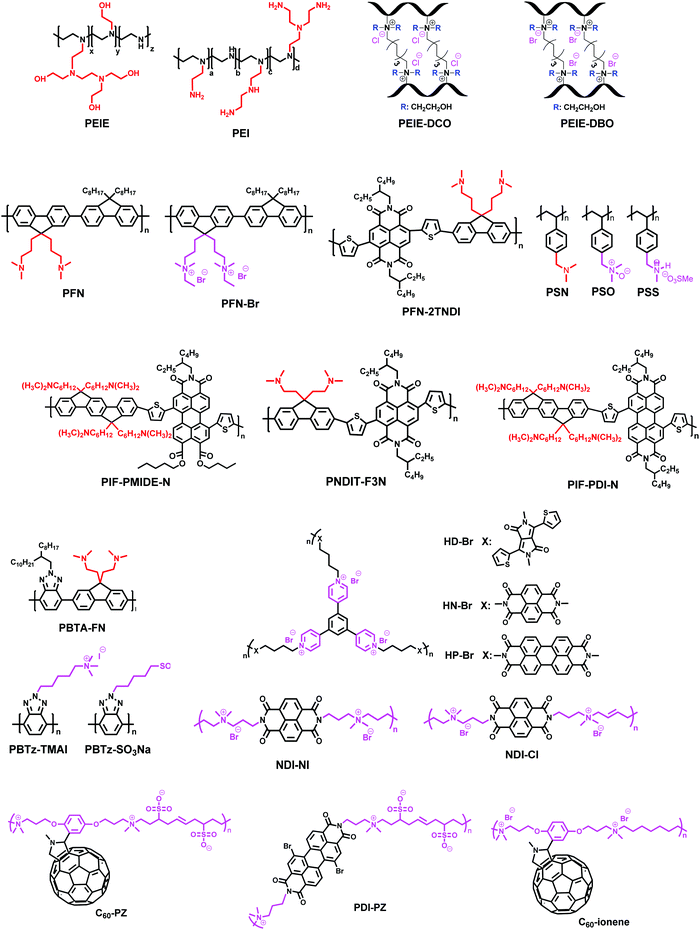
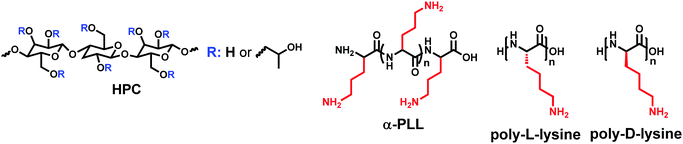
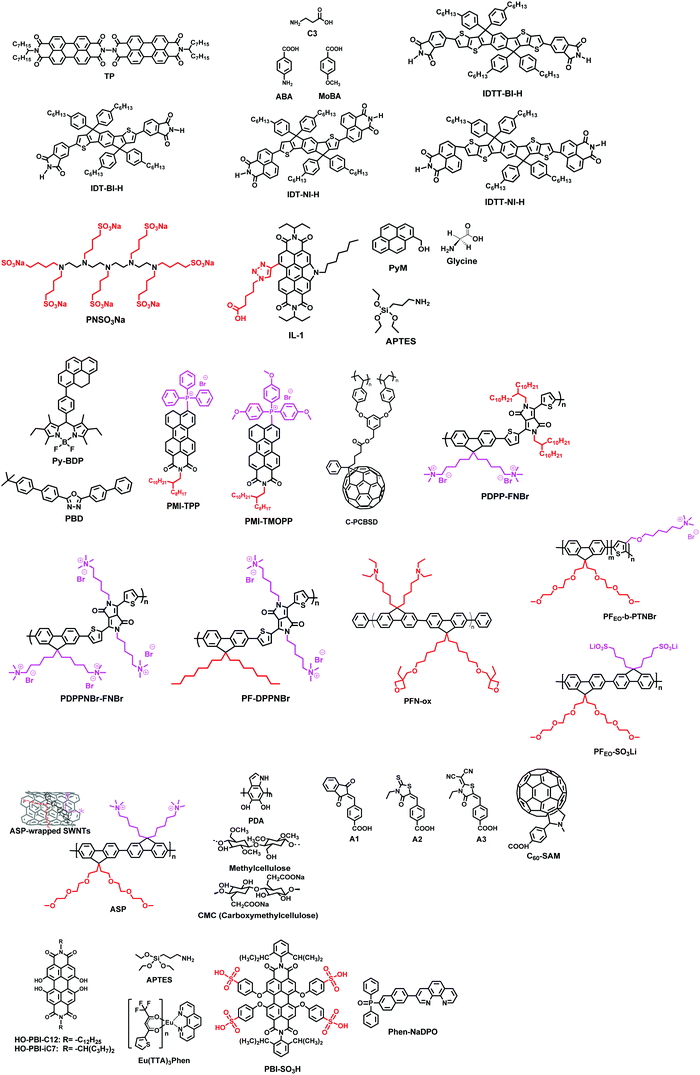
Interlayers for non-fullerene based polymer solar cells: distinctive features and challenges
Roberto
Sorrentino
 a,
Erika
Kozma
a,
Erika
Kozma
 a,
Silvia
Luzzati
a,
Silvia
Luzzati
 *a and
Riccardo
Po
*a and
Riccardo
Po
 b
b
aIstituto di Scienze e Tecnologie Chimiche “Giulio Natta”, Consiglio Nazionale delle Ricerche (SCITEC-CNR), Via A. Corti 12, 20133, Milano, Italy. E-mail: silvia.luzzati@scitec.cnr.it
bEni SpA, Renewable Energies and Environmental R&D, Istituto Guido Donegani, Via G. Fauser 4, 28100, Novara, Italy
First published on 10th December 2020
Abstract
Polymer solar cells based on fullerene acceptors have reached in recent years power conversion efficiencies (PCEs) approaching 13%. The advent of non-fullerene acceptors (NFAs) with the advantages of synthetic versatility, a strong absorption ability and high thermal stability has resulted in impressive PCEs of over 18% in single junction devices. The insertion of interlayers between the active components and electrodes plays a key role in charge collection, boosts the efficiency and improves the device stability. However, the mechanisms regulating the interaction between interlayer materials and active layers based on NFAs are not yet completely rationalized. This review article summarizes organic, inorganic and hybrid materials used as anode and cathode interlayers in conventional and inverted fullerene-free solar cells. Particular attention is paid to the distinctive features of the interlayers when used in non-fullerene solar cells. We will also comment on the fabrication processes with an emphasis on the transition from small area, lab devices to large area modules and on possible mechanisms which are behind.
Broader contextThe increase in global energy demand and the concern about climate change have triggered the interest in renewable energies, in particular, for photovoltaic technologies. Organic and polymer solar cells represent a promising technology thanks to the possibility of high throughput fabrication by low temperature, low-cost printing or coating processes from solvent-based inks, which are compatible with flexible plastic substrates. In recent years, organic photovoltaic devices have reached power conversion efficiencies exceeding 18% thanks to novel polymer donor materials and non-fullerene acceptors that, when properly combined, form photoactive blends. Still, to attain the desired performances also in large area modules, the overall device structure, including the choice and properties of the electrode interlayers used at both the anode and cathode interfaces, needs to be optimized and adapted to the newly developed active components. |
1. Introduction
The development of clean and renewable energy remains a crucial challenge for the scientific community and, particularly, the conversion of solar energy to electricity addresses the global energy issues. Many solar cell devices have been designed, like Si-based inorganic solar cells, and perovskite and dye sensitized based devices. Although these devices provide high efficiencies, their high production costs and the use of hazardous chemicals limit their applications.From this point of view, organic solar cells (OSCs) are ideal candidates and are without doubt moving rapidly towards commercialization. Still, transferring photovoltaic technologies from the laboratory scale to industrial applications needs further improvements in terms of performance, stability, and cost, keeping in mind as well their environmental impacts. OSCs are mostly fabricated through solution processing. Spin coating and, secondly, doctor blade coating – are mostly used on the laboratory scale, while slot die coating, gravure printing and screen printing are the deposition techniques suitable for roll-to-roll (R2R) processes, compatible also with flexible substrates.
Bulk heterojunction (BHJ)-type organic photovoltaics (OPVs) are the most efficient device structures in which the active layer is composed of a blend of a suitable donor and acceptor, sandwiched between the cathode and anode.1–4 OPVs are mainly based on polymer solar cells (PSCs), where the donor components are conjugated polymers. During the earlier development of OPVs, poly-3-hexylthiophene (P3HT), characterized by good π-delocalization properties and high thermal stability, was the most used semiconducting donor polymer. Blends of P3HT and [6,6]-phenyl-C61-butyric acid methyl ester (PC61BM) remain among the most extensively studied systems in the OPV field.5
Over the next years, the development of donor–acceptor conjugated low-band gap polymers has represented the real breakthrough for high efficiency OSCs. By using low-band gap polymer donors like poly[[4,8-bis[(2-ethylhexyl)oxy]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl][3-fluoro-2-[(2-ethylhexyl)carbo-nyl]thieno[3,4-b]thiophenediyl]] (PTB7)6 or its dithienyl derivative (PTB7-Th),7 device PCEs have increased from 4% to over 10% when combined with a fullerene-derivative acceptor, [6,6]-phenyl C61-butyric acid methyl ester (PC61BM) or [6,6]-phenyl C71-butyric acid methyl ester (PC71BM). Despite the development of conjugated donor polymers which definitely contributed towards enhancing the device efficiencies, fullerenes and their derivatives have been used almost exclusively as electron acceptors in polymer solar cells (PSCs), due to their high electron affinity, mobility and tendency to form percolated pathways for efficient electron transport.8 Still, the highly symmetric nature of fullerenes and their difficult synthetic tunability have resulted in weak absorption properties, affecting their photocurrent generation. Their high cost and multiple chemical synthesis steps are not compatible with the development of low cost photovoltaic technologies. It is also reported that their high photosensitivity and oxygen-induced degradation, along with the tendency to form macro-aggregates, could be responsible for different degradation mechanisms in PSCs.9,10
At this point, the necessity to seek and enlarge the library of alternative acceptor materials became mandatory.
The advent of non-fullerene acceptors (NFAs) (Scheme 1) has brought extremely important advantages in PSCs which blew up their efficiencies.11 Their high synthetic flexibility not only permitted improving their light harvesting properties but also enabled the realization of highly efficient PSCs with open circuit voltages exceeding unity and with substantially reduced voltage losses.12,13 The use of low-band gap ITIC derivatives led to PCE values of 6.8% when blended with a low-band gap polymer donor (PTB7)14 and to values over 11% when blended with a wide band gap polymer donor (PBDB-T) as a consequence of the better photoactive material's absorption.15 All these findings show that the main progress in PSC performances is mostly due to the outstanding research into active layer components.
However, for a reliable and high performing solar cell, charge carrier collection is of key importance, and this implies the insertion of interlayers (ILs). These ILs depending on their position with respect to the electrodes are called cathode or anode interlayers (CILs or AILs) and can selectively transport carriers.
In this review, instead of critically discussing the photoactive layer, we present the advances of interlayers used in NFA-based PSCs. We focus on the papers up to July 2020 found by searching in Google Scholar and the Web of Science with different keywords and various combinations of these keywords: non-fullerene, fullerene free, interlayers, polymer solar cells, cathode engineering, anode engineering, polyelectrolytes, zinc oxide, hybrid, and doping.
In Section 2, we expose the role and the specific features of interlayers in NFA-based PSCs. We choose to classify the buffer layers according to their working mechanisms, namely, (i) work function (WF) adjuster, (ii) doping and (iii) morphology modifier. We also emphasize that studies are still needed to shed light not only on stability issues due to possible reactions that might occur when the interlayers and the newly designed NFA photoactive materials are in contact, but also on the stability of materials themselves under light conditions. For both themes we propose possible strategies to follow and we also offer a discussion about the possible mechanisms involved, expressly highlighting the diatribes in the scientific community, hoping that clarity will be soon made.
In Section 3 we summarize the different classes of interfacial layers used in NFA-based PSCs. They are divided into anode and cathode interlayers and both categories are discussed in detail according to their chemical composition (organics, inorganics and hybrid materials).
Finally, in Section 4, we deeply discuss the possibility of the scale-up of the processes and we conclude with a brief overview of what has been found and the future challenges and perspectives for interlayers in NFA-based PSCs.
2. Role of interfacial layers
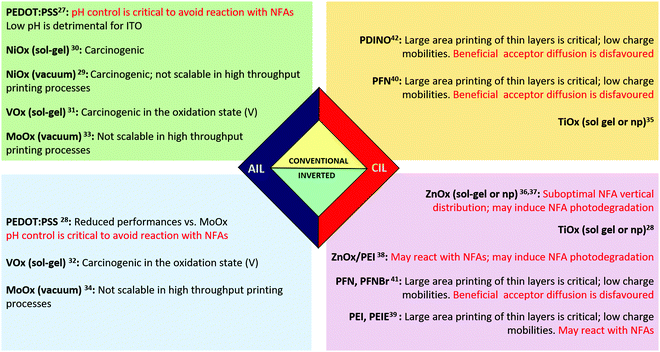
The understanding of the role of interfacial layers in solar cell devices comes from a know-how in the field developed during the last thirty years that connected chemists, physicists and materials scientists. From the very beginning of FA-based PSCs, the role of interfacial layers has been extensively discussed in many seminal papers and reviews.16–22 During these years, the community had to find a compromise between the high efficiencies and long-term stability of devices but looking ahead to the upscalability of the process. For these reasons, the main focus has been addressed to solution processable devices. Since PSCs are multilayered sandwiched devices, the use of orthogonal solvents is highly recommended. It permits the deposition of materials without ruining and/or dissolving the layer below. Usually, the photoactive layers are processed from non-polar solvents; due to that, water and alcohols became fundamental solvents for processing the interlayers in PSCs.23–26The number of interlayer materials that partially satisfy the needed features in terms of efficiency, stability and up-scalability is relatively low and these materials can be divided into conductive conjugated polymers, metal oxides and non-conjugated polymers. To help the readers, these “classical” IL materials27–42 are summarised in Fig. 1. Undoubtedly, an efficient IL for FA-based PSCs doesn’t necessarily mean an efficient IL for NFA-based PSCs. In this review, we discuss the roles of interfacial materials in PSCs with a particular focus on NFA-based devices. We also highlight the general drawbacks and the peculiar disadvantages once an IL is used with NFA materials. Particular attention is given to the comparison between the use of ILs in FA and NFA solar cell devices.
In BHJ solar cells, after the electrons and holes are separated at the donor–acceptor interface under illumination, the collecting electrodes should selectively drain the charges and move them to the external circuit. In this process, the organic/metal contacts play a critical role in device performances. Barrier-less (ohmic) contacts should be formed to avoid limitations on the charge extraction efficiency and open circuit voltage (VOC).43 The maximum VOC achievable, in BHJ devices, is determined by the difference between the quasi-Fermi levels (EF,h) and (EF,e), under illumination.44,45 However, if a Schottky barrier is formed at one of the electrodes, it creates a potential loss that decreases the VOC and the FF parameter.46 One of the reasons for this barrier is a bad location of the electrode work function. Following the ICT model, a Fermi level pinning of a conducting interfacial layer to organic semiconductors can only occur when spontaneous charge transfer between the two layers is possible, which is not the case when the electrode work functions are located within the BHJ energy levels.47 To address these issues, AIL and CIL materials should be inserted both to pin the Fermi energy levels of the electrodes to the quasi-Fermi energy levels of the BHJ and to selectively drain the charges. The charge selective behavior imparted by the ILs plays a crucial role in changing the device polarity, which provides flexibility to the device architecture, as shown in Fig. 2.
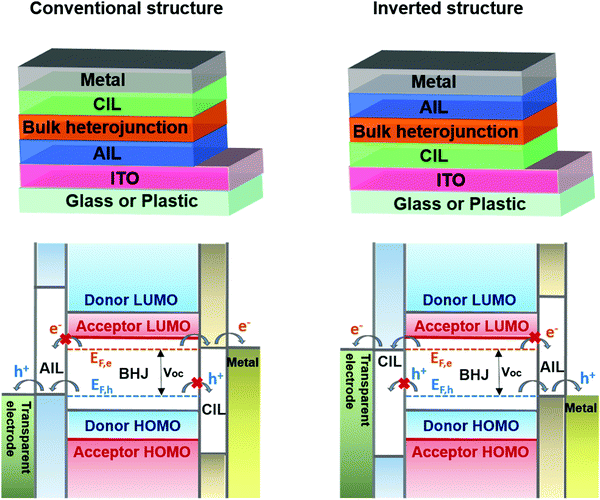 | ||
| Fig. 2 Schematic representation of organic solar cells with conventional (upper-left) and inverted (upper-right) structures and the corresponding energy level diagrams. Adapted from ref. 19 with permission from the Royal Society of Chemistry. | ||
In a conventional geometry (transparent anode/BHJ/cathode), the typical anode is a transparent conductive electrode, indium-doped tin oxide (ITO). Upon the ITO, a conductive conjugated polymer called PEDOT:PSS, which enables a shift of the ITO's work function (WF) from 4.7 eV to 5.1–5.3 eV,48 is usually deposited as an AIL. This energy level is suitable to obtain a pinning to the energy level of the most common donor polymers applied both in F-PSCs and in NF-PSCs. For this reason, on the anode side, energy level engineering is not going to be strongly affected in the switch from FAs to NFAs.49–51
In an inverted geometry (transparent cathode/BHJ/anode), the CIL can reduce the ITO's WF to values ranging between 4.3 and 3.5 eV. One of the most used CILs in PSCs is zinc oxide (ZnO), which is an n-type metal oxide semiconductor which has a WF of ca. 4.3 eV and optical transparency in the visible-wavelength region.52 Other cathode interlayers widely applied to inverted and conventional PSCs are amino-containing polymers (i.e.PEIE and PFN). These materials are rather effective in reducing the electrode WF at the inner interface with the active layer to values between 3.5 and 4.1 eV with a gain in electron selectivity of the electrodes.41,53,54 With these WF values, the Fermi levels of the modified cathode electrodes are pinned close to the LUMO energy levels if fullerene acceptors are used55,56 but not necessarily with NFAs.13,57 For this reason, it is expected that CILs designed for FA-based PSCs might not fit when NFAs are used.58
A metal electrode is frequently used in both conventional and inverted geometries. The most common metal electrodes are aluminum (Al) and silver (Ag) with WF values close to 4.2 and 4.3 eV, respectively.59 If an interfacial layer is used at the interface, it will increase the electron selectivity of the contact. These effects strongly reduce the barrier between the active layer and the electrode, improving the extraction, the transport and the collection of charges. In an inverted structure the most common buffer layer is molybdenum oxide (MoO3, MoOx). Even if it is usually evaporated and not solution processed, MoO3 is widely used thanks to its intrinsic properties. It is an n-type, wide bandgap, metal oxide semiconductor with a higher/similar electron affinity than/to the HOMO levels of many organic semiconductors. Efficient transfer from the HOMO level of the donor material to the 4d conduction band of MoO3 occurs.60–62
The organic/electrode interface plays a critical role in determining the device performances and a Schottky barrier to the electrode can be easily formed. As will be frequently discussed in the next sections, a non-conductive and thick IL, or chemical reaction at the interface, might result in a significant barrier. Since charge extraction is not allowed, charge accumulation at the electrode occurs. Due to this, a voltage drop is verified, leading to a typical flex, S-shape, close to the VOC in the J–V characteristics.63
The interfacial energy barrier between two layers can be reduced in different ways: in particular, (i) by optimizing the work function of the electrodes, obtaining a better energy level alignment;64–66 (ii) by minimizing the interfacial contact resistance, obtaining more ohmic contacts for efficient charge extraction;67 and (iii) by enhancing the built-in voltage to efficiently sweep out the charges at the electrodes, thus reducing recombination losses.68–70 The combination of the above functions improves the charge selectivity and the efficiency of charge collection, having a positive effect on the figures of merit of PSC devices, with major gains in FF and VOC values. Being correlated with each other, it is hard to differentiate these mechanisms and treat them individually. All these effects are discussed in Sections 2.1 and 2.2.
Besides energy level alignment, other specific features for interlayers discussed here are the doping effect and the morphology modification. For the first one, the abilities to efficiently transport the charges and to minimize the contact resistance are mandatory to promote charge extraction and reduce recombination losses. These characteristics are generally of key importance for efficient ILs but are becoming even more important in NFA-based PSCs. In fact, minimizing the recombination losses might be critical for devices’ performances because the recombination effects can be intensified with the increased number of photons captured in NFAs.71 In FA-based PSCs it has been suggested that one of the reasons to explain the excellent performances obtained using a p-type CIL like PFN, despite its quite low electron transport ability, is the possibility of fullerene molecules to diffuse into the polymeric matrix, doping the IL. It permits lowering the transport losses and improving electron collection.66,67,72 The intermixing is possible for small and symmetric fullerene molecules but not so easy for massive and anisotropic NFAs. The difference in shape between FAs and NFAs results in different working mechanisms of charge transport, and in NFAs electron extraction is, in general, more complicated.73 For these reasons, the doping of NFAs in contact with CILs, useful to reduce the contact resistance in the FA counterparts, does not necessarily work with the same effectiveness. These aspects are investigated and treated in Section 2.2.
The surface properties of interlayers on which the BHJ photoactive layer is deposited can influence the phase segregation and microstructure morphology of the donor/acceptor blend.74 The presence of an IL might induce a favorable vertical phase separation, leading to improvements in the charge transport ability and collection processes.16,75 An enhancement in charge selectivity at the corresponding electrodes might be achieved by ensuring donor (acceptor) enrichment at the anode (cathode) interfaces.19 In addition, the presence of ILs might also induce a favorable molecular orientation to improve the charge collection and reduce the recombination losses.76 Recalling the difference between FA and NFA geometries discussed before, we might expect that the morphologies of the photoactive materials won’t be similar. In Section 2.3 we focused our attention on the morphology modification of the photoactive material through the insertion of buffer layers.
Finally, the (in)stability of organic materials makes NFA-based PSCs not competitive in the current market yet. This is due to the fact that, frequently, materials present defects at the interfaces and these defects or traps can promote physical phenomena or chemical reactions between the active layer and the electrodes or the ILs, if present. To partially avoid these interactions, the presence of inert buffer layers might protect the integrity of the device by prohibiting chemical reactions at the interface and it can enhance the stability of the devices. For instance, some ILs are used as protective layers to prevent the degradation of the photoactive material due to oxygen and moisture exposure.77 Others are used to avoid the diffusion of metal atoms into the photoactive layer and to prevent corrosion problems of the active material at the interface.78 In fact, the deposition of the metal electrode, which is usually done by an evaporation process, or the continuous exposure to 1 sun intensity, due to the extremely high temperature reached, can cause the intercalation of metal atoms into the organic layers. This creates shunting or electrical shorts in the device and compromises the performances and the long-term stability. Regarding the lifetime of the devices, Brabec and coauthors studied the stability of solar cells based on PBDB-T and a series of ITIC acceptors, achieving an extrapolated T80 lifetime of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h (ca. 10 years) under continuous irradiation. They observed that the breaking of conjugation during the photo-aging leads to an increment of energetic traps and thus a continuous decrease of photovoltaic parameters. They claimed that, in general, the presence of a fluorinated end-group (IT-4F) stabilizes the molecules against light soaking, while adding methyl groups (IT-M) showed an opposite trend.79 As far as we know, the longest extrapolated lifetime in NFA-based devices has reached the extraordinary operational lifetime value equal to 34
000 h (ca. 10 years) under continuous irradiation. They observed that the breaking of conjugation during the photo-aging leads to an increment of energetic traps and thus a continuous decrease of photovoltaic parameters. They claimed that, in general, the presence of a fluorinated end-group (IT-4F) stabilizes the molecules against light soaking, while adding methyl groups (IT-M) showed an opposite trend.79 As far as we know, the longest extrapolated lifetime in NFA-based devices has reached the extraordinary operational lifetime value equal to 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h (ca. 22 years) reported by Xu et al.80 In the device structure, they used a fullerene self-assembled monolayer to modify the ZnO interface on the cathode side of the device that increased the morphological stability of the photoactive layer. The device became less susceptible to photodegradation thanks to the morphological stabilization of the active layer and the suppression of trap-assisted recombinations.80 The discussion about the stability of the active layer and the interlayer upon photo-irradiation (photostability) and photocatalytic effects is provided in Section 2.4. Here, we consider both the possible reactions between interlayers and active materials and the degradation upon UV, oxygen and moisture exposure. Since regarding these aspects the scientific community is nowadays trying to fix many issues, a lot of topics are still under debate and, for this reason, we purposely focus on the controversial analysis found in the literature.
000 h (ca. 22 years) reported by Xu et al.80 In the device structure, they used a fullerene self-assembled monolayer to modify the ZnO interface on the cathode side of the device that increased the morphological stability of the photoactive layer. The device became less susceptible to photodegradation thanks to the morphological stabilization of the active layer and the suppression of trap-assisted recombinations.80 The discussion about the stability of the active layer and the interlayer upon photo-irradiation (photostability) and photocatalytic effects is provided in Section 2.4. Here, we consider both the possible reactions between interlayers and active materials and the degradation upon UV, oxygen and moisture exposure. Since regarding these aspects the scientific community is nowadays trying to fix many issues, a lot of topics are still under debate and, for this reason, we purposely focus on the controversial analysis found in the literature.
2.1. Energy level alignment and work function modification
ZnO is the most common CIL in fullerene-based inverted solar cells and thus has been considered also for NFA-based PSCs. Although both FA- and NFA-based PSCs efficiently work with ZnO as a CIL,15,81 the efficiency of the cells can be further improved by modifying the WF of ZnO. Moreover, ZnO presents dangling bonds and surface defects that can trap the charges and thus diminish the solar cell performances. To efficiently improve the sweeping out of the charges, a widely used approach is to form bi-layered CILs. The double layer permits both modifying the ZnO WF and passivating the trap states. For example, the possibility to modify the WF of ITO/ZnO with a thin layer of cross-linkable PFN (PFN-ox) has been devised.82 This strategy is a consequence of a previous study done by the same group on FA-based PSCs; the cross-linked material has a lower solubility in the active layer's solvent, ensuring a more robust IL, but allows the fullerene to diffuse into the IL, increasing its conductivity.83 By ultraviolet photoelectron spectroscopy (UPS) it is possible to observe a WF modulation in the presence of PFN-ox; the WF shifts from 4.63 (ITO) to 3.95 eV (ITO/PFN-ox).As already said, polymers atop ZnO can efficiently passivate the defects on the metal oxide surface, resulting in fewer trap-states and, after being widely used for FA-based PSCs,84 carbon nanotubes were also used for NFA-based PSCs. In this case, a mixture of alcohol-soluble polyfluorene and single-walled carbon nanotubes (SWCNTs) on ZnO has been used. The polymer is used both to efficiently modulate the WF of the CIL in PM6:Y6 based devices and for trap passivation, while the presence of SWCNTs permits obtaining a higher conductivity of the CIL.85 Another pathway that has been followed for the CIL is the realization of a bilayer consisting of ZnO nanoparticles and ZnO sol–gel (ZnO-NP/ZnO). This bilayer exhibited a lower work function when compared to bare sol–gel ZnO (4.04 eV vs. 4.16 eV).86 The energy level mismatch between ZnO and ITIC is reduced with the proposed ZnO bilayer, lowering the barrier to electron extraction at the ITIC/ZnO interface (see Fig. 3). Since the energy level for fullerenes is in the range between 4.1 and 4.4 eV, the energy mismatch going from FA to NFA materials became more important.
