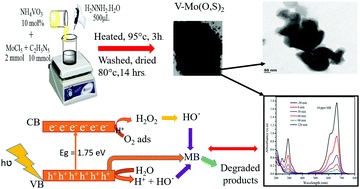
Discharging of organic pollutants from industrial activities into the water bodies is a common worldwide problem, which can adversely affect human life and the environment. Recently, many efficient, eco-friendly, and cost-effective photocatalytic methods have been investigated for environmental remediation. In this study, a simple and facile precipitation method at low temperature was proposed for the synthesis of V-doped Mo(O,S)2 oxysulfide. The crystallinity, morphology, elemental composition, and optical and electrical properties of the as-synthesized materials were characterized by XRD, Raman spectroscopy, FT-IR spectroscopy, FE-SEM, TEM, XPS, PL, and EIS. The photocatalytic performance of both bare and V-doped Mo(O,S)2 was evaluated in the degradation of methylene blue (MB) dye. The holes (h+) and hydroxyl radicals (OH˙) were identified as the main oxidative species for MB degradation. V-Mo(O,S)2-10 synthesized with 10% vanadium precursor showed the highest photocatalytic efficiency for MB degradation under visible light irradiation with an apparent rate constant of 0.028 min−1, which was much greater than that of bare Mo(O,S)2 at 0.0098 min−1. Besides, V-Mo(O,S)2-10 had the lowest recombination rate of electron–hole pairs and charge transfer resistance to enhance the photocatalytic activity, as evidenced by PL and EIS analyses. Therefore, V-doped Mo(O,S)2 can be a potential candidate for photocatalytic degradation of organic dyes for environmental remediation.