang, Z. Yang, Y. Y. Cai, D. C. Zhou, X. Yu, J. B. Qiu and X. H. Xu, Optical thermometry properties of silicate glass ceramics with dual-phase for spatial isolation of Er3+ and Cr3+, J. Lumin., 2020, 219, 116861 CrossRef CAS
.
- D. Q. Chen, S. Liu, Y. Zhou, Z. G. Wan, P. Huang and Z. G. Ji, Dual-activator luminescence of RE/TM:Y3Al5O12 (RE = Eu-3+,Eu- Tb3+, Dy3+; TM = Mn4+, Cr3+) phosphors for self-referencing optical thermometry, J. Mater. Chem. C, 2016, 4, 9044–9051 RSC
.
- Y. B. Chen, J. He, X. G. Zhang, M. C. Rong, Z. G. Xia, J. Wang and Z. Q. Liu, Dual-Mode Optical Thermometry Design in Lu3Al5O12:Ce3+/Mn4+ Phosphor, Inorg. Chem., 2020, 59, 1383–1392 CrossRef CAS PubMed
.
- C. Y. Xie, L. Zhao, B. Jiang, J. S. Mao, Y. Lin, P. Wang, X. T. Wei, M. Yin and Y. H. Chen, Dual-activator luminescence of LuAG:Mn4+/Tb3+ phosphor for optical thermometry, J. Am. Ceram. Soc., 2019, 102, 7500–7508 CrossRef CAS
.
- W. Piotrowski, K. Kniec and L. Marciniak, Enhancement of the Ln3+ ratiometric nanothermometers by sensitization with transition metal ions, J. Alloys Compd., 2021, 870, 159386 CrossRef CAS
.
- K. Elzbieciak-Piecka, M. Suta and L. Marciniak, Structurally induced tuning of the relative sensitivity of LaScO3:Cr3+ luminescent thermometers by co-doping lanthanide ions, Chem. Eng. J., 2021, 421, 129757 CrossRef CAS
.
- W. H. Fonger and C. W. Struck, Temperature dependences of Cr+ 3 radiative and nonradiative transitions in ruby and emerald, Phys. Rev. B: Solid State, 1975, 11, 3251–3260 CrossRef CAS
.
- S. Geschwind, P. Kisliuk, M. P. Klein, J. Remeika and D. L. Wood, Sharp-Line Fluorescence, Electron Paramagnetic Resonance, and Thermoluminescence of Mn 4+ in α-Al2O3, Phys. Rev., 1962, 126, 1684–1686 CrossRef CAS
.
- C. Tian, H. Lin, D. W. Zhang, P. P. Zhang, R. J. Hong, Z. X. Han, X. L. Qian and J. Zou, Mn-4(+) activated Al2O3 red-emitting ceramic phosphor with excellent thermal conductivity, Opt. Express, 2019, 27, 32666–32678 CrossRef CAS PubMed
.
- M. G. Brik, Y. X. Pan and G. K. Liu, Spectroscopic and crystal field analysis of absorption and photoluminescence properties of red phosphor CaAl12O19:Mn4+ modified by MgO, J. Alloys Compd., 2011, 509, 1452–1456 CrossRef CAS
.
-
B. Denker and E. Shklovsky, Handbook of Solid-State Lasers: Materials, Systems and Applications, Woodhead Publishing, Cambridge, 2013 Search PubMed
.
- F. Datchi, A. Dewaele, P. Loubeyre, R. Letoullec, Y. Le Godec and B. Canny, Optical pressure sensors for high-pressure-high-temperature studies in a diamond anvil cell, High Pressure Res., 2007, 27, 447–463 CrossRef CAS
.
- F. Anghel, C. Iliescu, K. T. V. Grattan, A. W. Palmer and Z. Y. Zhang, Fluorescent-Based Lifetime Measurement Thermometer for Use at Subroom Temperatures (200–300 K), Rev. Sci. Instrum., 1995, 66, 2611–2614 CrossRef CAS
.
- K. T. V. Grattan, Z. Y. Zhang, T. Sun, Y. H. Shen, L. M. Tong and Z. C. Ding, Sapphire-ruby single-crystal fibre for application in high temperature optical fibre thermometers: studies at temperatures up to 1500 degrees C, Meas. Sci. Technol., 2001, 12, 981–986 CAS
.