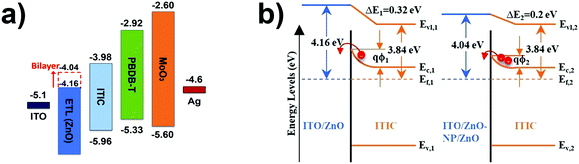 | ||
| Fig. 3 (a) Flat energy band diagram of ITO/ZnO/PBDB-T:ITIC/MoO3/Ag devices. (b) Energy level alignments of ITO/CIL/ITIC, depicting the band-bending phenomenon. Reprinted from ref. 86, Copyright (2020) with permission from Elsevier. | ||
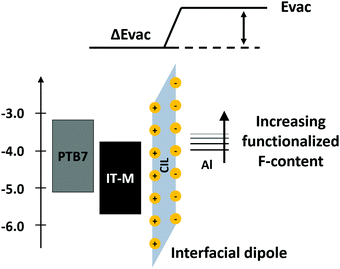
Besides polymers and inorganics, small molecules are also used as WF modifiers. In organic CILs, the presence of polar groups imparting processability in polar solvent induces dipoles at the interfaces that might tailor the WF of the electrode. This strategy is used with non-conjugated small molecules to efficiently exploit the dipole effect. The presence of a dipole permits the modulation of the WF and thus favors extraction of charges from NFA-based PSCs with conventional geometries.87Alq3 has been used as a core and modified with an increasing number of fluorene units (Alq3-F1, Alq3-F2, and Alq3-F3) substituted with alkyl ammonium bromide moieties.88 It is found that by increasing the amount of ammonium functionalized fluorene units, the interfacial dipole moment can be enhanced, lowering the metal electrode WF and thus decreasing the energy barrier between the active layer and the cathode (see Fig. 4).
PFN and PFN-Br are materials commonly used as CILs in FA-based PSCs and they became benchmarks for newly developed ILs for different fullerene-free PSCs. The insertion of a thin PFN layer induces a relevant increment in electron selectivity, when compared to bare Al due to a favorable dipole formation that reduces the energy offset at the electrode interface.89,90
When the PFN layer is kept ultrathin (∼5 nm), the charges can easily be extracted by tunnel effects (see Fig. 5), but as soon as the PFN film thickness increases, a huge barrier to electron extraction occurs and the SC performances drastically decrease. This is due to both the misalignment between the acceptor and IL and the characteristic poor conductivity of PFN-derivatives. Aside from PFN, PFN-2TNDI was also used as a CIL. It is an n-type naphthalene diimide based conjugated polymer with a lower LUMO level (−3.91 eV) with respect to PFN (∼2.14 eV).90PFN-2TNDI has been studied with three different acceptors, N2200, ITIC and IDT-2Br, with different LUMO energy levels (−3.84 eV, −3.83 eV and −3.69 eV, respectively). As a general trend, the devices prepared with PFN-2TNDI outperformed the ones prepared with PFN when ultrathin interlayers (∼5 nm) were used.
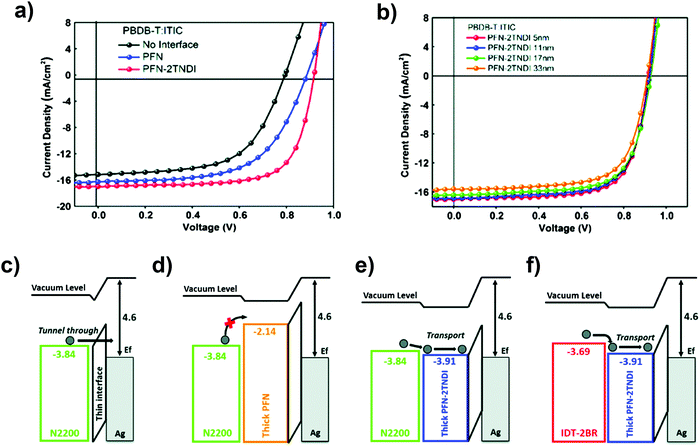 | ||
| Fig. 5 (a) J–V characteristics of PBDB-T:ITIC devices with different CILs with thicknesses ∼5 nm. (b) J–V characteristics of PBDB-T:ITIC devices with PFN-2TNDI as interlayers of various thicknesses. Energy level diagrams of PTB7-Th:N2200 BHJs with (c) thin and (d and e) thick CILs. (f) Energy level diagrams of a PTB7-Th:IDT-2Br BHJ with a thick CIL. Adapted from ref. 90 with permission from the Royal Society of Chemistry. | ||
Such behavior is due to the PFN-2TNDI electron transport properties and reduced energy offset with the NFA, which promote better extraction and reduce charge recombination losses when compared to PFN. Note that the same CILs applied to FA-based PSCs gave the same performances when the CIL was kept thin. Additionally, efficient devices with thicker interlayers could be achieved using N2200 and ITIC acceptors, but not with IDT-2Br due to the different energy level alignments between the IL and acceptors. In fact, when using a thick IL, the interface between NFAs and CIL becomes more important and a good energy level alignment between the IL and acceptors is needed to ensure efficient electron transport across the interface. This is the case for N2200 and ITIC but not for IDT-2Br (see Fig. 5).
A different behavior is observed in FA-based PSCs, where a thickness independence is observed. As will be discussed in the next paragraph, relevant n-doping between the fullerene acceptor and the PFN or PFN-2TNDI interlayers accounts for the different features.91
An approach to match the energy levels of CILs with those of NFAs is to tune the energy levels of the CILs by molecular design. It is possible to push the LUMO level up to 3.68 eV with a new n-type polymer CIL (PIF-PMIDE-N). This shift enables the reduction of the energy off-set and, at the same time, it modifies the material from an n-type to a p-type, which is detrimental to the CIL transport ability.
A more interesting strategy is to reduce the energy barriers by designing ILs with similar chemical structures to acceptor materials. As an example, an n-type small molecule electrolyte (N-IDTBR) with a similar backbone to the non-fullerene acceptor O-IDTBR was reported.92 This CIL not only provides an excellent energy match but also exhibits good transport properties. Moreover, the compatibility between the two materials is higher. As depicted in Fig. 6, a substantial CIL thickness invariance was obtained with this approach.
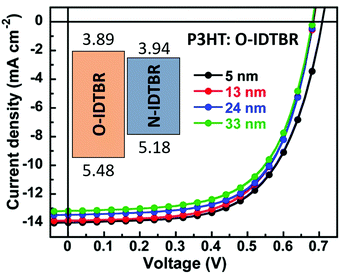 | ||
| Fig. 6 J–V curves of PSCs based on the P3HT:O-IDTBR active layer with various thicknesses of the N-IDTBR CIL. Adapted from ref. 92 with permission from the Royal Society of Chemistry. | ||
2.2. Doping effect
Besides the energy level alignment and WF modulation, having efficient charge transport in organic CIL materials is a critical issue to obtain high performance devices. For this reason, p- or n-type doping is commonly applied to organic semiconductors to improve their electrical conductivity. Herein, we are interested in shedding light on the various processes that govern the interactions between CILs and NFAs, and therefore, we will focus on n-type doping strategies. It was already demonstrated that the doping process between FAs and organic CILs represents an important mechanism to reduce the BHJ/electrode contact resistance in FA-based PSCs; thus, addressing the same doping also with fullerene-free devices was a natural consequence. It is unlikely that the roles played by the NFAs are not necessarily the same. Given the diversity of emerging NFAs, fundamental insights into the selection rules for n-dopants are still lacking.The n-type doping of organic semiconductors is an anion induced electron transfer (AIET)67 and involves charge transfer from electron-rich moieties to electron deficient π–acceptors.93 The effectiveness of AIET depends on both electronic and steric hindrance effects. This includes the anion's electron donating ability (Lewis basicity), the electron accepting ability of the π–acceptor and the physical interaction between these components. Effective AIET has been achieved for both FAs67 and NFAs94 using tetra-alkyl ammonium salts as extrinsic dopants. This strategy was demonstrated to be effective in boosting the conductivity of both FAs and NFAs and was applied to achieve quasi-ohmic contact in solar cells, without any CIL (Fig. 7).
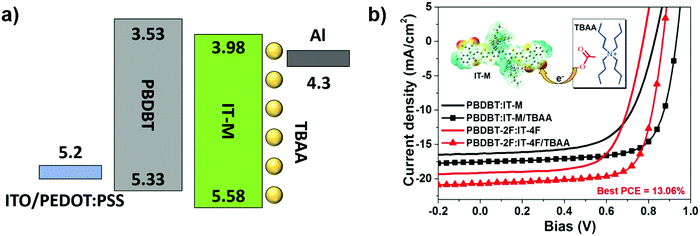 | ||
| Fig. 7 Scheme of the energy diagram of PBDBT:IT-M with TBAA doping (a) and illustration of electron transfer from TBAA to IT-M along with the effect of charge transfer on J–V characteristics (b). Adapted from ref. 71. Copyright (2020) with permission from Elsevier. | ||
It is extremely clear that the doping efficiency depends on both electrostatic interactions and steric effects between TXABr (tetraalkylammonium bromide) and acceptors. The preference of long chain dopants for NFAs is in contrast to the preference of short-chain dopants in FAs, most likely due to the difference in both intramolecular interactions and steric effects between TXABr and acceptors. Moreover, by changing the NFA from the ITIC family to Y6, an opposite trend was observed. Due to a more intimate contact, the doping of Y6 with THABr (tetrahexylammonium bromide) results in the most effective one. Differently from the spherical FA, the molecular configuration of the NFA is planar or quasi-planar. It leads to a more complicated interaction between the interlayers and NFA, which will influence the electron-transfer process.
Another approach used for the AIET process is to covalently attach anion dopants to π–acceptors through alkyl chains (self-doping). The enhanced stability, good solution processability and effective AIET provided OSCs with improved performances.95–97 In these systems the AIET efficiency can be finely tuned by modifying the electron acceptor capability and anions’ Lewis basicity.98
Another interesting doping phenomenon is the charge transfer which may occur between CILs and π–acceptors. By analyzing the effects of CILs constituted by the same polar groups flanked to alkyl chains attached to naphthalene diimide (NDI) or polyfluorene (PF) on FAs or NFAs, it is very obvious that the mechanisms which govern the interactions at the interface are extremely complex and it is rather difficult to imagine a model that can be adopted for both FAs and NFAs.
Two n-type naphthalene bisimides containing an alkyl-amino (NDI-N) and a tetraalkylammonium-bromide (NDI-Br) were used as CILs for NFA-based PSCs with IT-4F as an acceptor material (Fig. 8a).97 Both NDI CILs show similar n-doping effects evidenced by Electron Spin Resonance (ESR) spectroscopy. Additionally, NDI-N combines high crystallinity and good film-forming properties, endowing the semiconductor with outstanding electron-transport properties and good processability. Also, when NDI-N is used as a CIL, an interfacial doping effect between the amino substituent and IT-4F is observed. This process may induce an interfacial dipole layer at the IT-4F/NDI-N interface which facilitates the electron extraction from the active layer to the cathode (see Fig. 8b).
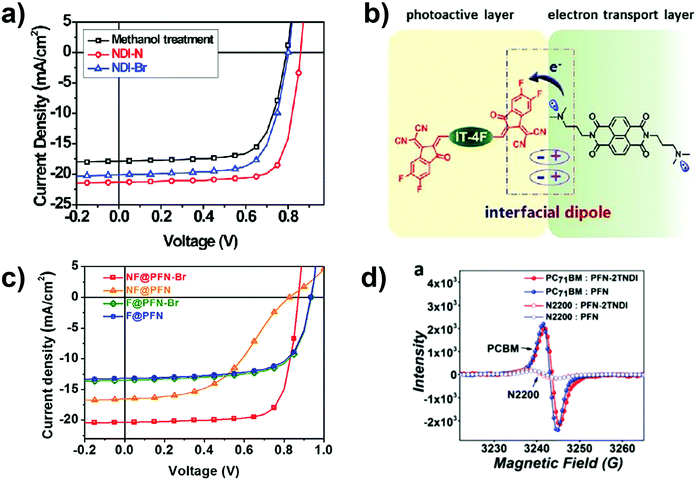 | ||
| Fig. 8 (a) J–V curves of PSCs with different CILs (none, NDI-N, NDI-Br). (b) Schematic illustration of an interfacial dipole formed by the doping between IT-4F and NDI-N. Reprinted from ref. 97. Copyright (2020) with permission from Elsevier. (c) J–V curves for FA- and NFA-based PSCs with different CILs. Reprinted from ref. 99. Copyright (2020) with permission from Elsevier. (d) Electron spin resonances of acceptor blends in solid states. Reproduced from ref. 90 with permission from the Royal Society of Chemistry. | ||
The same active layer is used to compare PFN and its derivative (PFN-Br).99 Fullerene-based solar cells are also investigated in this study. While the CILs PFN and PFN-Br exhibit quite similar performances when applied in FA-based PSCs, the same does not happen in the case of IT-4F devices (see Fig. 8c). With IT-4F as an acceptor material and PFN as a CIL, “S” shaped photovoltaic characteristics are observed. They result from an extraction barrier when using PFN as a CIL but are not observed when using PFN-Br.63 The results can be explained by excessive electron-transfer from PFN amino-groups to IT-4F. If this happens it can induce an unfavorable charge accumulation at the interface. Such an electronegative layer blocks the electron collection and it can cause serious charge carrier recombination at the interface. By replacing the amino side group of PFN with the ammonium bromide (PFN-Br) the excessive doping effect between the CIL and the acceptor material is eliminated and better performances are achieved.99 At first glance, this mechanism seems to be in contrast to the one suggested for the NDI-N, but it is important to consider the CILs’ electron transport capabilities.89 If we consider PFN and PFN-Br, although they have the same backbone, the presence of different side chains induces some differences: (i) PFN-Br has higher processability and therefore better thickness control100 and (ii) the dielectric constant of PFN-Br is higher, which guarantees better charge extraction. This explains why PFN-Br represents a benchmark CIL for both FA and NFA PSCs, while PFN shows good performances only in FA PSCs. The good results obtained when PFN is used as the CIL in FA PSCs is due to the combined effect of good charge transport properties and the capability of PCBM to interpenetrate into the CIL (Fig. 8d).90
Apart from the electronic factors which govern AIET, the steric effects are also important to ensure efficient charge transport properties in PSCs. The use of a non-conjugated biopolymer bearing primary amines in the side chain (α-PLL) is a winning approach.101 A doping occurs between α-PLL and IEICO-4F, promoting charge extraction and ultimately photon-harvesting, a trend which was not observed when PFN was used as an interlayer. The differences in interfacial doping effects were suggested to arise from conformational effects leading to a more favorable contact of the IEICO-4F molecules to the amino groups of the α-PLL rather than to the ones of PFN. Consequently, the α-PLL can be used indistinctly as a CIL in a variety of fullerene-free BHJ devices and, notably, inverted PSCs with α-PLL reached comparable PCEs with those based on ZnO, which is not by far the case of PFN.
Although many CIL materials have been developed, the proper selection of a CIL is still a great challenge due to the complicated working mechanisms of CILs in PSCs. Indeed, with many efficient active layer materials developed like high-performance non-fullerene acceptors, more complex factors need to be comprehensively considered for selecting the appropriate CIL to realize the best photovoltaic performance of the active layer.
Concerning organic CILs, it is clear from Sections 2.1 and 2.2 that the framework already used for the development of efficient interfacial layers for FA-based BHJs cannot be merely transposed when going to NFA-based SCs.
One of the “blessing” features for FAs is that it is possible to take advantage of the excellent interfacial doping with several ILs. This is due to the combination of a good physical contact between the IL material and the acceptor molecules and to an excellent electron accepting ability of FAs. This brings both an enhancement in the transport properties of the ILs and the reduction of the contact resistance between the IL and acceptor components. When going to NFAs, interfacial doping becomes more critical. The needs to address the tailoring of organic ILs can follow two main pathways: (i) enhance the electron conductivity of the ILs and (ii) favor both the physical and electronic contacts between the IL and the NFA to enhance the interfacial doping effect through the tailoring of the materials. Note that if the interfacial-doping and/or the electron transport properties are not fully optimized, much more attention should be given to the energy level alignment between ILs and NFAs.
2.3. Morphology modifiers
Few aspects that can drastically modify the morphology are (i) the presence of an interlayer under the photoactive material or (ii) additives in the photoactive layer. These variations can bring a change in wettability and/or in optical properties and they drastically affect the final performances of the device. A common method to improve the wettability is to reduce the surface tension of the CIL by engineering the design and synthesis. The surface tension modification leads to a vertical distribution of the acceptor molecules in the BHJ.102 Bai et al. used titanium(IV) oxide bis(2,4-pentanedionate) (TiO(acac)2) as a CIL and, by TOF-SIMS, they verified the effectiveness of the vertical phase distribution of donor/acceptor molecules in the bulk heterojunction. They observed that the PBDB-T rich phase is located at the air surface, while the ITIC rich phase is located at the bottom; this observation means that the presence of TiO(acac)2 induces the desired vertical component distribution. A similar vertical separation has been studied by Cheng et al. and by Liu et al.103,104 In the first work the authors realized a K doped ZnO that can interact with the acceptor molecule more efficiently than bare ZnO.103 In the second one, conjugated small molecules are deposited on ZnO to form SAMs. A similar surface energy between SAM-modified ZnO and ITIC, used as an acceptor, helps the segregation at the interface, inducing an acceptor rich phase on the cathode side.104 A similar segregation is found by Park et al. who used PFN-Br to induce a vertical segregation into a ternary device made with IT-4F and PC71BM acceptors, obtaining nano-domains suitable for improved performances.105 These results could be obtained because PC71BM, having a relatively high surface energy within the ternary active layer, accumulated in the PFN-Br layer by way of rearrangement during the film-formation process (Fig. 9).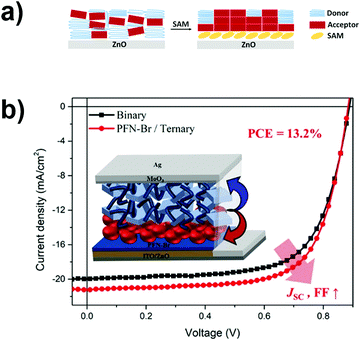 | ||
| Fig. 9 (a) Schematic illustrations of the composition gradients near the region of bare ZnO (left side) and SAM-modified ZnO (right side). (b) J–V characteristics and morphological effect on PCE. Reproduced with permission from ref. 104 and 105. Copyright (2020) American Chemical Society. | ||
Besides CILs, the surface energy of AILs can also be used to engineer the morphology of the active layer. Wang et al. used discotic liquid crystals 2,3,6,7,10,11-hexaacetoxytriphenylene (HATP) between PEDOT:PSS and the active layer to achieve 3D charge transportation.106HATP exhibits a columnar structure with a dominantly edge-on orientation and the significantly improved electron mobility indicates that it is easier for the charges to hop into HATP. Another study in which the AIL was modified was carried out by Wang et al.107 The authors found an approach to tune the surface free energy of the AIL by adding to PEDOT:PSS both poly(styrene sulfonic acid)sodium salts (PSSS) or nickel formate dihydrate (NFD). The change in surface free energy permitted improving the face-on molecular ordering in the BHJ, enhancing the FF and PCE.
Other than interlayers used to modify the bulk heterojunction morphology, additives dispersed in the photoactive layer are used. For example, Yang et al. added liquid-phase-exfoliated black phosphorus nanoflakes (BPNFs) to the bulk heterojunction as a morphology modifier.108 They observed that a PTB7-Th:IEICO-4F blend modified with BPNFs exhibited more ordered π–π stacking and their presence promoted domain purity. This contributed to the decrease of the charge transport resistance and suppression of the charge recombination within the bulk heterojunction (BHJ). On the other hand, Su et al. used P3HT-segment-based block copolymers as additives in a PDCBT:ITIC blend to improve the morphology and thus the performances of PSCs.109 In particular, the presence of these additives promotes the miscibility of the constituent components and prevents the large aggregation of domains of both PDCBT and ITIC, resulting in a more uniform intermixed network.
2.4. Interlayer/active layer reactions and photocatalytic effect
The stability issue is frequently discussed when ZnO is used as a CIL. The intrinsic instability upon UV exposure of this metal oxide leads to reactions with ITIC, used in the active layers110 and other acceptors such as IT-4F and IEICO-4F.111 To completely or partially avoid these reactions, the substitution of ZnO with less UV-reactive SnO2111 and the addition of different interlayers are both strategies proposed (Fig. 10).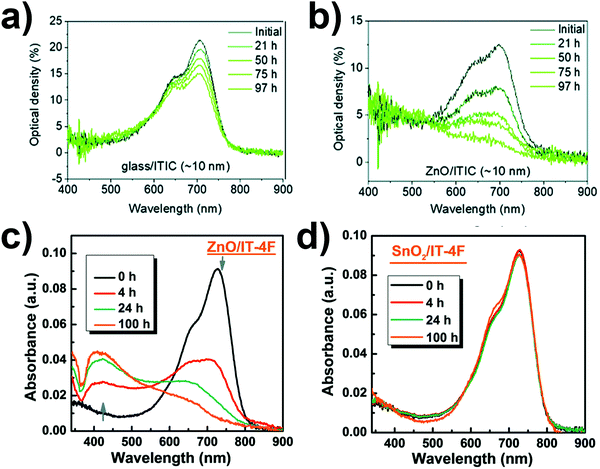 | ||
| Fig. 10 UV/vis absorption spectra upon AM 1.5 illumination under N2 of (a) glass/ITIC and (b) glass/ZnO/ITIC films (Reproduced from ref. 110 with permission from the Royal Society of Chemistry); and (c) ZnO/IT-4F and (d) SnO2/IT-4F films (Reproduced from ref. 111 with permission from the Royal Society of Chemistry). | ||
The presence of interlayers is not only a physical spacer between ZnO and the active layer. With an appropriate engineering of chemical groups attached, it can passivate the defects and traps at the interfaces between metal oxide and the photoactive layer, inhibiting the chemical reactions between the two materials.112 By decreasing the reactivity, the long term stability can be enhanced as shown by Soultati et al. All the figures of merit decrease upon continuous UV irradiation with and without an interlayer, underlining the importance of an interlayer between ZnO and the active material.
The same group claimed that Li–ZnO used as a CIL compared with bare ZnO permits increasing the stability of NFA-based devices. In fact, Li ions can passivate the defects at the interfaces of ZnO. The evolution of the performances with light exposure time is worse in the case of FAs, meaning more effective stability for NFAs.113 This statement is in partial contrast to that of Park et al., who claimed that FA-based devices are more stable than the NFA counterparts.110 A different thought was expressed by Guo et al., who analyzed the degradation mechanism of different acceptors upon light soaking and air exposure, and they claimed that the degradation mechanism is intrinsically due to the NFA instability and the reactions with the interlayer has a minimal overall effect.114 Moreover, the authors compared the stability of both NFA and fullerene-based photoactive materials, identifying a higher photoactive nature for NFAs and the possibility to efficiently stabilize the acceptors by introducing a nickel chelate that acts as a quencher of reactive oxygen species (ROS) and thus permits increasing the stability of the devices upon air exposure.
In a conventional geometry, generally, PEDOT:PSS is used as an AIL in PSCs. Having this conjugated polymer in contact with an ITO electrode, the latter can be etched due to the acidic nature of PEDOT:PSS and the low-work-function metal is sensitive to moisture and oxygen. These characteristics affect the long-term stability of the devices.115–117 The pH debate was addressed by Zhu et al.118 The authors studied the chemical stability of different efficient NFAs (ITIC, IT-M, IT-4F, IEICO-4F, Y6) under acidic or basic environments, finding that under basic conditions the OH− anion can attack the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. This reaction breaks the conjugation and the original intramolecular charge transfer, which significantly changes the optical absorption properties and destroys their photovoltaic performance. On the other hand, under acidic conditions, the NFAs become stable. It could be expected that an acidic interlayer should favor the stability of NFA-based PSCs. Indeed, a hint in this direction is provided by a previous paper that reports a better stability of NFA-based devices using as an AIL an acidic PEDOT:PSS layer rather than a pH-neutral PEDOT:PSS119 layer but an opposite trend is found for FA-based PSCs.120 The comparison is shown in Fig. 11.
C bonds. This reaction breaks the conjugation and the original intramolecular charge transfer, which significantly changes the optical absorption properties and destroys their photovoltaic performance. On the other hand, under acidic conditions, the NFAs become stable. It could be expected that an acidic interlayer should favor the stability of NFA-based PSCs. Indeed, a hint in this direction is provided by a previous paper that reports a better stability of NFA-based devices using as an AIL an acidic PEDOT:PSS layer rather than a pH-neutral PEDOT:PSS119 layer but an opposite trend is found for FA-based PSCs.120 The comparison is shown in Fig. 11.
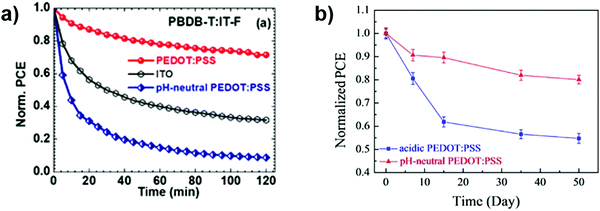 | ||
| Fig. 11 Stability (normalized PCE vs. time) for NFA-based PSCs (left side, reprinted from ref. 119. Copyright (2020) with permission from Elsevier) and FA-based PSCs (right side, reprinted with permission from ref. 120. Copyright (2020) American Chemical Society). | ||
Unlike PEDOT:PSS, PEI and PEIE cathode interlayers are usually basic. The aforementioned NFAs’ chemical reactivity in basic environments is found to be detrimental to the PV performances of NFA-based PSCs with PEI and PEIE used as CILs on ITO.121 The authors observed a poor electron collection in the devices that was related to an interfacial chemical reaction between the ITIC acceptor and the amino groups of these CILs. Later, the same group reported that the substituent groups in the ITIC's terminal acceptor moieties have an influence on the chemical reactivity to amino-containing interfacial layers.122 If fluorine is present (IT-4F) the chemical reactivity increases when compared to hydrogen or methyl substituents. As a result of this increase, more severe “S” shaped current density–voltage characteristics are observed when PEI is used as an interfacial layer, and a larger efficiency difference compared to reference devices with ZnO interlayers is obtained, Fig. 12.
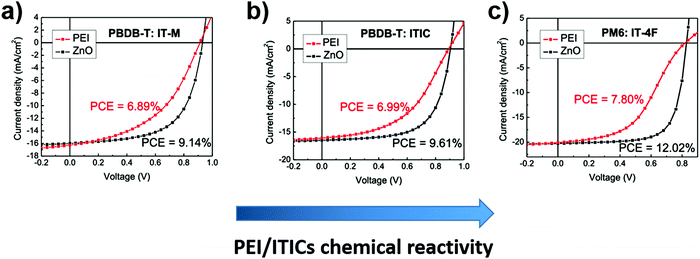 | ||
| Fig. 12 Comparison of the J–V characteristics of ITO/IL/active layer/MoO3/Ag, using PEI (red) vs.ZnO (black) as the CIL and IT-M (a), ITIC (b), and IT-4F (c) as acceptor components. Adapted from ref. 122. Copyright (2020) American Chemical Society. | ||
Conversely, the authors found a higher chemical stability to amino-containing interlayers for Y6, which is bearing a similar fluorine substituted terminal acceptor to IT-4F but a different donor core. Xiong et al. found an effective way to use PEIE on ITO by inhibiting the reaction between the CIL and the active layer; the inhibition is achieved by increasing the protonation of PEIE by adding carbon dioxide, acetic or phosphoric acids in aqueous PEIE solution. The protonated PEIE results in a more efficient CIL in PSCs and with a higher stability.123
3. Classes of interlayer materials
In this section the different classes of interfacial layers, according to their chemical composition, and their impact on photovoltaic performances will be discussed.First of all, anode IL materials will be examined: organic compounds (both small molecules and polymers), inorganic compounds and hybrid materials (mixtures of organics and inorganics). Then, cathode IL materials will be discussed: low-molecular weight organic compounds (including metallic organic salts), n-type dopants, polymers (synthetic and biopolymers), inorganic compounds, hybrid materials (multilayers and mixtures of organic–inorganic materials).
3.1. Anode interlayer materials
 | ||
| Scheme 2 Chemical structures of different materials used as AILs in non-fullerene organic solar cells. | ||
| Material | Solar cell structure | V OC (V) | FF (%) | J SC (mA cm−2) | PCE (%) | Material in the control device | PCE (%) control device | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Maximum values. | ||||||||
| F6-TCNNQ | ITO/ZnO/PBDB-T:ITIC/AIL/Ag | 0.87a | 67a | 17.01a | (9.97) | MoOx | 10.66 | 124 |
| PCP-H | ITO/AIL/J52:IT-M/PFN-Br/Al | 0.96a | 73a | 18.4a | 12.40 (12.80) | PEDOT:PSS | 12.30 (12.80) | 125 |
| PCP-Li | ITO/AIL/J52:IT-M/PFN-Br/Al | 0.93a | 70a | 18.3a | 11.20 (11.80) | PEDOT:PSS | 12.30 (12.80) | 125 |
| PCP-Na | ITO/AIL/J52:IT-M/PFN-Br/Al | 0.91a | 67a | 18a | 10.40 (11.00) | PEDOT:PSS | 12.30 (12.80) | 125 |
| PCP-K | ITO/AIL/J52:IT-M/PFN-Br/Al | 0.87a | 66a | 17.5a | 9.41 (9.99) | PEDOT:PSS | 12.30 (12.80) | 125 |
| PCP-Cs | ITO/AIL/J52:IT-M/PFN-Br/Al | 0.84a | 65a | 16.6a | 8.46 (9.10) | PEDOT:PSS | 12.30 (12.80) | 125 |
| PCP-H | ITO/AIL/PBDTTT-E-T:IEICO/PFN-Br/Al | 0.81a | 66a | 18.8a | 9.75 (10.10) | PEDOT:PSS | 9.79 (10.10) | 125 |
| PCP-Li | ITO/AIL/PBDTTT-E-T:IEICO/PFN-Br/Al | 0.82a | 66a | 18.8a | 9.81 (10.10) | PEDOT:PSS | 9.79 (10.10) | 125 |
| PCP-Na | ITO/AIL/PBDTTT-E-T:IEICO/PFN-Br/Al | 0.82a | 66a | 18.8a | 9.78 (10.20) | PEDOT:PSS | 9.79 (10.10) | 125 |
| PCP-K | ITO/AIL/PBDTTT-E-T:IEICO/PFN-Br/Al | 0.81a | 66a | 18.7a | 9.70 (10.00) | PEDOT:PSS | 9.79 (10.10) | 125 |
| PCP-Cs | ITO/AIL/PBDTTT-E-T:IEICO/PFN-Br/Al | 0.82a | 66a | 18.6a | 9.74 (10.10) | PEDOT:PSS | 9.79 (10.10) | 125 |
| PCP-H | ITO/AIL/PBDB-T-2F:IT-4F/PFN-Br/Al | 0.81a | 73a | 20.6a | 11.80 (12.2) | PEDOT:PSS | 13.10 (13.30) | 125 |
| PCP-Li | ITO/AIL/PBDB-T-2F:IT-4F/PFN-Br/Al | 0.74a | 68a | 20.3a | 9.45 (10.30) | PEDOT:PSS | 13.10 (13.30) | 125 |
| PCP-Na | ITO/AIL/PBDB-T-2F:IT-4F/PFN-Br/Al | 0.71a | 66a | 20.1a | 8.57 (9.32) | PEDOT:PSS | 13.10 (13.30) | 125 |
| PCP-K | ITO/AIL/PBDB-T-2F:IT-4F/PFN-Br/Al | 0.67a | 63a | 19.6a | 7.55 (8.26) | PEDOT:PSS | 13.10 (13.30) | 125 |
| PCP-Cs | ITO/AIL/PBDB-T-2F:IT-4F/PFN-Br/Al | 0.61a | 62a | 19.3a | 6.44 (7.32) | PEDOT:PSS | 13.10 (13.30) | 125 |
| HAPAN | ITO/AIL/PBDB-T:ITIC/Ca/Al | 0.90 | 65 | 16.61 | 9.51 (9.72) | PEDOT:PSS | 9.72 (9.93) | 81 |
| POE (dopant 3%) | ITO/ZnO/PBDB-T:ITIC:POE/Ag | 0.89 | 62 | 16.14 | 8.58 (8.97) | w/o dopant | 4.38 (5.00) | 77 |
| POE | ITO/ZnO/PBDB-T:ITIC/AIL/Ag | 0.90 | 62 | 16.33 | 8.94 (9.22) | w/o AIL | 4.38 (5.00) | 77 |
| POE (dopant 3%) | ITO/ZnO/PM6:IT-4F:POE/Ag | 0.80 | 72 | 19.50 | 11.12 (11.55) | w/o dopant | 6.40 (7.09) | 77 |
| POE | ITO/ZnO/PM6:IT-4F/AIL/Ag | 0.82 | 70 | 20.27 | 11.57 (11.88) | w/o AIL | 6.40 (7.09) | 77 |
An interesting approach has been reported by using an organic small molecule, 2,2-(perfluoronaphthalene-2,6-diylidene)dimalononitrile (F6-TCNNQ), to prepare a sequentially molecularly doped device with PBDB-T:ITIC as a photoactive layer.124 By using sequential deposition of F6-TCNNQ solutions with different concentrations on the top of the active layer, the penetration of the dopants can be controlled and optimized. By adopting this strategy, a PCE close to 10% is obtained with F6-TCNNQ doped PBDBT:ITIC devices without using MoOx. It was also observed that controlling the penetration depth, the hole extraction is more efficient and the recombination processes at the interface are suppressed to some extent.
Cui et al. demonstrated a method to modulate the p-type self-doping in a conjugated polymer by changing the counterions.125 It was found that the self-doping effect is enhanced with the decrease of the cation radius. By employing these interlayers (PCP-X, X = H, Li, Na, K, Cs) on top of ITO, the work function of the anodes is tuned from 4.78 to 5.11 eV.
Zhao et al. used a hydrochloric acid doped polyaniline (HAPAN) as an AIL as an alternative to PEDOT:PSS in a conventional device architecture, obtaining very similar efficiencies in a PBDB-T:ITIC based device,80 while Alharbi et al. modified PEDOT:PSS by the introduction of Nafion in PBDB-T:Y6, obtaining PCEs up to 16.31%.126
In a very recent report, a polyolefin elastomer (POE) was used as an AIL in different high performance NFA-based PSCs.77 The presence of POE improves the mechanical properties in comparison with those containing MoO3 and acts as an encapsulating layer, maintaining the device performances as high as 9.60% after 150 days of storing in air with 70% humidity.
| Material | Solar cell structure | V OC (V) | FF (%) | J SC (mA cm−2) | PCE (%) | Material in the control device | PCE (%) control device | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Maximum values. | ||||||||
| Nb2O5 | ITO/AIL/PBDB-T:ITIC/Ca/Al | 0.88 | 61 | 16.3 | 8.67 | PEDOT:PSS | 8.03 | 127 |
| NiOx | ITO/AIL/PBDTTT-ET:IEICO/PFN-Br/Al | 0.81a | 64a | 17.5a | 9.06 (9.12) | PEDOT:PSS | 8.77 (8.83) | 128 |
| Maghemite:Fe(OH)3 | ITO/AIL/PBDB-T:ITIC/Ca/Al | 0.90 | 70 | 17.12 | 10.75 (10.94) | PEDOT:PSS | 10.17 (10.25) | 129 |
| BiOCl nanoplates | ITO/AIL//PM6:Y6/PDINO/Al | 0.83 | 70 | 26.9 | 15.60 (16.11) | PEDOT:PSS | 15.11 (15.63) | 130 |
| s-MoOx | ITO/AIL/PB3T:IT-M/PFN-Br/Al | 0.98a | 69a | 17.8a | — (12.1) | PEDOT:PSS | — (11.9) | 131 |
| H3PW12O40 | ITO/AIL/PBDB-T-2F:Y6/PFN-Br/Al | 0.86 | 74 | 25.5 | 15.67 (16.04) | PEDOT:PSS | — (15.7) | 136 |
| H3PMo3W9O40 | ITO/AIL/PBDB-T-2F:Y6/PFN-Br/Al | 0.85 | 75 | 25.3 | 15.84 (16.09) | PEDOT:PSS | — (15.7) | 136 |
| H3PMo6W6O40 | ITO/AIL/PBDB-T-2F:Y6/PFN-Br/Al | 0.85 | 75 | 25.4 | 15.87 (16.11) | PEDOT:PSS | — (15.7) | 136 |
| H3PMo9W3O40 | ITO/AIL/PBDB-T-2F:Y6/PFN-Br/Al | 0.85 | 75 | 25.5 | 15.92 (16.34) | PEDOT:PSS | — (15.7) | 136 |
| H3PMo12O40 | ITO/AIL/PBDB-T-2F:Y6/PFN-Br/Al | 0.84 | 75 | 25.2 | 15.45 (15.79) | PEDOT:PSS | — (15.7) | 136 |
| WS2 | ITO/AIL/PBDB-T-SF:IT-4F/PFN-Br/Al | 0.88 | 74 | 20.6 | 13.1 (13.5) | PEDOT:PSS | 12.9 (13.1) | 137 |
| MoS2 | ITO/AIL/PBDB-T-SF:IT-4F:PC71BM/PFN-Br/Al | 0.84 | 71 | 20.0 | 11.8 (12.0) | PEDOT:PSS | 12.9 (13.1) | 137 |
| WS2 | ITO/AIL/PBDB-T-SF:Y6/PFN-Br/Al | 0.84 | 73 | 25.9 | 15.3 (15.8) | PEDOT:PSS | 14.9 (15.3) | 137 |
| WS2 | ITO/AIL/PBDB-T-SF:Y6:PC71BM/PFN-Br/Al | 0.84 | 78 | 26.0 | 16.5 (17.0) | PEDOT:PSS | 16.0 (16.4) | 137 |
| α-In2Se3 | ITO/AIL/PBDB-T:ITIC/Ca/Al | 0.88 | 65 | 16.7 | 9.58 | PEDOT:PSS | 9.50 | 138 |
Ammonium heptamolybdate dissolved in a water/ethylene glycol solution containing a surfactant was spin-coated and thermally annealed at 200 °C, forming a molybdenum oxide layer (s-MoOx).131 ITO/s-MoOx/PB3T:IT-M/PFN-Br/Al solar cells provided a power conversion efficiency of 12.1%, a value slightly higher than that measured for PEDOT:PSS-based devices (11.9%). The optimum thickness of the AIL was found to be 10 nm. However, a 130 nm thick layer still leads to devices with PCEs above 9%. Heteropolyacid based interfacial materials have been already reported for inverted fullerene-based solar cells.132–135 Kang et al. used tungsten–molybdenum heteropolyacids with different Mo/W atomic ratios as anode interlayers in PBDB-T-2F:Y6 conventional solar cells.136 An improvement of PCE of 0.64% in comparison to PEDOT:PSS was observed in the best case (Mo/W = 3). A notable advantage of these materials is their relatively low sensitivity to layer thickness, which makes possible facile deposition by blade coating: a 1 cm2 device with 14.3% PCE was obtained in this way. ITO/AIL/PBDB-T-SF:IT-4F/PFN-Br/Al solar cells with tungsten or molybdenum disulfide nanosheets as an AIL have been reported by Lin et al.137WS2 and MoS2 were obtained by exfoliation of the corresponding commercial powders in water/ethanol mixed solvent. The nanosheet suspension was deposited on ITO without thermal post-treatment, forming a uniform film in the case of WS2. Solar devices exhibited a PCE of 15.3%, slightly higher than that of the corresponding PEDOT:PSS based devices, while the PCE of MoS2-based devices was only 11.8%. This lower PCE was due to the reduced charge selectivity of MoS2 and the increased leakage current arising from the reduced ability to block minority carrier recombination, caused by the lower film uniformity. Solar cells with a WS2 AIL and a PBDB-T-2F:Y6 active layer achieved a similar PCE of 15.3%, which was further increased to 16.5% by using a PBDB-T-2F:Y6:PC71BM ternary blend.
Bidimensional nanosheets of indium selenide are used as an AIL with an optimum thickness of 13 nm in conventional ITO/α-In2Se3/PBDB-T:ITIC/Ca/Al solar cells.138 The photovoltaic performances were similar to those of the PEDOT:PSS devices. On the other hand, the layer of indium selenide nanosheets has the advantage of not requiring a thermal post-treatment. In addition, non-encapsulated devices exposed to air retained almost 60% of their initial PCE, compared to those containing PEDOT:PSS, which lost 96% of their efficiency.
| Material | Solar cell structure | V OC (V) | FF (%) | J SC (mA cm−2) | PCE (%) | Material in the control device | PCE (%) control device | Ref. |
|---|---|---|---|---|---|---|---|---|
| SnO2/PEDOT:PSS | ITO/AIL/PTB7:P(NDI2OD-T2)/MoO3/Al | 0.78 | 54 | 16.9 | — (7.07) | PEDOT:PSS | — (6.79) | 139 |
| WOx NPs:PEDOT:PSS | ITO/AIL/PBDB-TF:IT-4F/PFN-Br/Al | 0.87 | 80 | 20.7 | 14.37 (14.57) | PEDOT:PSS | 13.09 (13.29) | 140 |
| PEDOT:PSS:PSSS | ITO/AIL/PBDB-TF:IT-4F/PFN-Br/Al | — | — | — | ∼13.1 | PEDOT:PSS | ∼13.2 | 140 |
| PEDOT:PSS:NDF | ITO/AIL/PBDB-T:ITIC/PFN-Br/Al | 0.95 | 70 | 16.1 | 10.5 (11.1) | PEDOT:PSS | 10 | 140 |
| PEDOT:PSS:PSSS | ITO/AIL/PBDB-T:ITIC/PFN-Br/Al | — | — | — | ∼11 | PEDOT:PSS | ∼10 | 140 |
| PEDOT:PSS:NDF | ITO/AIL/PBDB-TF:IT-4F/PFN-Br/Al | 0.85 | 79 | 21 | 14 (14.08) | PEDOT:PSS | 13.2 | 140 |
| PEDOT:PSS:g-C3N4 | ITO/AIL/PM6:Y6/PFN-Br/Ag | 0.84 | 73 | 26.7 | 15.94 (16.38) | PEDOT:PSS | 14.84 (15.29) | 141 |
| PEDOT:PSS:Ti3C2TX | ITO/AIL/PBDB-T:ITIC/PFN-Br/Al | 0.91 | 71 | 17.1 | 11.02 | PEDOT:PSS | 9.72 | 142 |
| PEDOT:PSS:Ti3C2TX | ITO/AIL/PM6:Y6/PFN-Br/Al | 0.83 | 68 | 25.6 | 14.55 | PEDOT:PSS | 13.10 | 142 |
| PEDOT:PSS/HATP | ITO/AIL/PBDB-T:IT-M/PFN-Br/Al | 0.93 | 64 | 20.1 | 11.95 | PEDOT:PSS | 10.6 | 106 |
| PEDOT:PSS:Nafion | ITO/AIL/PBDB-TCl:IT-4F/PFN-Br/Al | 0.88 | 76 | 21.1 | 14.12 | PEDOT:PSS | 13.01 | 126 |
| PEDOT:PSS:Nafion | ITO/AIL/PBDB-TF:Y6/PFN-Br/Al | 0.84 | 75 | 25.76 | 16.31 | PEDOT:PSS | 15.54 | 126 |
The photoactive layer morphology modification is achieved also by using discotic liquid crystals 2,3,6,7,10,11-hexaacetoxytriphenylene (HATP) between PEDOT:PSS and the active layer. The HATP, having a columnar structure with a dominantly edge-on orientation, imparts to the photoactive layer both edge-on and face-on orientations, confirmed by the GIWAXS technique, which facilitates charge hopping. Moreover, HATP exhibits high charge mobility; thus the conductivity of the AIL is enhanced.106
Instead of improving the morphology in the photoactive layer, two dimensional materials are used to improve the conductivity of PEDOT:PSS.141,142 Thanks to their high carrier mobility characteristics, nanosheets of graphitic carbon nitride (g-C3N4) and transition metal carbides called MXenes (Ti3C2TX) are used as dopants for PEDOT:PSS in AIL PSCs.
3.2. Cathode interlayer materials (CILs)
In this section we describe the use of organic small molecules, polymers and biopolymers as CILs.
3.2.1.1. Small molecules. Small molecule interlayers exhibit intrinsic advantages over polymeric materials, in terms of facile and reduced synthetic steps and easy purification, better batch-to-batch reproducibility, monodispersity and well defined structures (Scheme 3). For these reasons, many small molecules have been designed to be used as interlayers for both fullerene25 and non-fullerene organic solar cells.
We will describe first the use of conjugated and non-conjugated small molecules as interlayers (Table 4), followed by a brief description concerning the utilization of Lewis bases as n-type dopants in non-fullerene organic solar cells (Table 5).
| Material | Solar cell structure | V OC (V) | FF (%) | J SC (mA cm−2) | PCE (%) | Material in the control device | PCE (%) control device | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Maximum values. | ||||||||
| Bis-FPPI/PFN-Br | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.85 | 69 | 17.09 | 9.80 (10.01) | ZnO | 9.37 (9.58) | 144 |
| Bis-FIMG | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Al | 0.91 | 72 | 16.57 | 10.65 (10.82) | PFN-Br | 9.73 (10.08) | 144 |
| Bis-FITG | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Al | 0.88 | 65 | 15.65 | 8.49 (8.99) | — | — | 144 |
| PDINO | ITO/PEDOT:PSS/PTB7:Bis-PDI-T-EG/CIL/Al | 0.84 | 55 | 8.46 | 3.88 (4.10) | — | — | 149 |
| PDINO | ITO/PEDOT:PSS/PTB7-Th:Bis-PDI-T-EG/CIL/Al | 0.87 | 58 | 11.14 | 5.65 (5.87) | — | — | 149 |
| PDINO | ITO/PEDOT:PSS/PBDT-TS1:Bis-PDI-T-EG/CIL/Al | 0.88 | 59 | 13.23 | 6.94 (7.24) | Ca/Al | 6.22 (6.42) | 149 |
| PDIN | ITO/PEDOT:PSS/J71:ITIC/CIL/Al | 0.90 | 65 | 15.28 | 8.93 (9.03) | — | — | 148 |
| P1PN | ITO/PEDOT:PSS/J71:ITIC/CIL/Al | 0.91 | 65 | 15.67 | 9.40 (9.45) | — | — | 148 |
| P2PN | ITO/PEDOT:PSS/J71:ITIC/CIL/Al | 0.91 | 67 | 15.78 | 9.65 (9.76) | — | — | 148 |
| P3PN | ITO/PEDOT:PSS/J71:ITIC/CIL/Al | 0.91 | 67 | 16.36 | 9.94 (10.1) | — | — | 148 |
| P4PN | ITO/PEDOT:PSS/J71:ITIC/CIL/Al | 0.91 | 65 | 16.10 | 9.44 (9.65) | — | — | 148 |
| PDI-NBr | ITO/PEDOT:PSS/J71:ITIC/CIL/Al | 0.93 | 48 | 15.85 | 6.85 (7.14) | — | — | 148 |
| P1P-NBr | ITO/PEDOT:PSS/J71:ITIC/CIL/Al | 0.92 | 58 | 16.05 | 8.65 (8.76) | — | — | 148 |
| P2P-NBr | ITO/PEDOT:PSS/J71:ITIC/CIL/Al | 0.93 | 65 | 16.08 | 9.68 (9.90) | — | — | 148 |
| P3P-NBr | ITO/PEDOT:PSS/J71:ITIC/CIL/Al | 0.94 | 67 | 16.47 | 10.29 (10.46) | — | — | 148 |
| P4P-NBr | ITO/PEDOT:PSS/J71:ITIC/CIL/Al | 0.93 | 55 | 16.45 | 8.34 (8.54) | — | — | 148 |
| P1P-NO | ITO/PEDOT:PSS/J71:ITIC/CIL/Al | 0.94 | 70 | 17.47 | 11.33 (11.56) | PDINO | 10.82 (11.09) | 148 |
| P2P-NO | ITO/PEDOT:PSS/J71:ITIC/CIL/Al | 0.93 | 65 | 17.38 | 10.45 (10.60) | — | — | 148 |
| P3P-NO | ITO/PEDOT:PSS/J71:ITIC/CIL/Al | 0.92 | 64 | 17.11 | 9.98 (10.27) | — | — | 148 |
| P4P-NO | ITO/PEDOT:PSS/J71:ITIC/CIL/Al | 0.91 | 65 | 16.21 | 9.44 (9.60) | — | — | 148 |
| PDINN | ITO/PEDOT:PSS/PM6:Y6/CIL/Al | 0.84 | 77 | 25.70 | 16.77 (17.23) | PDINO | 14.94 (15.17) | 150 |
| PMI-TPP | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Al | 0.75 | 58 | 11.75 | (5.18) | w/o CIL | (4.21) | 151 |
| PMI-TMOPP | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Al | 0.75a | 55a | 12.50a | (5.05) | — | — | 151 |
| PDIP | ITO/PEDOT:PSS/PCDTBT:EP-PDI/CIL/Al | 0.89 | 35 | 1.68 | 0.52 | Ca/Al | 0.29 | 152 |
| Alq3-F1 | ITO/PEDOT:PSS/PBTA-TF:IT-M/CIL/Al | 0.92 | 72 | 19.59 | 12.63 (12.89) | PFN-Br | 11.95 (12.24) | 88 |
| Alq3-F2 | ITO/PEDOT:PSS/PBTA-TF:IT-M/CIL/Al | 0.94 | 73 | 19.78 | 13.40 (13.75) | PFN-Br | 11.95 (12.24) | 88 |
| Alq3-F3 | ITO/PEDOT:PSS/PBTA-TF:IT-M/CIL/Al | 0.93 | 73 | 19.25 | 12.93 (13.20) | PFN-Br | 11.95 (12.24) | 88 |
| OAS | ITO/PEDOT:PSS/PBDB-T:IT-M/CIL/Al | 0.93 | 72 | 15.96 | 10.76 (10.84) | Ca/Al | 9.42 (9.47) | 87 |
| SAS | ITO/PEDOT:PSS/PBDB-T:IT-M/CIL/Al | 0.94 | 72 | 16.53 | 11.25 (11.30) | Ca/Al | 9.42 (9.47) | 87 |
| SOAS | ITO/PEDOT:PSS/PBDB-T:IT-M/CIL/Al | 0.93 | 66 | 16.04 | 9.82 (9.93) | Ca/Al | 9.42 (9.47) | 87 |
| NDI-N | ITO/PEDOT:PSS/PBDT-2F:IT-4F/CIL/Al | 0.86 | 76 | 21.3 | (13.9) | PFN-Br | (4.08) | 97 |
| NDI-Br | ITO/PEDOT:PSS/PBDT-2F:IT-4F/CIL/Al | 0.80a | 71a | 20.1a | (11.5) | — | — | 97 |
| AP-tBu-Cl | ITO/PEDOT:PSS/PBDB-T:IT-M/CIL/Al | 0.87 | 62 | 15.93 | 8.72 (8.78) | w/o CIL | 7.24 (7.34) | 153 |
| AP-tBu-BF | ITO/PEDOT:PSS/PBDB-T:IT-M/CIL/Al | 0.86 | 63 | 16.01 | 8.75 (9.02) | w/o CIL | 7.24 (7.34) | 153 |
| AP-tBu-TfO | ITO/PEDOT:PSS/PBDB-T:IT-M/CIL/Al | 0.89 | 63 | 15.88 | 9.03 (9.41) | w/o CIL | 7.24 (7.34) | 153 |
| AP-CF-Cl | ITO/PEDOT:PSS/PBDB-T:IT-M/CIL/Al | 0.82 | 60 | 15.61 | 7.75 (7.84) | w/o CIL | 7.24 (7.34) | 153 |
| AP-CO-Cl | ITO/PEDOT:PSS/PBDB-T:IT-M/CIL/Al | 0.81 | 61 | 15.81 | 7.86 (7.93) | w/o CIL | 7.24 (7.34) | 153 |
| DAP-tBu-Cl | ITO/PEDOT:PSS/PBDB-T:IT-M/CIL/Al | 0.92 | 66 | 16.43 | 10.14 (10.24) | w/o CIL | 7.24 (7.34) | 153 |
| ATF | ITO/PEDOT:PSS/PBDTS:IT-4F/CIL/Ag | 0.88 | 68 | 18.79 | 10.97 (11.30) | w/o CIL | 6.67 (7.08) | 154 |
| STF | ITO/PEDOT:PSS/PBDTS:IT-4F/CIL/Ag | 0.89 | 70 | 19.32 | 11.76 (12.12) | w/o CIL | 6.67 (7.08) | 154 |
| OTF | ITO/PEDOT:PSS/PBDTS:IT-4F/CIL/Ag | 0.89 | 74 | 19.89 | 12.85 (13.21) | w/o CIL | 6.67 (7.08) | 154 |
| N-IDTBR | ITO/PEDOT:PSS/P3HT:O-IDTBR/CIL/Ag | 0.76a | 59a | 12.59a | (6.01) | w/o CIL | (4.07) | 92 |
| S1 | ITO/PEDOT:PSS/PBDB-TF:BO-4Cl/CIL/Al | 0.81 | 70 | 25.27 | 13.77 (14.41) | PFN-Br | 15.68 (16.07) | 155 |
| S2 | ITO/PEDOT:PSS/PBDB-TF:BO-4Cl/CIL/Al | 0.82 | 71 | 25.40 | 14.24 (14.80) | PFN-Br | 15.68 (16.07) | 155 |
| S3 | ITO/PEDOT:PSS/PBDB-TF:BO-4Cl/CIL/Al | 0.84 | 76 | 25.97 | 16.01 (16.56) | PFN-Br | 15.68 (16.07) | 155 |
| N719 | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Al | 0.89 | 66 | 16.44 | 9.79 (10.15) | w/o CIL | 7.37 (7.86) | 157 |
| QPhPBr | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Al | 0.90 | 67 | 16.86 | 10.20 (10.63) | w/o CIL | 7.37 (7.86) | 157 |
| TPhPEtBr | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Al | 0.90 | 68 | 17.87 | 11.08 (11.38) | w/o CIL | 7.37 (7.86) | 157 |
| N719:QPhPBr | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Al | 0.91 | 67 | 17.54 | 10.74 (11.17) | w/o CIL | 7.37 (7.86) | 157 |
| N719:TPhPEtBr | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Al | 0.91 | 69 | 18.13 | 11.61 (12.03) | w/o CIL | 7.37 (7.86) | 157 |
| PDINO:QPhPBr | ITO/PEDOT:PSS/PBDT-T:IDTBR/CIL/Al | 0.98a | 62a | 13.45a | (8.27) | PDINO | (5.45) | 158 |
| Alq3 | ITO/PEDOT:PSS/PBDB-T:ITIC-Th/CIL/Al | 0.81 | 67 | 16.57 | 8.92 (9.17) | Ca/Al | 6.89 (7.32) | 159 |
| QPhPBr | ITO/PEDOT:PSS/PBDB-T:ITIC-Th/CIL/Al | 0.84 | 69 | 17.41 | 9.74 (10.15) | Ca/Al | 6.89 (7.32) | 159 |
| Alq3:QPhPBr | ITO/PEDOT:PSS/PBDB-T:ITIC-Th/CIL/Al | 0.86 | 69 | 18.38 | 10.03 (11.05) | Ca/Al | 6.89 (7.32) | 159 |
| CuCN | ITO/PEDOT:PSS/PBDB-T:IT-M/CIL/Al | 0.93 | 67 | 16.69 | 10.32 (10.55) | PFN | 9.93 | 160 |
| Dopant | Solar cell structure | V OC (V) | FF (%) | J SC (mA cm−2) | PCE (%) | Material in the control device | PCE (%) control device | Ref. |
|---|---|---|---|---|---|---|---|---|
| TBAA | ITO/PEDOT:PSS/PBDB-T:IT-M/dopant/Al | 0.92 | 73 | 17.54 | 11.78 | PFN-Br | 11.32 | 71 |
| TBAA | ITO/PEDOT:PSS/PBDB-T:ITTC/dopant/Al | 0.98 | 73 | 15.05 | 10.80 | w/o CIL | 7.80 | 71 |
| TBAA | ITO/PEDOT:PSS/PTB7-Th:IEICO-4F/dopant/Al | 0.72 | 60 | 23.23 | 10.04 | w/o CIL | 8.37 | 71 |
| TBAA | ITO/PEDOT:PSS/PBDB-2F:IT-4F/dopant/Al | 0.86 | 73 | 20.70 | 13.06 | w/o CIL | 9.82 | 71 |
| TMABr | ITO/PEDOT:PSS/PBDB-T-2F:IT-4F/dopant/Al | 0.82 | 66 | 20.58 | 11.16 | w/o CIL | 9.56 | 94 |
| TMABr | ITO/PEDOT:PSS/PBDB-T:ITIC/dopant/Al | 0.82 | 64 | 15.73 | 8.28 | w/o CIL | 6.34 | 94 |
| TMABr | ITO/PEDOT:PSS/PBDB-T:IT-M/dopant/Al | 0.82 | 67 | 16.04 | 9.76 | w/o CIL | 8.79 | 94 |
| TMABr | ITO/PEDOT:PSS/PBDB-T:ITCC/dopant/Al | 0.90 | 60 | 13.14 | 7.12 | w/o CIL | 5.97 | 94 |
| TMABr | ITO/PEDOT:PSS/PBDB-T-2F:Y6/dopant/Al | 0.82 | 70 | 23.73 | 13.63 | w/o CIL | 10.02 | 94 |
| TPABr | ITO/PEDOT:PSS/PBDB-T-2F:IT-4F/dopant/Al | 0.84 | 71 | 20.74 | 12.39 | w/o CIL | 9.56 | 94 |
| TPABr | ITO/PEDOT:PSS/PBDB-T:ITIC/dopant/Al | 0.86 | 65 | 16.33 | 9.16 | w/o CIL | 6.34 | 94 |
| TPABr | ITO/PEDOT:PSS/PBDB-T:IT-M/dopant/Al | 0.86 | 71 | 16.59 | 10.62 | w/o CIL | 8.79 | 94 |
| TPABr | ITO/PEDOT:PSS/PBDB-T:ITCC/dopant/Al | 0.94 | 63 | 13.73 | 8.21 | w/o CIL | 5.97 | 94 |
| TPABr | ITO/PEDOT:PSS/PBDB-T-2F:Y6/dopant/Al | 0.82 | 72 | 24.52 | 14.52 | w/o CIL | 10.02 | 94 |
| TAABr | ITO/PEDOT:PSS/PBDB-T-2F:IT-4F/dopant/Al | 0.86 | 73 | 20.86 | 13.18 | w/o CIL | 9.56 | 94 |
| TAABr | ITO/PEDOT:PSS/PBDB-T:ITIC/dopant/Al | 0.88 | 70 | 16.62 | 10.33 | w/o CIL | 6.34 | 94 |
| TAABr | ITO/PEDOT:PSS/PBDB-T:IT-M/dopant/Al | 0.88 | 72 | 16.92 | 11.22 | w/o CIL | 8.79 | 94 |
| TAABr | ITO/PEDOT:PSS/PBDB-T:ITCC/dopant/Al | 0.96 | 66 | 13.92 | 8.95 | w/o CIL | 5.97 | 94 |
| TAABr | ITO/PEDOT:PSS/PBDB-T-2F:Y6/dopant/Al | 0.82 | 75 | 24.50 | 15.05 | w/o CIL | 10.02 | 94 |
| THABr | ITO/PEDOT:PSS/PBDB-T-2F:IT-4F/dopant/Al | 0.85 | 54 | 20.90 | 9.64 | w/o CIL | 9.56 | 94 |
| THABr | ITO/PEDOT:PSS/PBDB-T:ITIC/dopant/Al | 0.88 | 55 | 15.67 | 7.64 | w/o CIL | 6.34 | 94 |
| THABr | ITO/PEDOT:PSS/PBDB-T:IT-M/dopant/Al | 0.88 | 52 | 14.81 | 7.18 | w/o CIL | 8.79 | 94 |
| THABr | ITO/PEDOT:PSS/PBDB-T:ITCC/dopant/Al | 0.96 | 58 | 13.23 | 7.40 | w/o CIL | 5.97 | 94 |
| THABr | ITO/PEDOT:PSS/PBDB-T-2F:Y6/dopant/Al | 0.82 | 73 | 25.42 | 15.34 | w/o CIL | 10.02 | 94 |
Conjugated organic small molecules possess delocalized electrons along the π-conjugated backbone, and to be suitable materials as interlayers, the presence of surfactant-like side groups like hydroxyl, sulfonic, phosphate, and amino is required. The roles of these side units are both to enhance their solution-processability and to improve the interaction between the active layer and electrode.