- D. D. Ragan, R. Gustavsen and D. Schiferl, Calibration of the Ruby R(1) and R(2) Fluorescence Shifts as a Function of Temperature from 0 to 600-K, J. Appl. Phys., 1992, 72, 5539–5544 CrossRef CAS
.
-
R. C. Powell, Physics of Solid-State Laser Materials, Springer-Verlag, New York, 1998 Search PubMed
.
- H. Riesen, T. Monks-Corrigan and N. B. Manson, Temperature dependence of the R-1 linewidth in Al2O3:Mn4+: A spectral hole-burning and FLN study, Chem. Phys. Lett., 2011, 515, 241–244 CrossRef CAS
.
- I. V. Gasenkova, N. I. Mukhurov, S. P. Zhvavyi, E. E. Kolesnik and A. P. Stupak, Photoluminescent properties of nanoporous anodic alumina doped with manganese ions, J. Lumin., 2017, 185, 298–305 CrossRef CAS
.
-
B. Henderson and G. F. Imbush, Optical Spectroscopy of Inorganic Solids, Oxford University Press, Oxford, 1989 Search PubMed
.
- M. G. Brik, S. J. Camardello, A. M. Srivastava, N. M. Avram and A. Suchocki, Spin-Forbidden Transitions in the Spectra of Transition Metal Ions and Nephelauxetic Effect, ECS J. Solid State Sci. Technol., 2016, 5, R3067–R3077 CrossRef CAS
.
- D. E. McCumber and M. D. Sturge, Linewidth and Temperature Shift of the R Lines in Ruby, J. Appl. Phys., 1963, 34, 1682–1684 CrossRef CAS
.
-
G. Blasse and B. C. Grambaier, Luminescence Materials, Springer-Verlag Berlin, Heidelberg, 1994 Search PubMed
.
- N. Ahmed, H. Kraus, H. J. Kim, V. Mokina, V. Tsiumra, A. Wagner, Y. Zhydachevskyy and V. B. Mykhaylyk, Characterisation of tungstate and molybdate crystals ABO4 (A=Ca, Sr, Zn, Cd; B=W, Mo) for luminescence lifetime cryothermometry, Materialia, 2018, 4, 287–296 CrossRef
.
- D. F. Nelson and M. D. Sturge, Relation between Absorption and Emission in the Region of the R- Lines of Ruby, Phys. Rev., 1965, 137, A1117–A1130 CrossRef
.
- Z. Y. Zhang, K. T. V. Grattan and A. W. Palmer, Temperature Dependences of Fluorescence Lifetimes in Cr-3+-Doped Insulating Crystals, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 7772–7778 CrossRef CAS PubMed
.
- Z. Lodziana and K. Parlinski, Dynamical stability of the alpha and theta phases of alumina, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 174106 CrossRef
.
- M. Back, J. Ueda, M. G. Brik and S. Tanabe, Pushing the Limit of Boltzmann Distribution in Cr3+-Doped CaHfO3 for Cryogenic Thermometry, ACS Appl. Mater. Interfaces, 2020, 12, 38325–38332 CrossRef CAS PubMed
.
- V. B. Mykhaylyk, H. Kraus, L. Bobb, R. Gamernyk and K. Koronski, Megahertz non-contact luminescence decay time cryothermometry by means of ultrafast PbI2 scintillator, Sci. Rep., 2019, 9, 5274 CrossRef CAS PubMed
.
- M. Back, J. Ueda, H. Nambu, M. Fujita, A. Yamamoto, H. Yoshida, H. Tanaka, M. G. Brik and S. Tanabe, Boltzmann Thermometry in Cr3+-Doped Ga2O3 Polymorphs: The Structure Matters!, Adv. Opt. Mater., 2021, 9, 2100033 CrossRef CAS
.
- M. Back, J. Ueda, J. Xu, K. Asami, M. G. Brik and S. Tanabe, Effective Ratiometric Luminescent Thermal Sensor by Cr3+-Doped Mullite Bi2Al4O9 with Robust and Reliable Performances, Adv. Opt. Mater., 2020, 8, 2000124 CrossRef CAS
.
- M. D. Dramićanin, B. Milićević, V. Đorđević, Z. Ristić, J. J. Zhou, D. Milivojević, J. Papan, M. G. Brik, C.-G. Ma, A. M. Srivastava and M. Wu, Li2TiO3:Mn4+ Deep-Red Phosphor for the Lifetime-Based Luminescence Thermometry, Chemistry Select, 2019, 4, 7067–7075 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2021 |