Fullerene derivatives are suitable materials as cathode interlayers due to their n-type semiconductor character and high electron mobility. Obviously, fullerene based interlayers have good energy matching with fullerene acceptors and have been commonly developed for fullerene organic solar cells.143 There are two reports on fullerene based materials used in fullerene-free solar cells.
Yan et al. developed fullerene surfactants containing different side units like bispyrrolidine pyridinium iodide (Bis-FPPI), bispyrrolidinium tris(methoxyethoxy)phenyl iodide (Bis-FIMG) and bispyrrolidinium tris(methoxytriglycol)phenyl iodide (Bis-FITG) as ETLs for organic solar cells in both conventional and inverted structures.144 Conventional devices using Bis-FIMG and Bis-FITG ILs and PBDB-T:ITIC as an active layer were fabricated. The OSCs containing Bis-FIMG exhibited better performance compared with those containing PFN-Br, while Bis-FITG gave lower PCEs mostly due to morphological issues (Table 4). Both devices had higher EQEs compared to PFN-Br, demonstrated by improved current densities and therefore an improved charge extraction of fullerene based ETLs. In the inverted solar cell, a dual blend of Bis-FPPI and PFN-Br was employed, because pristine Bis-FPPI in DMF has poor film processing properties. Using the same active layer, the device performances were slightly higher compared to the ZnO ETL and are attributed to the enhanced charge extraction capability of the fullerene containing ETL.
Another interesting approach was developed later by using a chemically doped PCBM with 1,3,5-trimethylhexahydro-1,3,5-triazine (TMHT).145 The TMHT doping improves substantially the conductivity and shifts the Fermi levels of PCBM by 0.3 eV. By increasing the level of doping up to 5% of TMHT, the charge collection is highly improved and the photostability of the devices containing doped PCBM is superior to those containing ZnO.
A representative class of conjugated materials is perylene diimides (PDIs), initially studied and designed as acceptors for organic solar cells146 and subsequently developed as promising materials for interlayers.
Following the first success reported by Zhang et al. on the use of two materials consisting of perylene diimides as cores and amino (PDIN) or amino-N-oxide (PDINO) as side chains as thickness insensitive small molecule based cathode interlayers in fullerene organic solar cells,42 many reports have been made regarding the development of PDI based materials to further improve the device performances and to adopt them to fullerene-free OSCs. Notably, the two perylene diimides reported by Zhang, PDIN and PDINO, are commonly used as buffer materials in different fullerene-free OSCs147 and as benchmarks for newly developed PDI materials for interlayers.148
For example, Zhang et al. selected three low-band gap polymers, PTB7, PTB7-Th and PBDT-TS133, as the donors and a Bis-PDI-T-EG dimer as a small molecule acceptor.149 It was demonstrated that the PCE value improved as the cathode interlayer was changed from Ca to PDINO, due to the higher electron mobility of PDINO in comparison to the Ca layer, which leads to a higher electrical current generation.
The presence of the interlayer can have beneficial effects on the device parameters through various mechanisms. Li et al. developed a series of PDIs with different sizes of side and terminal groups and showed that the regulation of the molecular structure may induce different molecular packings of the interlayer material and change the film morphology.148 These molecules contain dimethylamino (PDIN), trimethylammonium bromide (PDI-NBr) and amino N-oxide (PDINO) moieties at the imidic positions and phenyl (P1P-N, P1P-Br, P1P-NO), biphenyl (P2P-N, P2P-Br, P2P-NO), m-terphenyl (P3P-N, P3P-Br, P3P-NO) and tetraphenylethene groups (P4P-N, P4P-Br, P4P-NO) with gradually increased size in the bay-position to obtain out-of-plane structures. From theoretical calculations, SEM and XRD, it was demonstrated that the molecular conformation, packing state and film morphology have changed upon molecular design. Moreover, these PDIs exhibit self-doping behaviors, indicating that electrons can be transferred from the imidic moieties (amine groups or bromide ions) to the electron deficient PDI to generate PDI radical anions. The combined effect of a good molecular packing and the formation of radical anions improves the electron transport properties of the interfacial layer. In the PDI-NBr series, the WF values decrease dramatically, due to the formation of favorable interfacial dipoles between the PDI-NBr and the cathode surface, stimulating charge transport and extraction. In the PDI-NBr series, P3P-NBr exhibits optimized molecular packing with high electron mobility and an improved film morphology. Indeed, the OSC containing J71:ITIC as an active layer and P3P-NBr as a CIL shows the best PCE with an increase of more than 40% in comparison with that of PDI-NBr without any substituent in the bay position. Regarding the use of the PDI-NO series (P1P-NO, P2P-NO, P3P-NO, P4P-NO) as CILs in conventional OSCs containing J71:ITIC the performances are similar to those containing PDI-NO (without any substituent in the bay position), but the use of P1P-NO results in a high PCE, which is thickness independent, and the device exhibits enhanced long-term stability.
Very recently, Yao et al. reported on a low-cost hydrogen-bonding material based on an aliphatic amine-functionalized perylenediimide (PDINN), which modifies the WF of the cathode and ensures a good interfacial contact with the photoactive layer.150 It was also shown that a spin-coated solution of PDINN on PM6:Y6 generates a uniform film, most likely related to the formation of hydrogen bonds between the secondary amine moieties of PDINN and the active layer. Compared with the benchmark PDINO, PDINN enables better contact with the non-fullerene active layers, a higher conductivity and a stronger ability to reduce the WF of the cathode.
Qin et al. developed two perylene monoimide (PMI) based organic salts, PMI-triphenylphosphonium bromide (PMI-TPP) and PMI-trimethoxylphenylphosphonium bromide (PMI-TMOPP), as CILs for both conventional and inverted OSCs.151 Although the effect of these CILs on the device performances was extremely detailed for the inverted structure (see hybrid materials, Section 3), their role in the conventional structure was briefly described. When used as CILs, PMI-TPP and PMI-TMOPP led to an improvement of 20% in efficiency, because both molecules lower the active layer's surface tension, promoting a better film quality.
Lia et al. developed a phosphate containing PDI (PDIP) to improve the film morphology and to enhance the hydrophilicity of the active layer surface.152 Although the use of PDIP doubled the device efficiencies in comparison to those using Ca/Al or Al, the PCEs were very low.
Tris(8-hydroxyquinoline)aluminum(III) (Alq3)-based molecules were also reported as suitable materials for CILs for both fullerene and non-fullerene OSCs.88Alq3 was kept as the core, armed with an increasing number of fluorene units (Alq3-F1, Alq3-F2, and Alq3-F3) substituted with alkyl ammonium bromide moieties. By increasing the amount of ammonium functionalized fluorene units, the interfacial dipole moment is enhanced, lowering the metal electrode work function. Also, the ratio between the Alq3 unit and the polar group-grafted fluorenes influences the electron mobility. Alq3-F2 shows a good balance between the above two factors and exhibits the highest PCE in a device containing PBTA-TF:IT-M as a photoactive layer.
There is only one report on non-conjugated small molecules to be used as CILs.87 In this study, three small molecules containing amino cations and sulfonate anions with different core atoms like oxygen (OAS), sulfur (SAS), and sulfone (SOAS) were designed and employed as interlayers for non-fullerene OSCs. All non-conjugated molecules form interfacial dipoles at the active layer/Al interface, resulting in the decrease of the work function of the electrode, reducing the charge transport barrier and recombination at the interface. The non-conjugated SAS shows an excellent cathode modification ability and the highest PCE in a PBDB-T:IT-M based PSC.
Another strategy to enhance the device performances is the development of CILs with specific features. Kang et al. designed and synthesized a naphthalene diimide (NDI)-based small molecule, N,N-dimethylamino-propyl naphthalene diimide (NDI-N), exhibiting high crystallinity and compact molecular packing, along with good film-forming properties.97 It was also found that NDI-N can efficiently extract electrons from both non-fullerene acceptors and the polymer donors at the photoactive layer/CIL interface. Using NDI-N as a CIL with various active layers, high device performances were obtained. Additionally, NDI-N is prone to be processed by printing methods for large-area devices. A large-area NDI-N film of 1 cm2 was fabricated through blade-coating, affording PCEs higher than 13%.
Very recently, azaphenalene salts with self-doping features have been reported.153 These molecules contain different counterions with strong Lewis basicity, variable repeating units and end-capped substituent groups. All azaphenalene salts exhibit self-doping properties and, due to their good solubility in polar solvents, are suitable candidates for CILs. Indeed, all-solution-processed bulk heterojunction OSCs containing PBDB-T:IT-M as an active layer and azaphenalene salts as CILs generate device performances over 10%. It was claimed that free anion radicals delocalized over the π-conjugated systems are the origin of enhancing the charge carrier density and conductivity.
Wang et al. developed conjugated small molecule containing n-type 1,3,4-thiadiazole/1,3,4-oxadiazole side chains, featuring molecules with self-doping properties and high electron conductivities.154 The use of thiadiazole-oxadiazole based molecules (ATF, STF, OTF) lowers the work function of the cathode from 4.28 eV (Al) to 3.92, 4.12 and 4.18, due to the permanent interfacial dipole formed on the top of the Al electrode during CIL deposition. The lowered WF decreases the energy barrier between the photoactive layer and the electrode, boosting the charge extraction. Indeed, the PCEs of devices containing PBDTS-TDZ:IT-4F as an active layer are almost two times higher when ATF, STF and OTF are used as CILs in comparison to those without CILs.
A good interaction between the photoactive layer and CIL is definitely beneficial for the device performance. Indeed, very recently, a new molecular design strategy has been adopted for the preparation of new interlayer materials, by tailoring the electron acceptors of the photoactive layer.
Luo et al. designed and synthesized an n-type small molecule containing indacenodithiophene core with benzothiadiazole and rhodanine groups, substituted with alkylamino groups as side chains (N-IDTBR), thus having a similar backbone to the non-fullerene acceptor O-IDTBR used in the photoactive layer.92 The optimal device with N-IDTBR had a substantially enhanced performance and the PCEs of the devices remained at 5.85 ± 0.25% as the thickness of the N-IDTBR layer varied between 5 and 30 nm.
Liao et al. used the same approach to tailor the organic electron acceptor ITIC and developed three small molecules S-1, S-2 and S-3 to be used as CILs in non-fullerene OSCs.155 The molecules contain 1,3-indanedione, a moiety with a similar structure to the end-capping unit of ITIC, an amide group introduced to endow solubility and chlorine or methyl groups to tune the LUMO energy levels. The experimental and theoretical data showed an outstanding electron extraction capability of S-3 and proved that the large electrostatic potential difference between S-3 and PBDB-TF could induce an intermolecular electric field, promoting exciton dissociation.
To get a good alignment between the LUMO energy level of the acceptor material and the metal electrode work function value, accurate control of the latter is needed. The modification of the electrode work function depends largely on the CIL molecular structure. Using binary blends as interlayers, the work function of the Al cathode can be modulated due to the anion exchange between the binary components, generating a high gain in the PCE of fullerene based solar cells.156 The same strategy was adopted also for fullerene-free OSCs. Gupta et al. mixed N719 dye with two different organophosphorus derivatives, namely tetraphenylphosphonium bromide (QPhPBr) and 2-(1,3-dioxan-2-yl)ethyl-triphenylphosphonium bromide (TPhPEtBr).157 Using the PBDBT:ITIC blend active layer, remarkable enhancement in photovoltaic performances was obtained by using dual blends as CILs, compared to their individual CILs. The anion exchange between the organophosphorus and N719 dye is most likely the origin of the large tuning ability of the work function, which is ultimately reflected in high PCEs.
A further study of the same group used a different dual blend and confirmed that this approach could be an efficient way to increase the device performances.158 The binary blend is composed of the benchmark perylene diimide amino N-oxide (PDINO), having a suitable LUMO energy level for easy electron extraction, and tetraphenylphosphonium bromide (QPhPBr) with a low lying HOMO energy level for hole blocking. Using a conventional OSC with PBDB-T:IDTBR as an active layer and PDINO:QPhPBr as a CIL, the device exhibits higher exciton dissociation and more efficient charge collection than the individual CIL devices. The work function of the dual CIL is 3.95 eV, close to the corresponding LUMO energy level of the IDTBR acceptor (3.90 eV), which improves the charge extraction efficiency and reduces the recombination losses, boosting the PCEs, mainly for the improvement of VOC and FF.
It was also observed that better device parameters were achieved with a binary blended mixture of QPhPBr and aluminum tris(8-hydroxyquinoline) (Alq3), suggesting that organophosphorus compounds might be interesting candidates for fabricating dual blend molecules to be used as CILs for highly efficient non-fullerene OSCs.159 Biobased molecular materials like chlorophyllin (CuCN) were also used as CILs in PSCs having PBDB-T:IT-M as an active layer, to effectively improve the charge transport and collection abilities (Scheme 3 and Table 4).160 PCEs of 10.32% were obtained, mostly attributed to the improved electron mobility and better phase-separation in the morphology of the active layer induced by the deposition of CuCN from solution.
The chemical structure of the small molecules used as interlayers in PSCs has a direct influence on the photoactive layer. In the last few years, various small molecules with n-doping characteristics have been reported and their effects on the electronic properties have been studied. It was demonstrated that the use of tetraalkyl ammonium acetate71 or bromide94 salts containing a weakly electron donating anion as n-dopants in PSCs with non-fullerene acceptors represent an efficient strategy to mediate the electron extracting properties (Table 5).
Tetrabutylammonium acetate (TBAA) was explored as a dopant in a PSC containing a PBDB-T:IT-M photoactive layer.71 The acetate anions were chosen due to their moderate basicity, useful for better control on the doping efficiency with solution processing. TBAA was processed from solution and deposited on top of the active layer and electron transfer between IT-M and TBAA was demonstrated. Enhanced JSC and FF values were observed in the doped BHJ, due to a better charge extraction, achieving PCEs over 11%. By using the same dopant in PBDB-2F:IT-4F solar cells, PCEs over 13% were reported without the use of an electron transporting layer.
The same group made a more complete study by using tetraalkylammonium bromides with different chain lengths and tested the influence of these dopants in non-fullerene PSCs.94 It was observed that the doped devices without electron transporting layers exhibit comparable PCEs to those based on a conventional device structure containing ETLs. The authors claimed that the doping efficiency of the non-fullerene acceptors is correlated with the chain length of the dopants. Indeed, the devices based on ITIC type acceptors afford best efficiencies with the pentyl derivative dopant (TAABr), while in the case of the Y6 acceptor the device performances of the doped solar cells increase when longer alkyl chain dopants are used. Therefore, the use of tetrabutylammonium salts containing bromide or acetate anions could be a facile strategy which enables efficient PSCs without the need for ETLs.
3.2.1.2. Polymers. This section provides an overview of CIL materials composed of a polymeric single interlayer (Table 6). Polymers applied to multilayer or mixed hybrid cathode interlayers are treated in a different paragraph.
| Material | Solar cell structure | V OC (V) | FF (%) | J SC (mA cm−2) | PCE (%) | Material in the control device | PCE (%) control device | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Flexible PET substrate. b Maximum values. | ||||||||
| PEIE | PES/PH1000/CIL/P3HT:IDT-2Br/PH1000-T | 0.84 | 61 | 6.23 | 3.22 | — | — | 162 |
| PEI | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.87 | 45 | 16.11 | 6.38 (7.06) | ZnO | 9.28 (9.71) | 121 |
| PEIE | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.86 | 50 | 15.21 | 6.54 (7.11) | ZnO | 9.28 (9.71) | 121 |
| PEI | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.56 | 35 | 11.85 | 2.32 (2.57) | ZnO | 9.80 (10.37) | 121 |
| PEIE | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.56 | 39 | 14.01 | 2.91 (3.34) | ZnO | 9.80 (10.37) | 121 |
| i-PEIE | ITO/CIL/PCE-10/IEICO-4F/MoO3/Ag | 0.69b | 53b | 25.6b | — (9.4) | ZnO | — (12.6) | 123 |
| e-PEIE | ITO/CIL/PCE-10/IEICO-4F/MoO3/Ag | 0.69b | 56b | 25.1b | — (9.78) | ZnO | — (12.6) | 123 |
| m-PEIE | ITO/CIL/PCE-10/IEICO-4F/MoO3/Ag | 0.69b | 57b | 25.1b | — (9.97) | ZnO | — (12.6) | 123 |
| a-PEIE | ITO/CIL/PCE-10/IEICO-4F/MoO3/Ag | 0.7b | 69b | 27.2b | — (13.2) | ZnO | — (12.6) | 123 |
| a-PEIE | ITO/CIL/PCE-10/IEICO-4F/MoO3/Ag | 0.69b | 67b | 27.2b | — (12.5) | — | — | 123 |
| PEI | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.89b | 49b | 16.1b | — (6.99) | ZnO | — (9.61) | 122 |
| PEI | ITO/CIL/PM6:IT-4F/MoO3/Ag | 0.82b | 47b | 20.1b | — (7.8) | ZnO | — (12.02) | 122 |
| PEI | ITO/CIL/PBDB-T:IT-M/MoO3/Ag | 0.91b | 46b | 16.3b | — (6.89) | ZnO | — (9.14) | 122 |
| PEIE-DBO | ITO/CIL/PTB7-Th:IEICO-4F/MoO3/Al | 0.72b | 64b | 26.15b | — (12.05) | ZnO | — (11.69) | 163 |
| PEIE-DBO | ITO/CIL/PM6:IT-4F/MoO3/Al | 0.82b | 71b | 22.92b | — (13.34) | ZnO | — (13.05) | 163 |
| PEIE-DBO | ITO/CIL/PM6:Y6/MoO3/Al | 0.84b | 69b | 27.64b | — (15.74) | ZnO | — (15.37) | 163 |
| PFN | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.89 | 71 | 17.05 | 10.35 (10.77) | ZnO | 10.71 (11.30) | 81 |
| PFN-Br | ITO/PEDOT:PSS/PBDB-T-2F:IT-4F/CIL/Al | 0.87b | 76b | 20.37b | — (13.5) | — | — | 99 |
| PFN | ITO/PEDOT:PSS/PBDB-T-2F:IT-4F/CIL/Al | 0.87b | 47b | 16.55b | — (6.5) | — | — | 99 |
| PVP | ITO/CIL/PBDB-T:IT-M/MoO3/Al | 0.91 | 69 | 18.3 | 10.8 (11.5) | w/o CIL | 5.8 (6.6) | 164 |
| PVP | ITO/CIL/PBDB-TCl:IT-4F/MoO3/Al | 0.86 | 73 | 22.3 | 13.4 (14.0) | w/o CIL | 11.40 (11.4) | 164 |
| PVP | ITO/CIL/PBDTTT-T-E:IEICO/MoO3/Al | 0.81 | 59 | 16.5 | 7.3 (7.8) | w/o CIL | 3.7 (4.5) | 164 |
| PFN | ITO/CIL/PBDB-T:IT-M/MoO3/Ag | 0.94 | 69 | 17.05 | 10.69 (11.14) | — | — | 168 |
| PSN | ITO/PEDOT:PSS/PBDB-T-2F:IT-M/CIL/Al | 0.92a | 68a | 16.09a | 10.02 (10.11) | w/o CIL | 3.61 (3.74) | 169 |
| PSO | ITO/PEDOT:PSS/PBDB-T-2F:IT-M/CIL/Al | 0.89a | 63.1a | 16.44a | 9.10 (9.22) | w/o CIL | 3.61 (3.74) | 169 |
| PSM | ITO/PEDOT:PSS/PBDB-T-2F:IT-M/CIL/Al | 0.89a | 63.6a | 15.27a | 8.56 (8.74) | w/o CIL | 3.61 (3.74) | 169 |
| PFN-2TNDI | ITO/PEDOT:PSS/PTB7-Th:N2200/CIL/Ag | 0.78 | 58 | 13.60 | 6.2 (6.3) | w/o CIL | 4.2 (4.3) | 90 |
| PFN | ITO/PEDOT:PSS/PTB7-Th:N2200/CIL/Ag | 0.77 | 51 | 13.18 | 5.2 (5.4) | w/o CIL | 4.2 (4.3) | 90 |
| PFN-2TNDI | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Ag | 0.92 | 70 | 15.59 | 10.8 (11.1) | w/o CIL | 7.4 (7.8) | 90 |
| PFN | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Ag | 0.91 | 58 | 15.80 | 8.3 (8.6) | w/o CIL | 7.4 (7.8) | 90 |
| PFN-2TNDI | ITO/PEDOT:PSS/PTB7-Th:IDT-2Br/CIL/Ag | 1.04 | 57 | 13.60 | 7.9 (8.1) | w/o CIL | 5.2 (5.3) | 90 |
| PFN | ITO/PEDOT:PSS/PTB7-Th:IDT-2Br/CIL/Ag | 1.03 | 51 | 13.35 | 7.0 (7.2) | w/o CIL | 5.2 (5.3) | 90 |
| PIF-PMIDE-N | ITO/PEDOT:PSS/DT-ffBX-DT:SFPDI/CIL/Ag | 1.22 | 51 | 10.2 | 6.31 (6.38) | w/o CIL | 3.16 (3.27) | 170 |
| PNDIT-F3N | ITO/PEDOT:PSS/DT-ffBX-DT:SFPDI/CIL/Ag | 1.19 | 48 | 10.0 | 5.80 (5.95) | w/o CIL | 3.16 (3.27) | 170 |
| PIF-PDI-N | ITO/PEDOT:PSS/DT-ffBX-DT:SFPDI/CIL/Ag | 1.17 | 47 | 9.7 | 5.41 (5.52) | w/o CIL | 3.16 (3.27) | 170 |
| PIF-PMIDE-N:PNDIT-F3N | ITO/PEDOT:PSS/DT-ffBX-DT:SFPDI/CIL/Ag | 1.22 | 52 | 10.1 | 6.40 (6.42) | w/o CIL | 3.16 (3.27) | 170 |
| PIF-PMIDE-N | ITO/PEDOT:PSS/NT812:SFPDI/CIL/Ag | 0.95 | 42 | 6.45 | 2.59 (2.62) | PFN-Br | 2.53 (2.58) | 170 |
| PNDIT-F3N | ITO/PEDOT:PSS/NT812:SFPDI/CIL/Ag | 0.92 | 40 | 6.24 | 2.28 (2.33) | PFN-Br | 2.53 (2.58) | 170 |
| PIF-PDI-N | ITO/PEDOT:PSS/NT812:SFPDI/CIL/Ag | 0.86 | 38 | 6.13 | 2.03 (2.07) | PFN-Br | 2.53 (2.58) | 170 |
| PIF-PMIDE-N | ITO/PEDOT:PSS/PBDB-T:ITCC/CIL/Ag | 0.97 | 66 | 15.36 | 9.87 (10.0) | PFN-Br | 10.02 (10.13) | 170 |
| PNDIT-F3N | ITO/PEDOT:PSS/PBDB-T:ITCC/CIL/Ag | 0.95 | 63 | 15.22 | 9.07 (9.20) | PFN-Br | 10.02 (10.13) | 170 |
| PIF-PDI-N | ITO/PEDOT:PSS/PBDB-T:ITCC/CIL/Ag | 0.93 | 62 | 15.26 | 8.84 (8.90) | PFN-Br | 10.02 (10.13) | 170 |
| PIF-PMIDE-N | ITO/PEDOT:PSS/PTB7-Th:N2200/CIL/Ag | 0.79 | 53 | 14.33 | 5.97 (6.07) | PFN-Br | 6.00 (6.16) | 170 |
| PNDIT-F3N | ITO/PEDOT:PSS/PTB7-Th:N2200/CIL/Ag | 0.79 | 53 | 14.22 | 5.94 (6.02) | PFN-Br | 6.00 (6.16) | 170 |
| PIF-PDI-N | ITO/PEDOT:PSS/PTB7-Th:N2200/CIL/Ag | 0.79 | 53 | 14.22 | 5.97 (6.05) | PFN-Br | 6.00 (6.16) | 170 |
| PBTA-FN | ITO/PBDB-T-2F:IT-4F/CIL/Al | 0.88 | 73 | 19.05 | 12.18 | w/o CIL | 3.21 | 89 |
| PFN | ITO/PBDB-T-2F:IT-4F/CIL/Al | 0.86 | 71 | 18.15 | 11.03 | w/o CIL | 3.21 | 89 |
| PBTz-SO3Na | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Al | 0.89 | 65 | 16.6 | 9.6 | — | — | 172 |
| PBTBTz-TMAI | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Al | 0.9 | 64 | 18.3 | 10.5 | — | — | 172 |
| PBTBTz-SO3TBA | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Al | 0.92 | 67 | 14.7 | 9.1 | — | — | 174 |
| P-PDI-SB | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Ag | 0.73b | 60b | 15.85b | — (6.95) | w/o CIL | — (4.25) | 175 |
| P-C60-SB | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Ag | 0.91b | 67b | 16.66b | — (10.1) | w/o CIL | — (4.25) | 175 |
| C60− ionene | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Ag | 0.92 | 70 | 16.63 | 10.66 (10.82) | w/o CIL | 3.11 (3.89) | 175 |
| NDI-NI | ITO/PEDOT/PM6:Y6/CIL/Ag | 0.84 | 74 | 25.74 | 16.06 (16.27) | w/o CIL | 12.08 (12.63) | 177 |
| NDI-NI | ITO/PEDOT/PM6:Y6:PC71BM/CIL/Ag | 0.86 | 76 | 25.4 | 16.61 (16.86) | w/o CIL | 12.01 (12.21) | 177 |
| HD-Br | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Ag | 0.86a | 68a | 16.43a | 9.69 (9.77) | w/o CIL | 5.57 (5.8) | 178 |
| HN-Br | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Ag | 0.85 | 65 | 15.52 | 8.52 (8.66) | w/o CIL | 5.57 (5.8) | 178 |
| HP-Br | ITO/PEDOT:PSS/PBDB-T:ITIC/CIL/Ag | 86 | 69 | 16.58 | 9.84 (9.87) | w/o CIL | 5.57 (5.8) | 178 |
Many cathode interfacial polymer materials have been developed for F-based PSCs (Scheme 4).23,26
Among them, polyethylenimine (PEI) and ethoxylated polyethylenimine (PEIE) interlayers afforded good stability and efficient performances when applied to fullerene PSCs.53,161PEI and PEIE have also been considered for NF-PSCs. For example, a PEIE interlayer on top of a conductive PEDOT (Clevios PH1000 from Heraeus GmbH) was used to fabricate ITO-free semi-transparent flexible NFA-based PSCs, using a P3HT:IDT-2Br blend.162 These fullerene-free devices showed better performances and bending stability when compared to devices made with a standard PC61BM acceptor. As discussed in Section 2.4, the different reactivities of FAs and NFAs can be an issue when using PEI and PEIE interlayers. Zhou's group reported that the NF acceptors of the ITIC family have the tendency to react in a basic environment.118,122PEI and PEIE interfacial modifiers react as nucleophiles with the ITIC C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moiety, destroying the original electronic structure and the intramolecular charge transfer of the ITIC molecules. Hu et al. reported inverted NF-PSCs, with PBDT-T:ITIC as a photoactive layer, using PEIE or PEI as cathode interlayers.121 Decreases in the FF parameter and PCE were observed when using these amino-containing interlayers rather than a reference ZnO interlayer. An “S” shape of the J–V curves under illumination revealed a hindrance to charge extraction that was explained by an adverse reaction occurring at the interface between the NF active layer and the PEI or PEIE interlayers. Thermal annealing further pushed the PEI- or PEIE-ITIC reaction forward, with a drastic reduction of the PCEs. Later it was reported that the substituent groups in the ITIC's terminal acceptor moieties have an influence on the chemical reactivity to amino-containing interfacial layers.122 Fluorine substitution increased the chemical reactivity when compared to hydrogen or methyl substituents. Accordingly, the efficiency reduction compared to a reference ZnO device for BHJ cells made with IT-4F and using a PEI interlayer, was more pronounced than in ITIC or IT-M based devices. Interestingly, the chemical reactivity of the amine groups is also influenced by the donor core moieties. Y6, bearing similar fluorine substituted terminal acceptors to IT-4F but a different donor core, exhibits a higher chemical stability than the ITIC family. Xiong reported that by increasing the PEIE protonation it is possible to deactivate the interfacial chemical reaction and enhance the device performances.123 Inverted PCE-10:IECO-4F cells were prepared using different protic solvents (isopropanol, ethanol, methanol and water) to tune PEIE's protonation. The PEIE's highest protonation and best processing conditions were found using aqueous solutions, a-PEIE. The a-PEIE-based cells outperformed the reference cells with a ZnO interlayer. The lowest protonation, obtained with isopropanol, led to devices with the poorer electron collection properties and lower efficiencies. The different performances of the a-PEIE and i-PEIE cells are not ascribable to the different cathode electrode energy level alignments as the ITO work function modification was found to be substantially invariant with the PEIE's processing solvent. The efficient charge collection obtained in a-PEIE was attributed to the passivation of the chemical activity via amino group protonation. Note that in the other cited contribution of Zhou's group,121PEI and PEIE interlayer processing was carried out from isopropanol, i.e. in a condition where the interfacial chemical activity towards NF acceptors is more pronounced. Zhang et al. recently prepared a non-conjugated polyelectrolyte by the quaternization of PEIE with 1,8-dibromooctane, PEIE-BDO.163 This novel polyelectrolyte interlayer enabled an ITO work function reduction to 3.9 eV, which is suitable to minimize the energy misalignment between the active layer and the ITO electrode to afford good electron collection from both F- and NF-PSCs. Indeed, the effectiveness of the PEIE-BDO CIL material in an inverted device configuration was demonstrated for both F-PSCs and NF-PSCs. When using a PEIE-BDO interlayer in PTB7-Th:IEICO-4F, PM6:IT-4F and PM6:Y6 bulk heterojunctions, the efficiencies reached slightly higher values than with a reference ZnO interlayer. Another non-conjugated polymer used as a CIL in NFA-based PSCs is polyvinylpyrrolidone (PVP).164 The authors used the one-step technique involving the mixing of photoactive blend and interlayer polymers or molecules and taking advantage of the segregation of the materials in a quasi-sandwiched structure during or after the processing.165–167 Yang et al. analyzed the one step deposition on different active layers (PBDB-T:IT-M, PBDB-TCl:IT-4F and PBDTTT-T-E:IEICO), obtaining an increment in all the figures of merit of the solar cells, with respect to the devices without IL. These enhancements are mainly due to the high surface energy of PVP and its strong interaction with ITO. The same one-step technique was used by Lin et al. with PFN upon solvent vapor annealing.168 The organic blend composed of PBDB-T:IT-M and PFN tends to vertically separate when exposed to a solvent vapor-saturated atmosphere. The self-organized sandwiched structure yields solar cells with a significantly higher PCE (11.14%) with respect to the reference device (8.05%).
O moiety, destroying the original electronic structure and the intramolecular charge transfer of the ITIC molecules. Hu et al. reported inverted NF-PSCs, with PBDT-T:ITIC as a photoactive layer, using PEIE or PEI as cathode interlayers.121 Decreases in the FF parameter and PCE were observed when using these amino-containing interlayers rather than a reference ZnO interlayer. An “S” shape of the J–V curves under illumination revealed a hindrance to charge extraction that was explained by an adverse reaction occurring at the interface between the NF active layer and the PEI or PEIE interlayers. Thermal annealing further pushed the PEI- or PEIE-ITIC reaction forward, with a drastic reduction of the PCEs. Later it was reported that the substituent groups in the ITIC's terminal acceptor moieties have an influence on the chemical reactivity to amino-containing interfacial layers.122 Fluorine substitution increased the chemical reactivity when compared to hydrogen or methyl substituents. Accordingly, the efficiency reduction compared to a reference ZnO device for BHJ cells made with IT-4F and using a PEI interlayer, was more pronounced than in ITIC or IT-M based devices. Interestingly, the chemical reactivity of the amine groups is also influenced by the donor core moieties. Y6, bearing similar fluorine substituted terminal acceptors to IT-4F but a different donor core, exhibits a higher chemical stability than the ITIC family. Xiong reported that by increasing the PEIE protonation it is possible to deactivate the interfacial chemical reaction and enhance the device performances.123 Inverted PCE-10:IECO-4F cells were prepared using different protic solvents (isopropanol, ethanol, methanol and water) to tune PEIE's protonation. The PEIE's highest protonation and best processing conditions were found using aqueous solutions, a-PEIE. The a-PEIE-based cells outperformed the reference cells with a ZnO interlayer. The lowest protonation, obtained with isopropanol, led to devices with the poorer electron collection properties and lower efficiencies. The different performances of the a-PEIE and i-PEIE cells are not ascribable to the different cathode electrode energy level alignments as the ITO work function modification was found to be substantially invariant with the PEIE's processing solvent. The efficient charge collection obtained in a-PEIE was attributed to the passivation of the chemical activity via amino group protonation. Note that in the other cited contribution of Zhou's group,121PEI and PEIE interlayer processing was carried out from isopropanol, i.e. in a condition where the interfacial chemical activity towards NF acceptors is more pronounced. Zhang et al. recently prepared a non-conjugated polyelectrolyte by the quaternization of PEIE with 1,8-dibromooctane, PEIE-BDO.163 This novel polyelectrolyte interlayer enabled an ITO work function reduction to 3.9 eV, which is suitable to minimize the energy misalignment between the active layer and the ITO electrode to afford good electron collection from both F- and NF-PSCs. Indeed, the effectiveness of the PEIE-BDO CIL material in an inverted device configuration was demonstrated for both F-PSCs and NF-PSCs. When using a PEIE-BDO interlayer in PTB7-Th:IEICO-4F, PM6:IT-4F and PM6:Y6 bulk heterojunctions, the efficiencies reached slightly higher values than with a reference ZnO interlayer. Another non-conjugated polymer used as a CIL in NFA-based PSCs is polyvinylpyrrolidone (PVP).164 The authors used the one-step technique involving the mixing of photoactive blend and interlayer polymers or molecules and taking advantage of the segregation of the materials in a quasi-sandwiched structure during or after the processing.165–167 Yang et al. analyzed the one step deposition on different active layers (PBDB-T:IT-M, PBDB-TCl:IT-4F and PBDTTT-T-E:IEICO), obtaining an increment in all the figures of merit of the solar cells, with respect to the devices without IL. These enhancements are mainly due to the high surface energy of PVP and its strong interaction with ITO. The same one-step technique was used by Lin et al. with PFN upon solvent vapor annealing.168 The organic blend composed of PBDB-T:IT-M and PFN tends to vertically separate when exposed to a solvent vapor-saturated atmosphere. The self-organized sandwiched structure yields solar cells with a significantly higher PCE (11.14%) with respect to the reference device (8.05%).
PFN is a polyfluorene copolymer bearing amine groups in the alkyl-side chain and is another well-known polymeric CIL that enables high-efficiency in inverted F-PSCs.41 Despite the poor electron transport properties of this p-type conjugated polymer, a thin PFN layer deposited on top of an ITO electrode showed better performances when compared to an n-type ZnO electron transporting metal oxide semiconductor. As discussed in Section 2, such outstanding results have been widely debated in the literature. Interestingly, an opposite trend was observed in inverted NF-PSCs.81 Solar cells in an inverted configuration with a PBDB-T:ITIC photoactive layer showed a lower PCE of 10.35% when using PFN as a CIL, while a slightly higher efficiency of 10.77% was obtained in the devices prepared with a ZnO interlayer. This suggests that the cathode modification mechanism assessed for F-PSCs is not necessarily transposable to NF-PSCs and needs to be investigated.
Kang et al. studied and compared the interfacial engineering of PFN and its derivative, PFN-Br, when used in fullerene-free PSCs, taking F-PSCs for comparison.99 The devices were fabricated with a conventional geometry, using PBFB-2T-2F:IT-4F or PBFB-2T:PC71BM as active layers. The PV characteristics of the F-PSCs obtained using PFN or PFN-Br were very similar, indicating that both CILs worked well in the F-PSCs. However, the PFN and PFN-Br interlayers functioned quite differently in the NF-PSCs. Excessive electron-transfer from the PFN amino groups to IT-4F, i.e. an n-doping of the acceptor, induced an unfavorable charge accumulation at the interface. Such an electronegative IL might block the electron collection and cause serious recombination of charges at the interfaces. By replacing the amino side group of PFN with the ammonium bromide of PFN-Br, the excessive doping effect between the CIL and IT-4F was eliminated and any electronegative barrier at the interface could hamper the electron collection. As a result, a higher PCE of 13.5% was obtained with PFN-Br rather than 6.5% with PFN based devices.
To explore the relationship among the pendant polar groups of polymer CILs, the n-doping at the interface and the device performances, three alcohol-soluble non-conjugated polymers, PSN, PSO and PSM, with the same backbone but different pendant polar amino or ammonium groups (amine, ammonium oxide and ammonium mesylate, respectively) were devised and tested as cathode interlayers in PBDB-T:IT-M cells with a conventional geometry.169 Overall, the incorporation of these interlayers causes a remarkable improvement of the PV parameters, because their polar side groups formed dipoles at the cathode interface that reduced the electrode work function and improved electron collection. Additionally, transfer of unpaired electrons from the amino and ammonium oxide groups of the PSN and PSO materials to IT-M was detected from the EPR spectra. Here, the n-doping of the IT-M acceptors at the interface with the PSN and PSO interlayers improved the electrical contact, with beneficial effects for the efficiency of the devices. The strong withdrawing mesylate group of PSM lowered the electron donating capability of the CIL, resulting in no doping effects and lower PV performances.
Besides interfacial n-doping and dipole induced electrode work function reductions, to enhance charge collection in PSCs, a good matching of the energy levels between the cathode interlayer materials and the BHJ acceptor as well as decent electron transport properties are also important. Sun et al. reported on the interfacial engineering effects for NF-PSCs using two conjugated polymer CILs with different electronic properties and bearing similar alkyl-amino group side chains, PFN-2TNDI and PFN.90PFN-2TNDI is an n-type naphthalene diimide based conjugated polymer, with a LUMO energy level of 3.91 eV. PFN-2TNDI showed enhanced electron transport capability through self-doping effects and it was applied to fabricate efficient F-PSCs with interlayer thicknesses from 5 nm up to 100 nm.91PFN is a p-type electron rich polymer with a LUMO energy level situated at 2.14 eV. PFN shows low electron mobility and can only work efficiently as a cathode interlayer when a very thin film is applied (5 nm). PFN and PFN-2TNDI were tested in NF-PSCs with a conventional geometry using three different acceptors, namely an n-type polymer, N2200, and ITIC and IDT-2Br molecules, with the following LUMO energy levels: 3.84 eV (N2200), 3.83 eV (ITIC) and 3.69 eV (IDT-2Br). These acceptors were included in three photoactive layers PTB7-Th:N2200, PBDB-T:ITIC and PTB7-Th:IDT-2Br in devices with a conventional geometry. As a general trend, the PFN based devices delivered better PCE values than that of a bare aluminium cathode electrode, owing to the work function modification capability of PFN, which improved the energy level alignment at the electrode interface. Overall, the devices prepared with PFN-2TNDI outperformed the ones prepared with PFN. Such behavior was due to the PFN-2TNDI self-doping ability and reduced energy offset with the NF acceptors, which could promote better extraction and reduce charge recombination losses when compared to PFN. PFN-2TNDI functioned also as a light harvesting material, contributing to extra photocurrent generation and improved extraction properties at the interface. In addition, the good electron transport properties of PFN-2TNDI should allow the fabrication of efficient devices with relatively thick interlayers. This was indeed the case for the devices made with the N2200 and ITIC acceptors but not for the ones prepared with IDT-2Br, where the interlayer thickness should remain below 10 nm. Such differences were explained by the peculiar energy level match between the interlayer and the acceptors, rather good for N2200 or ITIC but not optimal when IDT-2Br was used. The LUMO energy levels of NF acceptors are usually closer to the vacuum level than that of PCBM. An energy mismatch between the CILs, designed for fullerenes, and the novel acceptors can thus occur. As the LUMOs of large-band gap NFAs keep shifting up, such an energy barrier becomes more severe. Yin et al. investigated the influence of interlayers with different LUMO energy levels in NF-PSCs.170 Three acceptors with different LUMO energies (SFPDI (3.71 eV), ITTCC (3.75 eV), and N2200 (3.98 eV)) were used together with four donor polymers, BDT-ffBX-DT, NT812, PBDB-T, PTB7-Th to fabricate NF-PSCs with a conventional geometry. A series of n-type conjugated polymer CIL materials with different LUMO energy levels, PIF-PMIDE-N (3.68 eV), PNDIT-F3N (3.89 eV), and PIFPDI-N (3.92 eV), were selected. These three CILs exhibited good interface modification properties when used in F-PSCs.171 The results shown by the authors indicate that the energy level-alignment between interlayers and acceptors plays a crucial role in achieving high open circuit voltages and efficient charge extraction. The PNDIT-F3N interlayers worked efficiently within a large thickness variation, due to their excellent transport properties, while the PIF-PMIDEN interlayers could work efficiently just using a thin film (5 nm). This feature stemmed from the up-shift of the LUMO level of PIF-PMIDEN. This shift enabled the reduction of the energy offset for optimized charge extraction but at the same time modified the CIL material from n-type to p-type, thus reducing the electron transport ability. To maintain together the good electron transport of PNDIT-F3N and the optimized charge extraction of PIF-PMIDE-N, these two CIL materials were blended together. With this binary CIL, it was possible to fabricate efficient single junction, double junction and triple junction homo-tandem solar cells with an ultra-high VOC of 1.22 V, 2.39 V and 3.21 V, respectively. Wang et al. reported an alcohol-soluble conjugated polymer based on fluorene and benzotriazole derivatives (PBTA-FN) with self-doping effects.89 It was suggested that PBTA-FN can reduce the work function of the metal electrode and enhance the built-in voltage. The use of PBTA-FN as a CIL in ITO/PEDOT:PSS/PBDB-T:IT-4F/CIL/Al generates better efficiencies in comparison to those containing PFN as a CIL. Shi designed a series of benzotriazole copolymers bearing two anionic and one cationic substituents, SO3Na, SO3TBA and TMAI.172–174 Overall, these conjugated polymers function properly when inserted as cathode interlayers in PBDB-T:ITIC devices with a conventional geometry. The polymer with the cationic substituent, TMAI, appeared to be slightly more efficient than the interlayer characterized by the presence of anionic groups SO3Na and SO3TBA.
The well-known n-type organic semiconductors, PDI and fullerene, were integrated into novel zwitterionic polymers, PDI-PZ and C60-PZ.175 The insertion of these CILs in PBDB-T:ITIC devices with a conventional geometry causes a relevant improvement of the PV parameters, due to the combined effect of electrode work function reduction and improved electric contact to the active layer. However, C60-PZ proved to be more efficient than PDI-PZ in modifying the interface and in achieving efficient electron extraction. As a result, the PCE values improved from 6.95% with PDI-PZ to 10.1% when using the C60-PZ interlayer. In addition, C60-PZ CILs exhibited excellent tolerance to interlayer thickness, which represents an important feature for processing consideration, discussed in Section 4. The same group integrated fullerenes in aliphatic ionenes in which the cations, here NBr, are positioned within the polymer backbone rather than pendant groups (C60-ionene).176 This novel polycation was tested as a CIL in PBDB-T:ITIC cells with a conventional geometry. The integration of fullerenes into the poly-cationic structure markedly improved the conductivity of ionene polymers with values from 1.2 × 10−6 to 6.4 × 10−4 S cm−1. This is a crucial factor in achieving thickness independent interlayer materials. The insertion of a 26 nm thick C60-ionene interlayer enabled efficient devices with a PCE of 10.66%, which just slightly decreased to 9.56% when using a 60 nm thick interlayer. Liu et al. reported on two naphthalene diimide based ionene polymers, NDI-NI and NDI-Cl,177 where the charged units are situated in the polymer backbone, rather than as pending moieties. The authors demonstrated that both ionenes have self-doping effects and are universal interlayers being used as CILs in fullerene, non-fullerene and ternary PSCs. Another group designed hyperbranched polyelectrolytes including diketopyrrolopyrrole, HD-Br, naphthalene diimide, HN-Br, and perylenediimide, HP-Br, and tested them as cathode interlayers in PBDB-T:ITIC devices with a conventional geometry.178 Promising PCE values of 9.77%, 8.66%, and 9.87% were obtained using relatively thick interlayers (20 nm) of, respectively, HD-Br, HN-Br and HP-Br. The EPR spectra showed that the most efficient interlayer, HP-Br, exhibited a pronounced self-doping ability that should enhance the electron transport to the electrode.
Recently, effective biopolymers recovered from natural sources were used as interlayers (Scheme 5). The use of environmentally friendly materials is highly indispensable in fabricating PSCs and promising results were obtained with cellulose nanopaper used as an optical trap in non-fullerene PSCs, reaching efficiencies as high as 16% (Table 7).179 In the last couple of years, the use of green CILs from natural products was also reported. Most of the natural materials possess polar groups like hydroxyl, amine, and carboxyl which can interact with both metal electrodes and metal oxides, modifying the active layer/electrode interface and tuning the work function.
| Material | Solar cell structure | V OC (V) | FF (%) | J SC (mA cm−2) | PCE (%) | Material in the control device | PCE (%) of control device | Ref. |
|---|---|---|---|---|---|---|---|---|
| Hydroxypropyl cellulose (HPC) | ITO/PEDOT:PSS/PM6:IDIC/CIL/Ag | 0.90 | 64 | 16.12 | 9.32 | — | — | 182 |
| α-PLL | ITO/PEDOT:PSS/PBDB-T:IT-M/CIL/Al | 0.94 | 74 | 17.13 | 11.92 | PFN | 1.24 | 101 |
| α-PLL | ITO/PEDOT:PSS/PDCBT:ITIC/CIL/Al | 0–94 | 65 | 16.58 | 10.17 | PFN | 4.57 | 101 |
| α-PLL | ITO/PEDOT:PSS/J52:IEICO-4F/CIL/Al | 0.72 | 62 | 25.95 | 11.74 (12.03) | PFN | 9.43 | 101 |
| α-PLL | ITO/CIL/PTB7-Th:IEICO-4F/MoO3/Al | 0.70 | 66 | 23.84 | 11.03 | PFN | 3.89 | 101 |
| Poly-L-lysine:poly-D-lysine | ITO/CIL/PBDBT:ITIC/MoO3/Ag | 0.71 | 51 | 16.3 | 5.82 (5.91) | — | — | 183 |
| Poly-L-lysine | ITO/CIL/PBDBT:ITIC/MoO3/Ag | 0.84 | 53 | 16.8 | 7.39 (7.49) | — | — | 183 |
| Poly-D-lysine | ITO/CIL/PBDBT:ITIC/MoO3/Ag | 0.84 | 52 | 16.7 | 7.28 (7.43) | — | — | 183 |
| Poly-L-lysine | ITO/CIL/PBDB-T-2Cl:IT-4F/MoO3/Ag | 0.85 | 60 | 19.7 | 9.86 (10.01) | — | — | 183 |
| DMEKL/PDIN | ITO/PEDOT:PSS/PM6:Y6/CIL/Al | 0.85 | 70 | 26.61 | 15.96 (16.02) | PDIN | 15.24 (15.41) | 184 |
Cellulose and cellulose derivatives are used as both ZnO modifiers (see hybrid materials, Section 3.2.3) in inverted PSCs109,180,181 and CILs in direct organic solar cells.182 Wang et al. used hydroxypropyl cellulose (HPC) as an interfacial layer in fullerene and non-fullerene solar cells. Due to the high proportion of hydroxyl groups anchored to HPC, it is possible to obtain a good contact between the active layer and the metal electrode and therefore to decrease efficiently the work function of the cathode, resulting in a lower interfacial geminate recombination. From experimental data it was also demonstrated that HPC forms a high-quality film on the active layer and enables better electron mobility. As a result, the device having PM6:IDIC as an active layer and HPC as a CIL generates a PCE of 9.32%. Zheng et al. used polyamino acid α-poly-L-lysine (α-PLL) to mediate charge extraction in non-fullerene OSCs in both conventional and inverted structures.101 For the direct structures, in all cases much better device performances were obtained when α-PLL was used as a CIL in comparison with those using PFN. Moreover, electron transfer between α-PLL and the IEICO-4F acceptor was demonstrated, suggesting the existence of an interfacial n-doping, promoting the electron extraction and transport. As a result, OSCs with a high JSC of 25.95 mA cm−2 and PCEs approaching 12% were reported. The effectiveness of α-PLL CILs also enables the production of high efficiency fullerene-free OSCs with inverted structures. Using PTB7-Th:IEICO-4F as a photoactive material, PCEs similar to those based on high-temperature processed ZnO CILs were obtained. An interesting study on the use of poly-lysine enantiomers as CILs for NFA-based PSCs was reported by Huang et al.183 The distinct configurations of poly-L-lysine and poly-L-lysine blended with poly-D-lysine, with different arrangements of the amino groups, create different surface dipoles on the ITO substrate and therefore alters the work function of the electrode. The surface energy shows that the hydrophobic nature of poly-L-lysine is favorable for improving the interfacial compatibility with the active layer. The higher surface potential of poly-L-lysine compared to the poly-lysine blend is an indication that the arrangements of amino groups generate a strong polarity. The photovoltaic performances of inverted geometry PSCs are significantly enhanced when using poly-L-lysine as a CIL in comparison to those containing the blend of the two enantiomers, reaching PCEs of 10% in the PBDB-T-2Cl:IT-4F system.
The use of biomass-based materials instead of fossil fuels as resources for PSCs was proposed by Chao et al.184 A demethylated kraft lignin (DMEKL) possessing uniformly distributed hydroxyl phenolic moieties shows an outstanding binding capacity to the amino terminal substituted perylene diimide (PDINO), generating an efficient isotropic electron transfer 3D network. Due to their particular structures and high compatibility, the use of biomass-based materials in binary blends reduces the issues of phase separation. Moreover, the blend combines the isotropic features of a 3D network of DMEKL with the high conductivity of PDINO. By using DMEKL:PDINO as a CIL and Y6:PM6 as a photoactive layer, PCEs very close to 16% were obtained. The high performances were attributed to suitable energy level alignment, improved electron transport and collection and good interfacial contact.
| Material | Organic solar cell structure | V OC (V) | FF (%) | J SC (mA cm−2) | PCE (%) | Material in the control device | PCE (%) control device | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Maximum values. | ||||||||
| ZnO-np/ZnO | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.94 | 65 | 19.4 | 11.87 (12.24) | ZnO | 10.53 (10.69) | 86 |
| EDTA-modified ZnO | ITO/CIL/PBDB-T:IT-M/MoO3/Ag | 0.95 | 73 | 17.1 | 11.67 (12.10) | ZnO | 10.56 (11.07) | 58 |
| Li-doped ZnO | FTO/CIL/PTB7-Th:IT-4F/MoOx/Al | 0.83a | 67a | 16.2a | 8.71 (8.96) | ZnO | 7.12 (7.47) | 113 |
| KOH-treated ZnO | ITO/CIL/PBDB-T-2Cl:IT-4F/MoO3/Ag | 0.84 | 74 | 20.0 | 12.4 (12.8) | ZnO | 11.4 (11.8) | 103 |
| KOH-treated ZnO | ITO/CIL/PBDB-T-2Cl:Y1-4F/MoO3/Ag | 0.84 | 63 | 23.0 | 12.2 (12.6) | ZnO | 11.0 (11.6) | 103 |
| KOH-treated ZnO | ITO/CIL/PBDB-T-2Cl:Y6/MoO3/Ag | 0.89 | 69 | 20.4 | 13.1 (13.5) | ZnO | 11.9 (12.2) | 103 |
| SnO2 | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.89 | 67 | 18.0 | 10.71 (10.77) | ZnO | 8.78 | 192 |
| SnO2 nc | ITO/CIL/PM6:IT-4F/MoO3/Ag | 0.85 | 77 | 21.3 | 13.8 (14.1) | ZnO | 12.8 (13.0) | 111 |
| SnO2 nc | ITO/CIL/PTB7-Th:ITIC/MoO3/Ag | 0.89 | 72 | 15.7 | 10.0 (10.5) | ZnO | 9.3 (9.6) | 111 |
| SnO2 nc | ITO/CIL/PTB7-Th:IEICO-4F/MoO3/Ag | 0.70 | 68 | 26.6 | 12.6 (13.1) | ZnO | 11.7 (12.0) | 111 |
| SnO2 NP | ITO/CIL/PTB7-Th:IEICO-4F/MoO3/Ag | 0.71 | 63 | 19.5 | 8.74 | — | — | 193 |
| SnO2 NP | ITO/CIL/PBDB-T:IEICO-4F/MoO3/Ag | 0.71 | 52 | 17.9 | 6.64 | — | — | 193 |
| SnO2 NP | ITO/CIL/PBDB-T-2F:ITIC-4F/MoO3/Ag | 0.79 | 72 | 19.7 | 11.2 | — | — | 193 |
| SnO2 NP | ITO/CIL/PBDB-T-2F:Y6/MoO3/Ag | 0.83 | 72 | 21.6 | 12.88 | — | — | 193 |
| SnO2 NP | ITO/CIL/J71:IT-M/MoO3/Ag | 0.93 | 61 | 12.8 | 7.23 | — | — | 193 |
| SnO2 NP | ITO/CIL/J71:IEICO-4F/MoO3/Ag | 0.77 | 52 | 11.1 | 4.45 | — | — | 193 |
| SnO2 NP/InP-ZnS QD | ITO/CIL/PM6:Y6/MoO3/Ag | 0.85a | 71a | 25.5a | — (15.22) | SnO2 NP | — (13.86) | 196 |
| Ti(i-OPr)2(acac)2 | ITO/CIL/PBDTBDD:IT-M/MoO3/Al | 0.94 | 71 | 17.5 | 11.7 | w/o CIL | 6.13 | 194 |
| Ti(i-OPr)2(acac)2 | ITO/CIL/PBDTTT-ET:IEICO/MoO3/Ag | 0.81a | 54a | 14.5a | 6.15 (6.26) | TiO2 | 6.73 (6.86) | 128 |
| TiO(acac)2 | ITO/CIL/PBDB-T:IT-M/MoO3/Al | 0.92 | 65 | 16.9 | 10.20 | — | — | 102 |
| TaO2FCx | ITO/PEDOT-PSS/PM6:IT-4F/CIL/Al | 12.77 (13.18) | PDNINO | — (12.04) | 195 | |||
| TaO2FCx | FTO/CIL/PM6:IT-4F/MoOx/Al | 0.85a | 76a | 21.6a | — (14.14) | ZnO | — (13.03) | 195 |
Zinc oxide is the most common CIL in fullerene-based inverted solar cells. Thus, it is obvious that it is also the first option for NFA-based devices. Besides “conventional” sol–gel ZnO and ZnO nanoparticles,86 some tailored ZnO materials have been developed for NFA devices. Upama et al. found that a bilayer comprising ZnO nanoparticles and sol–gel ZnO (ZnO-NP/ZnO) exhibited a lower work function than sol–gel ZnO and improved electron-extracting capability.86 The authors also observed that the combination of the superior crystallinity of ZnO NPs and the smoothness of ZnO contributed to a better overall morphology. The bilayer device with a PBDB-T:ITIC active blend exhibited a PCE of about 12%, higher than that of the single CIL. Sol–gel zinc oxide contains a high density of defects.185 These defects act as recombination centers for photogenerated carriers, leading to reduced performances. To decrease the density of defects, ethylenediamine-N,N,N′N′-tetraacetic acid (EDTA) has been added into the precursor solution along with the conventional complexing agent (ethanolamine).58 Because EDTA is a stronger complexing agent for metal sites than ethanolamine, it coordinates with zinc, thus completely passivating the defects both on the surface and in the bulk. Moreover, EDTA decreases the work function of ZnO (4.05 eV compared to 4.09 eV). The value is closer to the LUMO of IT-M (3.98 eV), thus leading to a higher VOC. As a result, the final PBDB-T:IT-M devices exhibit an increased PCE (11.67% vs. 10.56%). By adding lithium chloride to the zinc oxide precursor solution, a Li-doped ZnO was developed.113 Lithium ions intercalated within the ZnO lattice as dopants, replacing interstitial zinc defects that act as trap states. The increased electron conductivity improves the device performances. The optimum LiCl concentration was 5% w/w. Because zinc interstitial defects are responsible for oxygen adsorption and they promote oxidative degradation,186 the suppression by lithium doping also has a positive effect on device stability. The PCE of PTB7-Th:IT-4F devices with the Li-doped CIL is 81% of the initial value after 1000 h storage in the dark, compared to 60% of the initial value in the case of undoped ZnO. Cheng et al. found that surface modification of zinc oxide with potassium increased the binding energy difference between NFAs and polymer donors compared to untreated zinc oxide.103 As a consequence, a favorable vertical phase separation with the acceptor concentrated near the cathode and the donor concentrated near the anode was induced, resulting in much more efficient charge extraction. Accordingly, the ZnO CIL was treated with a diluted (1 mM) KOH solution and inverted cells with three different active blends (PBDB-T-2Cl:IT-4F, PBDB-T-2Cl:Y1-4F, and PBDB-T-2Cl:Y6) were fabricated. Increased PCE, JSC and FF values were found in all cases, consistently with the favorable vertical phase segregation, which was experimentally demonstrated by means of time-of-flight secondary ion mass spectrometry (TOF-SIMS) measurements.
As discussed in Section 2.4, photodegradation of NFAs occurs at the ZnO interlayer/active layer interface.110,111 For this reason, alternatives to ZnO have been considered. The most obvious is tin oxide (SnO2), because it is less sensitive to UV light due to its wider band gap as compared to ZnO. Tin oxide has been seldom used in OPV devices.187–191 The suitable energy level alignment in ITO/SnO2/PBDB-T:ITIC/MoO3/Ag devices guarantees a driving force for electron collection and improved short-circuit current and power conversion efficiency.192 Jiang et al. studied in detail the photocatalytic effect of ZnO on the durability of solar cells based on the PM6 donor and different NFAs (IT-4F, ITIC and IEICO-4F).111 They observed a slow photodecomposition of the three NFAs deposited on ZnO films upon continuous UV illumination (365 nm, 5 mW/cm2). Correspondingly, the solar devices exhibited poor stability under continuous AM1.5 illumination. Tin oxide is less sensitive than ZnO to UV light due to its wider band gap. Thus, by replacing ZnO with SnO2 (obtained from nanocrystal dispersions) the PCE of PM6:IT-4F devices increased from 12.8% to 13.8% but – more importantly – the loss of performance after 24 h continuous illumination was strongly reduced. In addition, the SnO2 interlayer-based cells showed a negligible dependence on their annealing temperature and film thickness. SnO2 nanocrystal dispersion was also found to be easily printable, with advantages from the point of view of scaleup, as will be discussed in Section 4. The wide band gap of SnO2 and its high transmittance in the region 300–900 nm, combined with the multicolor features of polymer:NFA blends, make the devices based on this material very attractive for semitransparent PV applications. Bai et al. reported multi-colored semitransparent inverted organic solar cells with efficiencies up to 12.9% and 25.6% average visible transmittance (AVT) for a PBDB-T-2F:Y6 device, a performance previously unattained with fullerene based devices.193 Titanium oxide films were prepared from a colloidal precursor solution with an optimal thickness of 10 nm. TOF-SIMS analysis on a PBDTTT-ET:IEICO blend showed that the NFA tends to accumulate on the surface of the active film.128 The LUMOs of titanium(IV) (diisopropoxide) bis(acetylacetonate) (−3.9 eV) and TiO2 (−4.2 eV) are close to that of IEICO (−3.95); thus they are suitable CILs for this active blend, because electrons can be easily injected into the cathode. Nevertheless, inverted PBDTTT-ET:IEICO solar cells exhibited PCEs lower than conventional cells, mainly due to their lower JSC. On the other hand, by using a PBDTBDD:IT-M active layer, a PCE of 11.7% was obtained, much higher than those of conventional devices or inverted devices without CIL.194 In a later study, the authors demonstrated that the surface free energy of a titanium(IV) oxide bis(acetylacetonate) CIL can be tuned by changing the annealing temperature.102 In particular, they found that the surface free energy increases with annealing temperature and achieves an optimum value of 48.7 mJ cm−2 at 90 °C, close to the value of IT-M. Consequently, this titanium(IV) oxide bis(acetylacetonate) film induces a desired vertical component distribution in PBDB-T:IT-M films, facilitating charge transport. PBDB-T:IT-M inverted solar cells reach the best values in terms of VOC, JSC, FF and PCE, compared to devices based on CIL films annealed at other (lower or higher) temperatures.
Vasilopoulou et al. used a vacuum-deposited carbon-doped tantalum dioxyfluoride (TaO2FCx) as a CIL material in ITO/PEDOT-PSS/PM6:IT-4F/TaO2FCx/Al solar cells.195 The proper energy level alignment between TaO2FCx and IT-4F led to a PCE of 12.77%, higher than that of the reference device with perylenediimide amino N-oxide (PDINO) used as an IL. Moreover, the hydrophobic characteristic of TaO2FCx contributes to the improved stability of the device, with a limited loss of efficiency (<10%) after 500 h of exposure to air and UV light. Inverted FTO/TaO2FCx/PM6:IT-4F/MoOx/Al devices were also fabricated, with improvements compared to ZnO-based devices, similar to those observed for conventional solar cells. In the literature there is only one example of bilayer inorganic CILs. Peng et al. developed a SnO2 NP/InP-ZnS QD material which solves the issue caused by the presence of surface defects on the solution-processed SnO2 nanoparticles.196InP-ZnS QDs form a very thin layer (the estimated thickness is less than 10 nm) on top of 40 nm tin oxide. The PM6:Y6 based device with n-SnO2 as CIL shows a low efficiency of 13.86%, along with a poor FF value. After using InP/ZnS QDs to passivate the tin oxide surface, the PCE was remarkably improved to 15.22%, with a FF of around 70%.
| Materiala | Solar cell structure | V OC (V) | FF (%) | J SC (mA cm−2) | PCE (%) | Material in the control device | PCE (%) control device | Ref. |
|---|---|---|---|---|---|---|---|---|
| a —: SAM; /: bilayer. b Maximum values. | ||||||||
| ZnO/twisted perylene | ITO/CIL/TP:PBDTTT-CT/MoO3/Ag | 0.76b | 50b | 11.9b | — (4.6) | ZnO | 3.99 (4.02) | 197 |
| ZnO/PDIN | ITO/CIL/PTB7-Th:B-DPDI/MoO3/Ag | 0.78 | 62 | 13.0 | 6.13 (6.29) | ZnO | 5.23 (5.52) | 199 |
| ZnO–C3 | ITO/CIL/PBDB-T:ITIC/MoO3/Al | 0.89 | 73 | 16.9 | 10.70 (10.90) | ZnO | 9.87 (10.03) | 217 |
| ZnO–ABA | ITO/CIL/PBDB-T:ITIC/MoO3/Al | 0.89 | 72 | 16.6 | 10.47 (10.60) | ZnO | 9.87 (10.03) | 217 |
| ZnO–MoBA | ITO/CIL/PBDB-T:ITIC/MoO3/Al | 0.90 | 72 | 16.4 | 10.50 (10.73) | ZnO | 9.87 (10.03) | 217 |
| ZnO/IDT-BI-H | ITO/CIL/PBDB-T:ITIC/MoO3/Al | 0.90 | 69 | 17.1 | 10.62 (10.65) | ZnO | 9.94 (10.04) | 203 |
| ZnO/IDT-NI-H | ITO/CIL/PBDB-T:ITIC/MoO3/Al | 0.89 | 66 | 16.1 | 9.44 (9.52) | ZnO | 9.94 (10.04) | 203 |
| ZnO/IDTT-BI-H | ITO/CIL/PBDB-T:ITIC/MoO3/Al | 0.90 | 70 | 16.6 | 10.47 (10.53) | ZnO | 9.94 (10.04) | 203 |
| ZnO/IDTT-NI-H | ITO/CIL/PBDB-T:ITIC/MoO3/Al | 0.89 | 66 | 16.4 | 9.68 (9.70) | ZnO | 9.94 (10.04) | 203 |
| ZnO/PNSO3Na | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.87 | 68.7 | 18.7 | 11.2 | ZnO | 9.9 | 206 |
| ZnO/HOOC5 triazole PDIN hex | ITO/CIL/tPDI2N-EH:QX1/MoO3/Ag | 1.04 | 58 | 10.5 | 6.3 (6.4) | ZnO | 4.7 (4.8) | 200 |
| ZnO/HOOC5 triazole PDIN hex | ITO/CIL/PPDT2FBTH:IT-M/MoO3/Ag | 0.91 | 59 | 13.3 | 7.1 (7.2) | ZnO | 6.6 (6.9) | 200 |
| ZnO/HOOC5 triazole PDIN hex | ITO/CIL/PPDT2FBT:tPDI2N-EH/MoO3/Ag | 1.04 | 53 | 11.8 | 6.4 (6.4) | ZnO | 5.8 (6.4) | 200 |
| ZnO–A1 | ITO/CIL/PBDB-T:ITIC/MoO3/Al | 0.88 | 71 | 16.3 | 10.52 (10.59) | ZnO | 9.87 (9.95) | 218 |
| ZnO–A2 | ITO/CIL/PBDB-T:ITIC/MoO3/Al | 0.87 | 71 | 16.0 | 10.16 (10.23) | ZnO | 9.87 (9.95) | 218 |
| ZnO–A3 | ITO/CIL/PBDB-T:ITIC/MoO3/Al | 0.69 | 52 | 15.6 | 5.82 (6.25) | ZnO | 9.87 (9.95) | 218 |
| ZnO–A1 | ITO/CIL/PBDB-T-SF:IT-4F/MoO3/Al | 0.86 | 72 | 20.4 | 12.70 (13.25) | ZnO | 11.51 (12.18) | 218 |
| ZnO/PyM | ITO/CIL/PBDB-T:ITIC/MoO3/Al | 0.90 | 66 | 18.5 | 10.9 | ZnO | 9.9 | 204 |
| ZnO/PyM | ITO/CIL/PBDB-T:IT-M/MoO3/Al | 0.91 | 68 | 18.5 | 11.5 | ZnO | 10.4 | 204 |
| ZnO/APTES | ITO/CIL/PBDB-T:IT-M/MoO3/Al | 0.91 | 69 | 18.4 | 11.21 (11.53) | ZnO | 10.04 (10.19) | 219 |
| ZnO/glycine | ITO/CIL/PM6:IT-4F/MoO3/Al | 0.85 | 75 | 22.0 | 13.8 (14.0) | ZnO | 12.7 (12.9) | 205 |
| ZnO/Py-BDP | ITO/CIL/PM6:IT-4F/MoOx/Al | 0.81 | 70 | 19.8 | 11.22 (11.80) | ZnO | 10.01 (10.41) | 112 |
| ZnO/PBD | ITO/CIL/PBDB-T:IT-M/MoO3/Ag | 0.94 | 74 | 16.7 | 11.3 (11.6) | ZnO | 10.7 (10.8) | 209 |
| ZnO/PMI-TPP | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.88 | 69 | 17.6 | 10.42 | ZnO | 9.49 | 151 |
| ZnO/PMI-TMOPP | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.88 | 65 | 17.3 | 9.87 | ZnO | 9.49 | 151 |
| ZnO/C-PCBSD | ITO/CIL/PBDB-TF:DTC(4Ph)-4FIC/MoO3/Al | 0.94 | 70 | 20.2 | 13.12 (13.36) | ZnO | 12.58 (12.95) | 210 |
| ZnO–C60-SAM | ITO/CIL/PTB7-Th:IEICO-4F/MoO3/Ag | 0.71 | 60 | 22.7 | 9.86 (10.00) | ZnO | 9.30 (9.46) | 80 |
| ZnO/PDPP-FNBr | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.86 | 69 | 15.6 | 9.38 (9.59) | ZnO | 8.92 (9.03) | 222 |
| ZnO/PF-DPPNBr | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.87 | 70 | 16.3 | 9.88 (10.13) | ZnO | 8.92 (9.03) | 222 |
| ZnO/PDPPNBr-FNBr | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.87 | 71 | 16.5 | 10.31 (10.57) | ZnO | 8.92 (9.03) | 222 |
| ZnO/PFN-ox | ITO/CIL/PTB7-Th:IDT-BT-R/MoO3/Ag | 1.02 | 55 | 13.9 | 7.82 (8.17) | ZnO | 9.20 (9.39) | 82 |
| ZnO/PFEO-b-PTNBr | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.87b | 70b | 17.8b | 10.8 | ZnO | 9.7 | 223 |
| ZnO/PFEO-SO3Li | ITO/CIL/PBDB-T:IT-M/MoO3/Al | 0.91 | 68 | 18.6 | 11.6 (11.7) | ZnO | 10.7 (10.9) | 224 |
| ZnO/PEIE | ITO/CIL/PTB7-Th:T2-OEHRH:EH-IDTBR/MoOx/Ag | 1.04 | 64 | 17.90 | 11.89 (12.10) | — | — | 269 |
| ZnO/PFN-Br | ITO/CIL/P(Cl–Cl) (BDD = 0.2):IT-4F/MoO3/Ag | 0.86 | 70 | 20.4 | 12.4 (12.7) | ZnO | 11.9 (12.1) | 105 |
| ZnO/polyfluorene:LD-SWCNTs | ITO/CIL/PM6:Y6/MoO3/Ag | 0.80 | 66 | 17.56 | 9.30 (9.45) | ZnO | 7.77 (7.97) | 85 |
| Polydopamine/ZnO | ITO/CIL/PBDB-T:ITIC/MoO3/Al | 0.88 | 71 | 17.9 | 11.1 | ZnO | 10.2 | 225 |
| Polydopamine/ZnO | ITO/CIL/PBDB-T:ITCC/MoO3/Al | 0.98 | 65 | 16.5 | 10.5 | ZnO | 10.0 | 225 |
| Polydopamine/ZnO | ITO/CIL/PBDB-T:IT-M/MoO3/Al | 0.92 | 67 | 17.6 | 10.9 | ZnO | 10.2 | 225 |
| ZnO/methyl cellulose | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.87 | 67 | 16.6 | 9.62 (9.89) | ZnO:PFN | 9.14 (9.30) | 180 |
| ZnO/methyl cellulose | ITO/CIL/PDCBT:ITIC:P4/MoO3/Ag | 0.91 | 62 | 12.2 | 6.84 (7.11) | ZnO | 5.08 (5.30) | 109 |
| ZnO/methyl cellulose | ITO/CIL/PDCBT:IT-M:P4/MoO3/Ag | 0.91 | 57 | 14.9 | 7.67 (8.04) | ZnO | 6.00 (6.21) | 109 |
| ZnO/carboxymethyl cellulose sodium | ITO/CIL/PBDB-T:IT-M/MoOx/Ag | 0.94b | 72b | 17.9b | 11.96 (12.01) | ZnO | 10.92 (10.97) | 181 |
| SnO2/PeNWs | ITO/SnO2/PeNWs/PBDB-T-SF:IT-4F/MoO3/Ag | 0.82b | 55b | 23.9b | 10.72 | SnO2 | 9.53 | 232 |
| SnO2–ethanolamine | ITO/CIL/PBDB-TF:IT-4F/MoO3/Ag | 0.82b | 74b | 20.5b | — (12.45) | SnO2 | — (10.71) | 221 |
The most widely used CILs in inverted structure PSCs are metal oxides. The most common ones are zinc oxide (ZnO) and tin oxide, usually deposited as nanoparticles (SnO2 NPs). These materials present high electron mobility, suitable energy levels with an ITO electrode and high transparency across the visible spectral range. On the other hand, metal oxides present a high concentration of defect sites and, to passivate these trap states, other materials are used to enhance the metal oxide properties. The passivation of metal oxides’ defects through other materials has been reported over the years; this technique not only allows tuning the properties of the CILs, in terms of both work function and level alignment but also permits modifying the morphology of the active layers atop. All these adjustments bring improvements in device performances. The approaches followed by researchers and scientists for defect passivation are mainly two. The first method involves a multilayered stack of materials; to the conventional metal oxide interlayer is added another layer, forming hetero-materials, multi-layered CILs. With this strategy it's easy to tune the superficial properties of CILs. The second method involves mixing two or more materials, forming blends and yielding one CIL consisting of hetero-materials. With this technique it's easy to modify in a massive way the bulk properties of CILs such as conductivity and carrier selectivity.
3.2.3.1. Bilayer materials. In this section, the first combination discussed is the family of small molecules used as ZnO modifiers. Shivanna et al. investigated the role of a nanopatterned ZnO morphology in fullerene-free PSCs. Thanks to the addition of an acceptor interlayer of twisted perylene (TP) that planarized the CIL and reduced the electron extraction barrier, the authors were able to increase the overall performances by 40% and to modify the work function.197 After this work, thanks to the easy tailoring of rylene molecules and their excellent solubility in water/alcohol solvents, many others succeeded in rylene-based CILs.151,198–200 The chemical engineering and design allowed an easy tuning of the HOMO–LUMO levels, permitting the employment of PDI-based CILs in different active materials. To enhance the figures of merit of a PTB7-Th:B-DIPDI-based solar cell, Yu et al. used a ZnO sol–gel modified with a PDIN layer.199 The energy levels of PDIN perfectly match with the acceptor's ones; the PCEs have been improved mainly with an enhancement of the FF (from 55% to 62%) and a reduction in the sheet resistance (from 8.6 to 4.2 Ω sq2). These results confirm not only the fact that PDIN has good electron-transporting properties but also that PDIN can modify the morphology of the bulk heterojunction atop, favoring the more crystalline face-on orientation in the photoactive layer, as confirmed by GIWAXS analysis. Moreover, the PDIN might induce interfacial doping at the PDIN/B-DIPDI interface as previously reported.201 Other PDI-based CILs are discussed as ZnO modifiers by Abd-Ellah et al., using a N-annulated perylene diimide molecule functionalized with carboxylic acid, (HOOC5-triazole)-PDIN-hex.200 The pendant carboxylic acid moiety has a minimal impact on optical and electrochemical properties but allows the molecule to anchor on the ZnO surface. The multilayer CIL permitted a more suitable electrode's WF modification (from 4.5 to 4.1 eV). Moreover, the presence of the ZnO modifier increased the hydrophobicity that leads to a photoactive layer morphology improvement by inducing favorable vertical phase segregation.41,201,202 These modifications in the device allow improving both the JSC and FF values in the BHJ formed by a quinoxaline-based donor polymer and a PDI-based acceptor material (QX1:tPDI2N-EH). The more promising results are also found with another donor polymer (PPDT2FBT) blended with different acceptor materials (PC61BM, IT-M and tPDI2N-EH) used to prove the versatility and the effectiveness of this CIL. Qin et al. synthetized two perylene monoimide organic phosphonium bromide salts (PMI-TPP and PMI-TMOPP) by the Suzuki reaction with different pendant groups.151 The presence of a ZnO-modified CIL increased the PCEs thanks to both an enhancement of photoconductivity and a more efficient hole injection block. Moreover, the surface tension of the multi-layered CIL is reduced. This reduction helps in the wetting of chlorobenzene during the photoactive coating deposition and it further promotes the morphology quality. Besides rylene-based small molecules, other conjugated small molecules are used in the literature. Wu et al. used J-aggregates acting as ZnO modifiers as CILs. These molecules have an IDT/IDTT core and BI-H/NI-H end groups which tend to self-assemble with hydrogen bonds and with π–π interactions. The conjugated molecules tend to absorb high energy photons and can efficiently transfer the energy to low energy excitons. As a result, the authors found an enhancement in EQE at long wavelengths. Thanks also to the high electron mobility and facile electron extraction, the PCE increased from 9.94% for bare ZnO to 10.62% for ZnO/IDT-BI-H.203 In the second example, Li et al. used a conjugated organic small-molecule (1-pyrenemethanol, PyM) with an extremely simple chemical structure and high solubility in methyl alcohol, which has been used to modify ZnO.204 The presence of hydrogen bonds between PyM and the metal oxide surface restrains the superficial defects. The increased VOC, JSC and FF result in higher PCE. In addition, the authors studied the thickness dependence of PyM by increasing the concentration of the small molecule before deposition and the PCE varied by less than 1% from few to hundreds of nanometers, rendering the CIL thickness insensitive. This aspect plays a key role in the scale-up process (discussed later, in Section 4). To show the effectiveness of this small molecule, two photoactive materials are used (PBDB-T:ITIC and PBDB-T:IT-M). Zhu et al. used another small molecule, glycine, on ZnO to modify the work function (from 4.11 to 4.02 eV) and to increase the built-in electric field, thus improving electron extraction. In fact, the carboxyl group can interact with ZnO, changing the surface properties, while the amino group can tune the interaction between the CIL and active layer. The presence of glycine on ZnO decreases the defect state and facilitates charge extraction. This multi-layered CIL is used in PM6:Y6 blend-based devices, with PCE reaching 13.8%.205
The tailoring of small molecules allowed Zhou et al. to obtain hyperbranched self-assembled small molecule electrolyte (SME), called PNSO3Na, as a ZnO modifier.206 It induces, besides a work function modification, also interfacial dipoles between the CIL and active layer, favoring charge extraction. Other types of small molecules are used aside from SME. Recently, a pyrene bodipy (Py-BDP) was used as a ZnO modifier to suppress the photocatalytic activity in both FA (PTB7-Th:PC71BM) and NFA (PM6:IT-4F)-based PSCs.112 In fact, as deeply explained by Jiang et al.111 and reported in Section 2.4, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds present in the photoactive materials might react with the ZnO under UV light conditions. To mitigate this effect, ZnO modified with Py-BDP has been used, resulting in work function reduction, favoring charge collection, and increasing the PCE value for the NFA-based photoactive material from 10.01% to 11.22%. The authors further studied the photostability of unencapsulated devices and observed a huge drop of performances for the device with bare ZnO with respect to the ZnO/Py-BDP multi-layer CIL. This study perfectly underlines the importance of an inert CIL for long term stability PSCs. An efficient way to improve the conductivity of CILs is to combine ZnO with another electron-transporting material, i.e. 2-(4-tert-butylphenyl)-5-(4-biphenyl)-1,3,4-oxadiazole (PBD). This small molecule based on the oxadiazole is a well-known material in Organic Light Emitting Diodes (OLEDs); this molecule has been used since 1989 and it presents good electron affinity, high carrier mobility and a good charge injection ability.207,208 Even if it is common in OLED application, it is rarely used in other optoelectronic branches. As reported by Fanady et al., the presence of PBD increased the conductivity of the CIL.209 Moreover, it decreased the roughness of the bare ZnO produced by the sol–gel method, rendering the multi-layered CIL more uniform and with a more intimate contact with the photoactive layer. Chen et al. used as an electron transport material a cross-linkable fullerene-based unsaturated compound (C-PCBSD) upon ZnO. For NFA-based PSCs, the structure analyzed is ITO/ZnO/C-PCBSD/PBDB-TF:DTC(4Ph)-4FIC/MoO3/Al, and the multi-layered CIL, being hydrophobic, can block moisture and can minimize the interfacial erosion of the ZnO layer.210 Previously, C-PCBSD has been used for both FA-based PSCs and perovskite-based SCs, achieving improvements in many aspects such as the stability, morphology and final performances of the devices.211,212
O bonds present in the photoactive materials might react with the ZnO under UV light conditions. To mitigate this effect, ZnO modified with Py-BDP has been used, resulting in work function reduction, favoring charge collection, and increasing the PCE value for the NFA-based photoactive material from 10.01% to 11.22%. The authors further studied the photostability of unencapsulated devices and observed a huge drop of performances for the device with bare ZnO with respect to the ZnO/Py-BDP multi-layer CIL. This study perfectly underlines the importance of an inert CIL for long term stability PSCs. An efficient way to improve the conductivity of CILs is to combine ZnO with another electron-transporting material, i.e. 2-(4-tert-butylphenyl)-5-(4-biphenyl)-1,3,4-oxadiazole (PBD). This small molecule based on the oxadiazole is a well-known material in Organic Light Emitting Diodes (OLEDs); this molecule has been used since 1989 and it presents good electron affinity, high carrier mobility and a good charge injection ability.207,208 Even if it is common in OLED application, it is rarely used in other optoelectronic branches. As reported by Fanady et al., the presence of PBD increased the conductivity of the CIL.209 Moreover, it decreased the roughness of the bare ZnO produced by the sol–gel method, rendering the multi-layered CIL more uniform and with a more intimate contact with the photoactive layer. Chen et al. used as an electron transport material a cross-linkable fullerene-based unsaturated compound (C-PCBSD) upon ZnO. For NFA-based PSCs, the structure analyzed is ITO/ZnO/C-PCBSD/PBDB-TF:DTC(4Ph)-4FIC/MoO3/Al, and the multi-layered CIL, being hydrophobic, can block moisture and can minimize the interfacial erosion of the ZnO layer.210 Previously, C-PCBSD has been used for both FA-based PSCs and perovskite-based SCs, achieving improvements in many aspects such as the stability, morphology and final performances of the devices.211,212
When the layer thickness is reduced to the minimum possible physical value and the layer consists of only one molecule, it is called a self-assembled monolayer (SAM). Self-assembled monolayers (SAMs) of polar organic molecules represent an interesting approach for tuning the energetics at the inorganic/organic interface.213,214 SAMs are able to form very thin and highly ordered dipole layers. If they have the appropriate direction, these dipoles can tune the WF of the interlayer material, minimizing the energy barriers for charge extraction/injection.215,216 In general, more efficient charge dissociation and weaker trap-assisted bimolecular recombination are observed. SAM-modified electrodes and interlayers have also been frequently used in F-based PSCs.18 Zhang et al. reported PBDB-T:ITIC inverted solar cells containing ZnO as a CIL with different organic acids on top as SAMs: 3-aminopropionic acid (C3), p-aminobenzoic acid (ABA) and p-methoxybenzoic acid (MoBA).217 Similar PCEs of 10.5–10.7% were measured in all cases, higher than those of the reference cells with bare zinc oxide (about 9.9%). Liu et al. prepared a series of carboxylic acids to modify the ZnO surface: 4-((1,3-dioxo-1,3-dihydro-2H-inden-2-ylidene)methyl)benzoic acid (A1), (E)-4-((3-ethyl-4-oxo-2-thioxothiazolidin-5-ylidene)methyl)benzoic acid (A2) and (E)-4-((2-(dicyanomethylene)-3-ethyl-4-oxothiazolidin-5-ylidene)methyl)benzoic acid (A3).104 These compounds have different electron-withdrawing strengths of terminal groups (A3 > A2 > A1) and both the energy levels and the intramolecular dipoles are modulated accordingly. The PCE values of ITO/CIL/PBDB-T:ITIC/MoO3/Al follow the trend ZnO–A1 > ZnO–A2 > bare ZnO ≫ ZnO–A3 that is governed mainly by the similarity in surface energy between SAM-modified ZnO and ITIC. To test the general applicability of the interfacial approach, ITO/ZnO–A1/PBDB-T-SF:ITIC-4F/MoO3/Ag solar cells were fabricated, achieving a PCE of 12.7% vs. 11.5% of the devices with bare ZnO. Notably, the passivation of the photocatalytic activities of ZnO upon SAM modification led to an improvement of device stability. Xie et al. used (3-aminopropyl)triethoxysilane (APTES) as a SAM on ZnO. They prepared the SAM by dipping the sol–gel ZnO into a dilute solution of APTES, observing an increment of PCE.219 A paper of Xu et al. claims the achievement of 10% PCE and a 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours extrapolated lifetime (T80) for inverted PTB7-Th:IEICO-4F devices with a ZnO:C60-SAM cathode interlayer.80 Surface treatment of zinc oxide with ethanolamine is a well known process used for decreasing the contact barrier for electron transport and extraction.220 The enhanced electron mobility, suppression of bimolecular recombination and reduction of the contact resistance and series resistance lead to an increase of the photovoltaic parameters. Similarly, surface treatment of tin oxide leads to PBDB-TF:IT-4F solar cells with improved performances.221
000 hours extrapolated lifetime (T80) for inverted PTB7-Th:IEICO-4F devices with a ZnO:C60-SAM cathode interlayer.80 Surface treatment of zinc oxide with ethanolamine is a well known process used for decreasing the contact barrier for electron transport and extraction.220 The enhanced electron mobility, suppression of bimolecular recombination and reduction of the contact resistance and series resistance lead to an increase of the photovoltaic parameters. Similarly, surface treatment of tin oxide leads to PBDB-TF:IT-4F solar cells with improved performances.221
The second category discussed is ZnO modified with polymers. The polymers, due to their easy design and facile synthesis, are used massively to modify the properties of the metal oxides in PSCs. There are many papers about the use of conjugated polyelectrolytes82,85,105,222–224 and biopolymers109,180,181,225 used as CILs in NFA-based PSCs.
Tan et al. used different types of self-doped n-type conjugated polyelectrolytes (n-CPEs) based on a diketopyrrolopyrrole (DPP) alternated with fluorene framework.222 The polar groups anchored on the DPP unit led to more efficient electron transfer from polar groups to the backbone, a tunable work function, interfacial interactions and a modification in the conductivity of the CIL. Moreover, as shown by EPR measurement, these materials can be efficiently doped and the formation of dipoles at the interface between the n-CPEs and the active layer allow more efficient charge extraction. Another n-CPE (PFEO-b-PTNBr) was used by Zhou et al. in PBDB-T:ITIC devices. This ZnO modifier is composed of a polyfluorene and polythiophene backbone, quaternary ammonium salt and ethylene oxy polar side chains. The chemical structure permits the modulation of the interfacial contact and the tuning of the morphology of the active layer atop. The major effect on the figures of merit in the device is the increased FF (from 64% to 70%).223 Li et al. used a polyfluorene-based CPE modified with lithium ions (PFEO-SO3Li) as a ZnO modifier.224 The ultraviolet photoelectron spectroscopy technique confirms the energy barrier reduction between the CIL and photoactive layers (PBDB-T:IT-M and PBDB-T:ITIC), which can be explained by the formation of interfacial dipoles, and the lithium ions present may move toward the ZnO layer, passivating the surface defects, increasing the charge transport ability of the CIL. In fact, as previously reported by Cheng et al., the intrinsic surface defects of ZnO are dramatically restrained if Li ions are present.226 Park et al. used a PFN-Br interlayer on ZnO for a multilayered CIL.105 The authors modified the interfacial resistance in the devices both by using a ternary active layer through the insertion of PC71BM in a P(Cl–Cl) (BDD = 0.2):IT-4F blend and by using a conjugated polyelectrolyte (PFN-Br). The first strategy goes behind the scope of this review but the presence of PFN-Br at the interface is treated. PFN-Br creates a better interfacial contact with the active layer and nanoscale phase control over its morphology with a well-dispersed vertical phase, verified by GIWAXS. The possibility to modify the PFN terminal groups led to the possibility to obtain a cross-linkable polyelectrolyte (PFN-ox) as a ZnO modifier for a PTB7-Th:IDT-BT-R based device.82 The proposed CIL improves the morphology quality for the donor/acceptor blend layer, it increases the carrier mobility and it reduces the recombination of charges at the interfaces. Here the PCE increased by 30% with respect to bare ZnO. An innovative pathway for NFA-based PSCs’ CIL modification was proposed by Lee et al. They proposed a mixture of single-walled carbon nanotubes (SW-CNTs) and polyfluorene-based conjugated polyelectrolyte produced by the sonication method on a ZnO layer.85 The CIL has been used for both FA (PTB7-Th:PC71BM) and NFA (PM6:Y6) PSCs. The authors analyzed the light intensity dependence of the performance of the devices to study the possible recombination involved. In common organic semiconductors the JSC is proportional to PSin, where Pin is the power of the incident light and S is the power-law exponent. If S tends to 1, it suggests that free carriers are efficiently swept out and collected at the electrodes prior to recombination; otherwise, if S < 1, it indicates that the limitation of the charge transport is due to bimolecular recombination.227 In fact, the authors reported S values equal to 0.97 and 0.99 for ZnO nanoparticles and ZnO/polyfluorene/SW-CNTs, respectively, underlying the low contribution of bimolecular recombination.
Recently, few papers using biopolymer-based interlayers came out thanks mainly to the cheapness, sustainability and easy processability of some biopolymers. There are two studies reporting on the use of methylcellulose as a ZnO modifier.109,180 The authors showed an improvement of the active layer morphology, resulting in increased FF and VOC values. Moreover, Su et al. introduced poly(3-hexylthiophene) (P3HT)-segment-based block copolymers acting as compatibilizers in the bulk heterojunction. These molecules increased further the thermal stability of the devices. Instead of using the bare methylcellulose as a ZnO modifier, Wu et al. used carboxymethyl cellulose sodium (CMC) obtained from rice straw residues in PBDB-T:IT-M based devices.181 The presence of CMC led to a modification in terms of work function and it increased the absorbance and the interfacial contact. Another biopolymer used in NFA-based PSCs is the polydopamine (PDA), obtained through self-polymerization of dopamine. In a paper of Ahmad et al. for the first time ZnO is located atop the ZnO modifier.225 The authors verified an enhancement in all the figures of merit of the devices even with a different geometry (ITO/polydopamine/ZnO/active layer/MoO3/Al structure). The enhancement is in part due to the suitable electron transfer between PDA and ZnO, underlying the presence of a doping effect induced by the presence of the biopolymer. The strategy has been demonstrated for three different blends as photoactive materials (PBDB-T:ITIC, PBDB-T:ITCC, PBDB-T:IT-M).
As already discussed in Section 2.4, despite the impressive optoelectronic properties of ZnO, it suffers from instability under UV light228 and it intrinsically presents defects.229–231 For these reasons, other metal oxides have been used as CILs in NFA-based PSCs. One of the most relevant is tin oxide (SnO2). Besides being used as a bare CIL, discussed in Section 3, SnO2 has been coupled with perovskite-based nanowires (PeNWs).232 The investigated structure is ITO/SnO2/PeNWs/PBDB-T-SF:IT-4F/MoO3/Ag. The presence of the low-dimensional structure between the metal oxide and the active layer enhanced the exciton dissociation and the absorption ability.
3.2.3.2. Mixed/composite materials. In this section composite materials used as CILs are discussed (Table 10).
| Material | Solar cell structure | V OC (V) | FF (%) | J SC (mA cm−2) | PCE (%) | Material in the control device | PCE (%) control device | Ref. |
|---|---|---|---|---|---|---|---|---|
| ZnO:HO-PBI-C12 | ITO/CIL/PBDB-T-2Cl:IT-4F/MoO3/Al | 0.86 | 76 | 20.4 | 13.26 (13.30) | ZnO | 12.04 | 198 |
| ZnO:HO-PBI-C12 | ITO/CIL/PBDB-T-2Cl:IT-4F/MoO3/Al | 0.85 | 76 | 20.2 | 12.99 (13.17) | ZnO | 12.04 | 198 |
| ZnO:HO-PBI-iC7 | ITO/CIL/PBDB-T-2F:Y6/MoO3/Al | 0.83 | 75 | 25.3 | 15.73 (15.95) | ZnO | 14.87 | 198 |
| ZnO:APTES | ITO/CIL/PBDB-T:ITIC/MoO3/Ag | 0.89 | 65 | 17.7 | 10.2 (10.3) | ZnO | 9.6 (9.7) | 234 |
| ZnO:PBI:SO3H | ITO/CIL/PBDB-T-2Cl:IT-4F/MoO3/Al | 0.85 | 72 | 21.6 | 13.32 (13.6) | ZnO | 11.16 (11.4) | 233 |
| ZnO:PBI:SO3H | ITO/CIL/PM6:Y6/MoO3/Al | 0.84 | 73 | 24.7 | 15.20 (15.4) | ZnO | 13.97 (14.2) | 233 |
| ZnO:Eu(TTA)3Phen | ITO/CIL/PBDB-T-2F:IT-4F/MoO3/Ag | 0.76 | 65 | 17.5 | 8.85 (9.01) | ZnO | 7.91 (8.09) | 236 |
| ZnO:PFN-Br | ITO/CIL/PBDB-T:IT-4F/MoO3/Al | 0.87 | 79 | 20.02 | 13.62 (13.82) | ZnO | 12.26 (12.56) | 235 |
| ZnO:bipyramidal-Au | ITO/CIL/PBDTBDD:ITIC/MoO3/Al | 0.88 | 66 | 18.9 | 10.88 | ZnO | 9.09 | 237 |
| ZnO:bipyramidal-Au | ITO/CIL/PBDB-TF:IT-4F/MoO3/Al | 0.85 | 72 | 22.4 | 13.67 | ZnO | 11.86 | 235 |
| Alq3:QPhPBr | ITO/PEDOT:PSS/PBDB-T:ITIC-Th/CIL/Al | 0.86 | 70 | 18.4 | 10.83 (11.05) | w/o CIL | 3.95 (4.38) | 159 |
| Phen-NaDPO:Sn(SCN)2 | ITO/PEDOT:PSS/PM-6:IT-4F/CIL/Al | 0.87 | 76 | 20.0 | 13.2 (13.5) | Phen-NaDPO | 12.2 (12.6) | 238 |
Wen et al. adopted a strategy to mix ZnO with a perylene bisimide dye modified with hydroxy functional groups in the bay position; the interaction between the OH-group and Zn2+ ions present in sol–gel ZnO films permits photosensitizing the semiconducting material.230 By adding the HO-PBI into the zinc solution, the obtained layer is a homogenous composite material with the coexistence of both materials. The authors used the innovative CIL for both FA- and NFA-based PSCs but observed major improvements for NFA-based PSCs.198 The same approach is shown by Luo et al. They modified a perylene bisimide dye with benzenesulfonic acid functional groups. In this way, the authors increased the solubility in water and the possibility for these groups to create bonds with ZnO. The obtained CIL presents a higher conductivity and an increased charge carrier selectivity.233 A similar defect passivation occurs also if 3-aminopropyltriethoxysilane (APTES) is added to ZnO NPs thanks to the formation of covalent and hydrogen bonds between the two materials.234 Differently from the previously reported work,105 Zheng et al. proposed a method to adjust the surface free energy of ZnO nanoparticles by blending different proportions of PFN-Br. The most efficient composite material obtained presents a surface free energy of 63.89 mN m−1, corresponding to a ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFN-Br ratio equal to 90
PFN-Br ratio equal to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. This composite material is used as a CIL in an ITO/ZnO:PFN-Br/PBDB-T:IT-4F/MoO3/Al device structure. Besides increased device performances the stability is also affected; the enhanced stability is mainly due to the lower degree of aggregation of ZnO nanoparticles when blended with PFN-Br, as the authors clearly show in TEM images.235 Generally, an efficient approach to increase the PCE is to absorb a wider range of wavelengths. Bu et al. blended ZnO with a lanthanide-based down-converter, Eu:(TTA)3Phen.236 This material can absorb UV radiation and re-emit photons with an energy that well matches with that of PBDB-T-2F (used as a donor) blended with IT-4F (used as an acceptor) in the active material of the device. This strategy permitted effectively enhancing the portion of radiation absorbed in the UV region and thus increasing the PCE. Moreover, the absorbance of the UV wavelength by the Eu-based complex permitted increasing the stability of the device under irradiation and inhibiting the photodegradation of ZnO and the active layer, as frequently happens with the metal oxides (see Section 2.4). To increase the portion of NIR photoresponse, core–shell nanoparticles have been used as the CIL.237 The core is formed by bipyramid-Au nanoparticles and the shell is composed of ZnO. The devices take advantage of the plasmonic effect of sharp-edged metal nanoparticles, increasing NIR region absorption, while the shell has an excellent electron collection ability. Thanks to the core–shell CIL, the JSC of the devices is increased. A completely different approach has been used by other authors.139,184,238 In these studies, the proposed CILs are not used as metal oxide modifiers but they are composite materials used in conventional structure PSCs (ITO/PEDOT:PSS/active layer/CIL/Al) atop the active layer.159 Here the CIL is composed of a mixture of aluminum tris(8-hydroxyquinolinate) (Alq3) and tetraphenylphosphonium bromide (QPhPBr) deposited by a solution-processing technique; in this CIL inter-ionic interactions are verified and the formation of dipoles between the active layer and metal cathode favors the charge extraction ability. Finally, the composite material used as a CIL is formed by organic 3-[6-(diphenylphosphinyl)-2-naphthalenyl]-1,10-phenanthroline (Phen-NaDPO) and inorganic tin(II) thiocyanate (Sn(SCN)2) small molecules.238 The synergetic effect and the chemical interactions between the two different molecules led to enhancement in device performances and to significant narrowing in the spread of cell parameters thanks mainly to the reduction of trap-assisted recombination across the active layer and CIL interface.
10. This composite material is used as a CIL in an ITO/ZnO:PFN-Br/PBDB-T:IT-4F/MoO3/Al device structure. Besides increased device performances the stability is also affected; the enhanced stability is mainly due to the lower degree of aggregation of ZnO nanoparticles when blended with PFN-Br, as the authors clearly show in TEM images.235 Generally, an efficient approach to increase the PCE is to absorb a wider range of wavelengths. Bu et al. blended ZnO with a lanthanide-based down-converter, Eu:(TTA)3Phen.236 This material can absorb UV radiation and re-emit photons with an energy that well matches with that of PBDB-T-2F (used as a donor) blended with IT-4F (used as an acceptor) in the active material of the device. This strategy permitted effectively enhancing the portion of radiation absorbed in the UV region and thus increasing the PCE. Moreover, the absorbance of the UV wavelength by the Eu-based complex permitted increasing the stability of the device under irradiation and inhibiting the photodegradation of ZnO and the active layer, as frequently happens with the metal oxides (see Section 2.4). To increase the portion of NIR photoresponse, core–shell nanoparticles have been used as the CIL.237 The core is formed by bipyramid-Au nanoparticles and the shell is composed of ZnO. The devices take advantage of the plasmonic effect of sharp-edged metal nanoparticles, increasing NIR region absorption, while the shell has an excellent electron collection ability. Thanks to the core–shell CIL, the JSC of the devices is increased. A completely different approach has been used by other authors.139,184,238 In these studies, the proposed CILs are not used as metal oxide modifiers but they are composite materials used in conventional structure PSCs (ITO/PEDOT:PSS/active layer/CIL/Al) atop the active layer.159 Here the CIL is composed of a mixture of aluminum tris(8-hydroxyquinolinate) (Alq3) and tetraphenylphosphonium bromide (QPhPBr) deposited by a solution-processing technique; in this CIL inter-ionic interactions are verified and the formation of dipoles between the active layer and metal cathode favors the charge extraction ability. Finally, the composite material used as a CIL is formed by organic 3-[6-(diphenylphosphinyl)-2-naphthalenyl]-1,10-phenanthroline (Phen-NaDPO) and inorganic tin(II) thiocyanate (Sn(SCN)2) small molecules.238 The synergetic effect and the chemical interactions between the two different molecules led to enhancement in device performances and to significant narrowing in the spread of cell parameters thanks mainly to the reduction of trap-assisted recombination across the active layer and CIL interface.
4. Scale-up and challenges
Although in the last couple of years OSCs have achieved remarkable efficiencies (over 18%) and improved stability at the laboratory scale, a significant performance gap still exists with large area modules.239 The highest reported efficiencies to date are 11.7% (certified)240 for mini-modules and 7.32% for small area R2R printed cells.241 Unless (and until) the existing gap is filled, the OPV technology will not be able to fully demonstrate its enormous potential of development and widespread application.242 Scaling up lab-scale devices requires a change of paradigm:243 materials, processes and device architectures should be in part re-designed to meet the criteria for the transfer to the industrial context. In particular, (i) low cost/synthetically accessible polymer donors244 and non-fullerene acceptors13,245 should be developed; (ii) materials should be formulated in non-hazardous solvents and processable at low temperatures; (iii) deposition techniques compatible with R2R production should be used (Fig. 13);246 and (iv) the module layout should be designed in such a way to minimize electrical and optical losses and to maximize the geometrical fill factor.247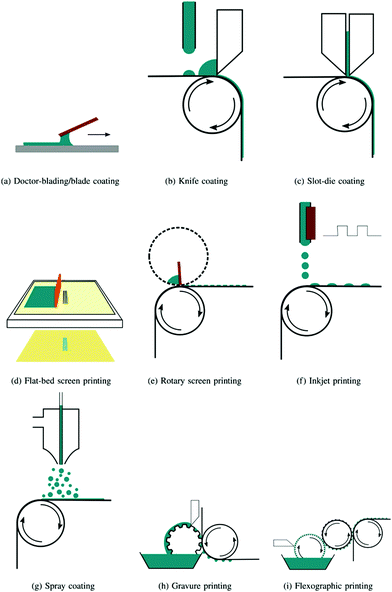 | ||
| Fig. 13 Overview of partly or fully scalable deposition techniques (reprinted with permission from ref. 246. Copyright 2020 The Authors). | ||
From these points of view, the availability of suitable cathode and anode interfacial materials is a key factor. However, only a relatively limited number of studies address the aspect of scalability (Table 11). In the following section we will review the literature on NFA-based solar cells and modules where at least one interlayer is fabricated with partly (doctor blade coating, wire bar coating, D-bar coating, spray coating) or fully (slot die coating and gravure printing, in either a sheet-to-sheet or roll-to-roll fashion) scalable deposition techniques. First of all, AIL materials will be discussed; then CIL materials will be reviewed.
| Architecture | Cell or module | Area (cm2) | CIL deposition | AL deposition | AIL deposition | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Glass/ITO/ZnO np/PEIE/PCDB-T:ITIC/MoO3/Ag | Cell | n.d. | Slot die coating | Slot die coating | Evaporation | 9.76 | 270 |
| Glass/ITO/SnO2 nc/PM6:IT-4F/MoO3/Ag | Cell | 0.04 | Slot die coating | Doctor blade coating | Evaporation | 12.5 | 111 |
| Glass/ITO/PEDOT:PSS/PTB7-Th:FOIC/PDINO/Al | Cell | 0.048 | Electrospray | Electrospray | Ultrasonic spray | 8.34 | 262 |
| Glass/ITO/ZnO/PBDB-T:IT-M/MoOx/Ag | Cell | 0.09 | Doctor blade coating | Doctor blade coating | Doctor blade coating | 10.15 | 257 |
| Plastic/ITO/ZnO/PTB7-Th:PC71BM:COi8DFIC/MoO3/Ag | Cell | 0.14 | R2R gravure printing | R2R slot die coating | Evaporation | 9.57 | 266 |
| PET/Ag/PEDOT:PSS (PH1000)/ZnO/PTB7-Th:IEIC/PEDOT:PSS/Ag | Cell | 0.66 | — | Slot die coating | Slot die coating | 1.26 | 255 |
| PET/ITO/ZnO/PTB7-Th:IEIC/PEDOT:PSS/Ag | Cell | 0.98 | Slot die coating | Slot die coating | Slot die coating | 1.79 | 255 |
| PET/PEDOT:PSS (PH1000)/ZnO/P3HT:F(DPP)2B2/PEDOT:PSS (F010)/PEDOT:PSS (4083)/Ag grids | Cell | 1 | — | Slot die coating | Slot die coating | 0.65 | 254 |
| Glass/ITO/s-MoOx/PB3T:IT-M/PFN-Br/Al | Cell | 1 | Spin coating | Spin coating | Wire bar coating | 11.9 | 131 |
| Glass/ITO/SnO2 nc/PM6:IT-4F/MoO3/Ag | Cell | 1 | Slot die coating | Doctor blade coating | Evaporation | 11.5 | 111 |
| Glass/ITO/PEDOT:PSS/PBDB-T-2F:IT-4F/NDI-N/Al | Cell | 1 | Doctor blade coating | Doctor blade coating | Doctor blade coating | 13.2 | 97 |
| PET/ITO/ZnO np/PEIE/PCDB-T:ITIC/MoO3/Ag | Cell | 10 | R2R slot die coating | R2R slot die coating | Evaporation | 7.11 | 270 |
| Glass/ITO/ZnO/TPD-3F:IT-4F/PEDOT:PSS/Ag | Module | 20.4 | Doctor blade coating | Doctor blade coating | Doctor blade coating | 6.77 | 249 |
| Plastic/ITO/ZnO/PTB7-Th:PC71BM:COi8DFIC/MoO3/Ag | Module | 30 | Slot die coating | Slot die coating | Evaporation | 8.6 | 266 |
| Glass/ITO/ZnO np/PEIE/PTB7-Th:EH-IDTBR:T2-OEHRH/MoOx/Ag | Module | 55.5 | D-bar coating | D-bar coating | Evaporation | 9.32 | 269 |
| Glass/ITO/ZnO np/P3HT:o-IDTBR/PEDOT:PSS/Ag nw | Module | 60 | Doctor blade coating | Doctor blade coating | Doctor blade coating | 5 | 265 |
| Glass/ITO/ZnO np/P3HT:o-IDTBR/PEDOT:PSS/Ag nw | Module | 60 | Slot die coating | Slot die coating | Slot die coating | 4.4 | 265 |
| PET/ITO/ZnO/SMD2:ITIC-Th/PEDOT:PSS/Ag | Module | 80 | R2R slot die coating | R2R slot die coating | R2R slot die coating | 2.02 | 256 |
| PET/ITO/ZnO/SMD2:ITIC-Th/WO3/PEDOT:PSS/Ag | Module | 80 | R2R slot die coating | R2R slot die coating | R2R slot die coating | 5.25 | 256 |
4.1. Anode interlayers
For devices fabricated with laboratory techniques (spin coating and vacuum evaporation), the anodic buffer layer consists typically of PEDOT:PSS in conventional solar cells or evaporated molybdenum oxide (MoO3, or e-MoOx) in inverted solar cells, which is the preferred configuration for scaling-up.246,248An example of a conventional device, where PEDOT:PSS was deposited by doctor blade coating, was reported by Kang et al.97 They demonstrated a PBDB-T-2F:IT-4F solar cell (1 cm2) with 13.2% efficiency, close to the value of the reference 3.7 mm2 spin coated device (13.9%). An interesting printable alternative to PEDOT:PSS reported in the literature is an ink based on ammonium heptamolybdate dissolved in water/ethylene glycol solution (s-MoOx).131 The optimization of the AIL thickness was done on spin coated cells. In the scaled devices (1 cm2), the interlayer was deposited by wire bar coating and incorporated in ITO/s-MoOx/PB3T:IT-M/PFN-Br/Al solar cells. A power conversion efficiency of 11.9% was obtained, which is higher than the value measured for the PEDOT:PSS device (11.3%). However, the annealing conditions necessary to transform ammonium heptamolybdate into MoOx (200 °C for 20 minutes) make this interlayer material unsuitable for high-throughput processes on plastic substrates.
Moving on to the inverted architecture, the overwhelming majority of publications on laboratory-scale devices report e-MoOx as AILs (combined with an evaporated silver anode), thanks to their good hole transport properties and energy level matching with common donor polymers. In a much smaller number of papers, spin-coated PEDOT:PSS aqueous suspensions are used. In the latter case, results compared to e-MoOx are often disappointing, independently of the type of active material. With the exception of the P3HT:PCBM active blend, significant losses of performances are observed for other active systems.135,249–253 In detail, by substituting e-MoOx with PEDOT:PSS, Jeon et al. observed a decrease of PCE from 7.81% to 2.7% in ITO/ZnO/PTB7:PC71BM/AIL/Ag spin coated devices.251 For ITO/ZnO/PBN-S:IT-4F/AIL/Ag spin coated solar cells the decrease was even more evident, from 11.02% to 3% and lower.250
As for NFA-based inverted devices with AILs processed by R2R compatible techniques, the very first example dates back to 2015.254 The P3HT:F(DPP)2B2 active layer and the PEDOT:PSS anode interlayer were deposited on a commercial PET/high conductivity PEDOT:PSS/ZnO ITO-free electrode, leading to a maximum PCE of 0.65%, with a very low FF and JSC. This result was due in part to the low performing donor (P3HT) and acceptor (an A–D–A compound prior to the development of highly performing NFAs). However, the main reason for the poor performances can be very likely attributed to the PEDOT:PSS used as an AIL, in spite of the fact that an additional PEDOT:PSS layer (a different grade) was used to improve the compatibility at the interface. In the following study,255 where the more performing PTB7-Th donor and IEIC acceptor were used, 1.8% PCE was obtained for an ITO-free device and over 2.2% for a cell with an ITO electrode, values that remain unsurprisingly low. Nevertheless, these two studies represent a milestone for the demonstration of the potential of non-fullerene acceptors for large scale production of flexible devices.
More recently, Strohm et al. reported the fabrication of 60 cm2 P3HT:O-IDTBR modules with ZnO nanoparticles and PEDOT:PSS interfacial layers. These devices exhibited 5% and 4.4% efficiency for blade coating and slot die coating deposition, respectively. Notably, using a green solvent, o-methylanisole, instead of a chlorobenzene:bromoanisole mixture, a respectable PCE of 4.7% was obtained. These performances are the result of a careful optimization of the ink formulation based on the Hansen solubility parameter evaluation to select the best solvent candidates, with the right boiling temperature to ensure the appropriate drying rate. Liao et al. examined in detail the factors leading to the decrease of performances for ITO/ZnO/TPD-3F:IT-4F/AIL/Ag solar cells fabricated under scalable conditions.249 When they used e-MoOx in spin coated solar cells, they obtained a PCE of 13.8%. By changing the AIL to PEDOT:PSS, a 5.88% PCE was measured, and the authors attributed the drastic decrease to the energy-level mismatch between the AIL (−5.0 eV) and the HOMO level of the donor polymer (−5.62 eV). By using a modified PEDOT:PSS with a deeper Fermi level (−5.2 eV), the PCE was partially recovered (10.6%). At the end, a blade coated 20.4 cm2 module with 6.77% PCE was fabricated.
A further demonstration of the scaling-up issues caused by PEDOT:PSS was provided by Han et al.256 SMD2:ITIC-Th inverted modules consisting of 10 series interconnected cells were fabricated by roll-to-roll slot die coating of the active and interlayers, and screen printing of the silver anode. The module with PEDOT:PSS alone exhibited a PCE of about 2%, while a WO3/PEDOT:PSS bilayer led to an improved efficiency of 5.25%. Guo et al. fabricated small-area PBDB-T:IT-M solar cells using a doctor bladed molybdenum oxide anode interlayer.257 They obtained a remarkable efficiency above 10%, using an evaporated silver anode. However, in a fully scaled process, thick anode interlayers are needed to prevent the penetration of silver ink solvent into the photoactive layer, which would have deleterious consequences on the final performances.258 In fact, in typical R2R processes the thickness of the anode interlayer is hundreds of nanometers259–261 to guarantee a good protection. Therefore, the practical integrability of a simple molybdenum oxide layer in a production process needs to be demonstrated, because the thickness is not reported by the authors.
Other anode interlayer materials processable with scalable techniques are reported in the literature that, in perspective, could be good candidates for efficient NFA-based solar cells. One example is the polyoxometallate salt/PEDOT:PSS double layer successfully tested in slot die coated PV2001:fullerene devices.135 In perspective, the PEDOT:PSS layer should provide the protection of the active layer from silver ink solvents. The obtained efficiencies (7.2%) were even higher than those of solar cells with evaporated molybdenum oxide (6.7%). Another potential anode interlayer material, described in Section 3.1.2, which has the potential to be printed consists of molybdenum or tungsten sulfides.137
4.2. Cathode interlayers
As discussed in the previous paragraphs, the prototypical CIL materials used in laboratory devices are PFN or PFN-Br in conventional cells, and ZnO in inverted SCs.Starting with conventional devices, the very low thickness needed by PFN and PFN-Br makes the deposition difficult with techniques different from spin coating. N,N-(Dimethylamino)propyl naphthalene diimide (NDI-N) is a compound with high crystallinity and good film-forming properties at the same time.97 In addition, NDI-N exhibits an outstanding tolerance to thickness variation in the fabrication of thin film devices. When the NDI-N thickness changed from 5 to 30 nm, the PCE only decreased from 13.5% to 10.2% (11.6% for a thickness of 30 nm) in small area devices. Noticeably, the fill factor maintained a high value of 74% at 50 nm thickness. For these reasons, NDI-N is a good candidate as a printable CIL, and in fact a 1 cm2 doctor bladed coated ITO/PEDOT:PSS/PBDB-T-2F:IT-4F/NDI-N/Al solar cell exhibited an outstanding efficiency of 13.2%. Aminopropyl (PDIN) and aminopropyl N-oxide (PDINO) perylene diimides have similar thickness-insensitive electrical properties,42 thus representing good candidates as CILs for large area devices. Indeed, the coating of PDINO has been recently reported by Chang et al.262 They fabricated ITO/PEDOT:PSS/PTB7-Th:FOIC/PDINO/Al small area cells, achieving a 8.34% PCE, by electrospraying the CIL (10 nm) and the active layer, after careful optimization of the solvent systems to tune the evaporation times. The PEDOT:PSS layer (35 nm) was deposited by ultrasonic spraying. Exactly as in spin coated solar cells, zinc oxide is widely used in the fabrication of printed inverted devices, based on both fullerene261,263,264 and non-fullerene acceptors.249,255–257,265,266 The advantages of zinc oxide are that it can be very easily printed at the desired thickness using different techniques (among others, slot die coating,256 gravure printing267 and flexographic printing268) and it is available in the form of either nanoparticle dispersions or sol–gel precursor solutions. Its WF and adhesion properties can be tuned using a thin layer of PEIE on top269,270 and it is relatively cheap.
As discussed in Section 2.4, however, both zinc oxide and PEIE exhibit issues of stability due to undesired side reactions with the NFAs. Moreover, the low thicknesses required by PEIE could be challenging on large areas. The most promising alternative to ZnO is SnO2, used also previously in printed F-based SC devices.191 Nevertheless, the literature on printed inverted NFA devices with CILs is still scarce. Predictably, the number of studies on this subject will increase in the near future. The only paper, to date, where the use of slot die coated tin oxide is demonstrated describes ITO/SnO2/PM6:IT-4F/MoO3/Ag solar cells.111 A small area (0.04 cm2) solar cell exhibited a VOC value of 0.84 V, a JSC value of 20.3 mA cm−2 and a FF of 73%, with an efficiency of 12.5%, which slightly decreased to 11.5% upon increasing the area to 1 cm2.
5. Conclusions and perspectives
Thanks to their high efficiencies, low cost and easy fabrication, NFA-based PSCs have gained growing interest in recent years. An increasing number of materials used as interfacial layers have been tested and clarity has necessarily been achieved. In this review we have discussed the impact of interfacial layers on the performances of NFA-based PSCs, clarifying, where possible, the specific features and the working behavior mechanisms of the IL in the device. Diverse interfacial engineering approaches have been used to enhance the charge collection in NFA-PSCs and the different features to the FA counterparts are highlighted. This implies that distinctive solutions are needed to attain efficient interlayers for NFA-PSCs. NFA based devices have more critical issues with regard to the effectiveness of interlayer doping, as compared to FA-based devices, where such an effect is known to reduce the contact resistance and to enhance the interlayer conductivity. Consequently, special care should be given to the NFA/interlayer energy level alignment and to the enhancement of the interlayer's electron transport properties. Still, it should also be considered to enhance the interfacial contact as well as the probability to transfer electrons between the NFA and IL. Comprehensive studies are also needed to improve the understanding about the interaction between NFAs and IL materials. Not only n-doping, but also chemical reactions could eventually occur, as shown for PEIE interlayers. Moreover, the ILs can work as the active layer's morphology modifier, favoring a vertical distribution of the NFA in the photoactive blend. All these specific behaviors are able to tune the performances of the solar cell devices.Fig. 14 shows a meta-analysis of the literature discussed in this review. In conventional architecture devices, having PEDOT:PSS as a reference material, inorganic and hybrid AILs are more effective than organic ones (Fig. 14a). Hybrid AILs are on average slightly better performing than inorganic AILs. This confirms that the opportunity of combining the properties of two different materials is advantageous and represents a research line that should be further explored. For CIL materials, the reference devices were those having calcium or nothing as the IL, because most of the references report this type of comparison (Fig. 14b). In this case, the PCE increments are higher, as expected, and no substantial differences in trends exist among the various classes. Considering that the different active layers play their role in determining the final PCE, however, one can note that n-type dopants afford better performing devices than small molecules that, in turn, on average behave better than polymeric ILs.
 | ||
| Fig. 14 Power conversion efficiency variations in NFA polymer solar cells: (a) conventional devices with different anode interlayers as the substitutes of PEDOT:PSS (data taken from Tables 1–3); (b) conventional devices with different cathode interlayers compared to devices without interlayer (data taken from Tables 4–6); and (c) inverted devices with different cathode interlayers as the substitutes of zinc oxide (data taken from Tables 4–10). Data refer to average values, unless the original references provide the maximum values only. | ||
As for inverted devices (the most suited for scaling up), there are only a few papers reporting innovative AIL materials, very likely due to the fact that it is very difficult to find convenient alternatives to evaporated molybdenum oxide, as discussed in Section 4. However, in consideration of the development of the technology toward large-area, R2R printed modules, this is an area where investigation should be considered a priority. Compared to zinc oxide, one can see that, apart from organics, novel CIL materials provide moderate increments of efficiencies (Fig. 14c), with no visible distinctions between inorganics and hybrids. However, the devices with the highest PCE values appear to be those containing composite hybrid materials. In order to guarantee a smooth processability, both AIL and CIL materials should be thickness-insensitive. This is especially true for the AIL materials in inverted solar cells which have the function to protect the active layer from the solvents contained in the anodic silver ink, when fully printed devices are involved.258–261
Besides the performances, another key aspect to consider is the stability of the devices upon irradiation, which is probably more important than efficiency for commercialization. In fact, a durable module with moderate efficiency is by far preferable to a module which loses to sun exposure its performances in a few months, if not weeks. NFAs, as recent materials, have not been studied intensively in this aspect in combination with novel IL materials: just twenty papers listed in Table 12 report a value about the stability of the device. To fill this gap, in the next few years, systematic studies on the stability of different materials and device architectures are surely needed and urgent to highlight the bright aspects of this technology. Moreover, because a range of different aging conditions are used to evaluate the stability, standard protocols should be desirably applied by the R&D community.271,272
| AIL or CIL | Device | Ageing conditions | Ageing time | Efficiency retention (%) | Control device | Ref. |
|---|---|---|---|---|---|---|
a Extrapolated T80 lifetime: 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours (control device with ZnO: 2500 h). 000 hours (control device with ZnO: 2500 h).
|
||||||
| n-SnO2/InP/ZnS QDs | ITO/CIL/PM6:Y6/MoO3/Ag | Nitrogen | 100 h | >80 | PEDOT:PSS: 20%, after 20 h | 196 |
| PEDOT:PSS/Ti3C2TX | ITO/AIL/PBDB-T:ITIC/PFN-Br/Al | Nitrogen, r.t. | 300 h | 79 | PEDOT:PSS: 69% | 142 |
| P1PNO | ITO/PEDOT:PSS/J71:ITIC/CIL/Al | Nitrogen | 400 h | ∼85 | PDINO: ∼50% | 148 |
| NiOx | ITO/AIL/PBDTTT-ET:IEICO/PFN-Br/Al | Nitrogen | 16 days | 85 | PEDOT:PSS: 70% | 128 |
| ZnO np:PFN-Br | ITO/CIL/PBDB-TF:IT-4F/MoO3/Al | Nitrogen | 30 days | ∼90 | ZnO: ∼80% | 235 |
| Ti(i-OPr)2(acac)2 | ITO/CIL/PBDTBDD:IT-M/MoO3/Al | Nitrogen | 1440 h | 85 | None: 71% | 194 |
| ZnO/PFEO-SO3Li | ITO/CIL/PBDB-T:IT-M/MoO3/Al | Nitrogen | 250 days | ∼89 | ZnO: ∼85% | 224 |
| ZnO:Li | ITO/CIL/PTB7-Th:IT-4F/MoOx/Al | Desiccator | 1000 h | 81 | ZnO: 60% | 113 |
| Poly-L-lysine + poly-D-lysine | ITO/CIL/PBDBT:ITIC/MoO3/Ag | 60 °C | 400 h | 80 | Poly-L-lysine: 73%; none: 60% | 183 |
| ZnO/C60-SAM | ITO/CIL/PTB7-Th:IEICO-4F/MoO3/Ag | 85 °C | 168 h | ∼98 | ZnO: ∼98% | 80 |
| Alq3:QPhPBr | ITO/PEDOT:PSS/PBDB-T:ITIC-Th/CIL/Al | Air | 2 h | 86 | Alq3: 82%; QPhPBr: 75%; none: ∼40% | 159 |
| α-In2Se3 | ITO/AIL/PBDB-T:ITIC/Ca/Al | Air | 48 h | 51 | PEDOT:PSS: 4% | 138 |
| POE | ITO/ZnO/PM6:IT-4F/AIL/Ag | Air, 70% RH | 150 days | 81 | MoO3: 71% | 77 |
| PCBM:TMHT | ITO/CIL/PM6:IT-4F/MoO3/Ag | White LED, 78 mW cm−2 | 100 h | 93 | ZnO: 90% | 145 |
| ZnO/C60-SAM | ITO/CIL/PTB7-Th:IEICO-4F/MoO3/Ag | LED, 100 mW cm-2 | 2000 h | ∼99a | ZnO: ∼88% after 1800 h | 80 |
| ZnO/Py-BDP | ITO/CIL/PM6:ITIC-4F/MoOx/Al | AM 1.5G, 100 mW cm−2 | 9 h | 66 | ZnO: 24% | 112 |
| PCBM:TMHT | ITO/CIL/PM6:IT-4F/MoO3/Ag | AM 1.5G, 100 mW cm−2 | 100 h | 53 | ZnO: 22% | 145 |
| Poly-L-lysine + poly-D-lysine | ITO/CIL/PBDBT:ITIC/MoO3/Ag | AM 1.5G, 100 mW cm−2 | 400 h | 83 | Poly-L-lysine: 70% | 183 |
| TaO2FCx | FTO/CIL/PM6:IT-4F/MoOx/Al | UV light, ambient conditions | 240 h | ∼92 | ZnO: 10% after 140 h | 195 |
| TaO2FCx | ITO/PEDOT:PSS/PM6:IT-4F/CIL/Al | UV light, ambient conditions | 240 h | ∼90 | PDINO: ∼58% | 195 |
| ZnO/APTES | ITO/CIL/PBDB-T:ITIC/MoO3/ITIC | Unspecified conditions | 10 days | 88 | ZnO: ∼80% | 234 |
| SnO2/PeNWs | ITO/CIL/PBDB-T-SF:IT-4F/MoO3/Ag | Unspecified conditions | 20 days | ∼90 | SnO2: ∼75% | 232 |
While non-fullerene acceptors have boosted the efficiencies of organic solar cells, OPV technology is at a crossroads. To succeed and achieve a large diffusion, and not to remain a laboratory curiosity, the development and implementation of effective (i.e., efficient, stable, processable and cheap) active and ancillary OPV materials is an urgent matter.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
R. S. and S. L. acknowledge ENI s.p.a. (Italy) for financial support (grant no. 3500047796).References
- J. J. M. Halls, C. A. Walsh, N. C. Grenham, E. A. Marseglia, R. H. Friend, S. C. Moratti and A. B. Holmes, Nature, 1995, 375, 498–500 CrossRef.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789–1791 CrossRef CAS.
- S. Günes, H. Neugebauer and N. S. Sariciftci, Chem. Rev., 2007, 107, 1324–1338 CrossRef PubMed.
- Solar Cells – New approaches and reviews, ed. L. A. Kosyachenko, IntechOpen, 2015, ISBN: 978-953-51-2184-8, eBook (PDF) ISBN: 978-953-51-6393-0 Search PubMed.
- M.-T. Dung, L. Hirsch, G. Wantz and J. D. Wuest, Chem. Rev., 2013, 113, 3734–3765 CrossRef.
- Y. Liang, Z. Xu, J. Xia, S.-T. Tsai, Y. Wu, G. Li, C. Ray and L. Yu, Adv. Mater., 2010, 22, E135–E138 CrossRef CAS.
- Z. He, B. Xiao, F. Liu, H. Wu, Y. Yang, S. Xiao, C. Wang, T. P. Russell and Y. Cao, Nat. Photonics, 2015, 9, 174–179 CrossRef CAS.
- R. Ganesamoorthy, G. Sathiyan and P. Sakthivel, Sol. Energy Mater. Sol. Cells, 2017, 161, 102–148 CrossRef CAS.
- H. K. H. Lee, A. M. Telford, J. A. Röhr, M. F. Wyatt, B. Rice, J. Wu, A. de Castro Maciel, S. M. Tuladhar, E. Speller, J. McGettrick, J. R. Searle, S. Pont, T. Watson, T. Kirchartz, J. R. Durrant, W. C. Tsoi, J. Nelson and Z. Li, Energy Environ. Sci., 2018, 11, 417–428 RSC.
- T. Heumueller, W. R. Mateker, A. Distler, U. F. Fritze, R. Cheacharoen, W. H. Nguyen, M. Biele, M. Salvador, M. von Delius, H.-J. Egelhaaf, M. D. McGehee and C. J. Brabec, Energy Environ. Sci., 2016, 9, 247–256 RSC.
- P. Meredith, W. Li and A. Armin, Adv. Energy Mater., 2020, 20, 2001788 CrossRef.
- D. Baran, T. Kirchartz, S. Wheeler, S. Dimitrov, M. Abdelsamie, J. Gorman, R. S. Ashraf, S. Holliday, A. Wadsworth, N. Gasparini, P. Kaienburg, H. Yan, A. Amassian, C. J. Brabec, J. R. Durrant and I. McCulloch, Energy Environ. Sci., 2016, 9, 3783–3793 RSC.
- A. Wadsworth, M. Moser, A. Marks, M. S. Little, N. Gasparini, C. J. Brabec, D. Baran and I. McCulloch, Chem. Soc. Rev., 2019, 48, 1596–1625 RSC.
- Y. Lin, J. Wang, Z.-G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170–1174 CrossRef CAS PubMed.
- W. Zhao, D. Qian, S. Zhang, S. Li, O. Inganäs, F. Gao and J. Hou, Adv. Mater., 2016, 28, 4734–4739 CrossRef CAS PubMed.
- L.-M. Chen, Z. Xu, Z. Hong and Y. Yang, J. Mater. Chem., 2010, 20, 2575–2598 RSC.
- R. Steim, F. R. Kogler and C. J. Brabec, J. Mater. Chem., 2010, 20, 2499–2512 RSC.
- R. Po, C. Carbonera, A. Bernardi and N. Camaioni, Energy Environ. Sci., 2011, 4, 285–310 RSC.
- H.-L. Yip and A. K.-Y. Jen, Energy Environ. Sci., 2012, 5, 5994–6011 RSC.
- T.-H. Lai, S.-W. Tsang, J. R. Manders, S. Chen and F. So, Mater. Today, 2013, 16, 424–432 CrossRef CAS.
- C.-C. Chueh, C.-Z. Lia and A. K.-Y. Jen, Energy Environ. Sci., 2015, 8, 1160–1189 RSC.
- Z. Yin, J. Wei and Q. Zheng, Adv. Sci., 2016, 3, 1500362 CrossRef PubMed.
- C. Duan, K. Zhang, C. Zhong, F. Huang and Y. Cao, Chem. Soc. Rev., 2013, 42, 9071–9104 RSC.
- F. Wang, Z. Tan and Y. Li, Energy Environ. Sci., 2015, 8, 1059–1091 RSC.
- X. Li, W. Zhang, K. Usman and J. Fang, Adv. Energy Mater., 2018, 8, 1702730 CrossRef.
- Y. Wu, Y. Liu, T. Emrick and T. P. Russell, Prog. Polym. Sci., 2020, 103, 101222 CrossRef CAS.
- G. Li, V. Shrotriya, J. S. Huang, Y. Yao, T. Moriarty, K. Emery and Y. Yang, Nat. Mater., 2005, 4, 864–868 CrossRef CAS.
- C. Waldauf, M. Morana, P. Denk, P. Schilinsky, K. Coakley, S. A. Choulis and C. J. Brabec, Appl. Phys. Lett., 2006, 89, 233517 CrossRef.
- M. D. Irwin, B. Buchholz, A. W. Hains, R. P. H. Chang and T. J. Marks, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 2783–2787 CrossRef CAS.
- K. X. Steirer, P. F. Ndione, N. E. Widjonarko, M. T. Lloyd, J. Meyer, E. L. Ratcliff, A. Kahn, N. R. Armstrong, C. J. Curtis, D. S. Ginley, J. J. Berry and D. C. Olson, Adv. Energy Mater., 2011, 1, 813–820 CrossRef CAS.
- K. Zilberberg, S. Trost, H. Schmidt and T. Riedl, Adv. Energy Mater., 2011, 1, 377–381 CrossRef CAS.
- C.-P. Chen, Y.-D. Chen and S.-C. Chuang, Adv. Mater., 2011, 23, 3859–3863 CAS.
- Y. Sun, C. J. Takacs, S. R. Cowan, J. H. Seo, X. Gong, A. Roy and A. J. Heeger, Adv. Mater., 2011, 23, 2226–2230 CrossRef CAS PubMed.
- C. M. Amb, S. Chen, K. R. Graham, J. Subbiah, C. E. Small, F. So and J. R. Reynolds, J. Am. Chem. Soc., 2011, 133, 10062–10065 CrossRef CAS PubMed.
- J. Y. Kim, S. H. Kim, H. H. Lee, K. Lee, W. L. Ma, X. Gong and A. J. Heeger, Adv. Mater., 2006, 18, 572–576 CrossRef CAS.
- M. S. White, D. C. Olson, S. E. Shaheen, N. Kopidakis and D. S. Ginley, Appl. Phys. Lett., 2006, 89, 143517 CrossRef.
- S. K. Hau, H.-L. Yip, N. S. Baek, J. Zou, K. O’Malley and A. K. Y. Jen, Appl. Phys. Lett., 2008, 92, 253301 CrossRef.
- S. Woo, W. Hyun Kim, H. Kim, Y. Yi, H.-K. Lyu and Y. Kim, Adv. Energy Mater., 2014, 4, 1301692 CrossRef.
- H. Kang, S. Hong, J. Lee and K. Lee, Adv. Mater., 2012, 24, 3005–3009 CrossRef CAS PubMed.
- Z. He, C. Zhang, X. Xu, L. Zhang, L. Huang, J. Chen, H. Wu and Y. Cao, Adv. Mater., 2011, 23, 3086–3089 CrossRef CAS PubMed.
- Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 591–595 CrossRef.
- Z.-G. Zhang, B. Qi, Z. Jin, D. Chi, Z. Qi, Y. Li and J. Wang, Energy Environ. Sci., 2014, 7, 1966–1973 RSC.
- P. W. M. Blom, V. D. Mihailetchi, L. J. A. Koster and D. E. Markov, Adv. Mater., 2007, 19, 1551–1566 CrossRef CAS.
- B. Gregg, J. Phys. Chem. B, 2003, 107, 4688 CrossRef CAS.
- N. K. Elumalai and A. Uddin, Energy Environ. Sci., 2016, 9, 391–410 RSC.
- V. D. Mihailetchi, P. W. M. Blom, J. C. Hummelen and M. T. Rispens, J. Appl. Phys., 2003, 94, 6849–6854 CrossRef CAS.
- S. Braun, W. R. Salaneck and M. Fahlman, Adv. Mater., 2009, 21, 1450–1472 CrossRef CAS.
- X. Deng, R. Nie, A. Li, H. Wei, S. Zheng, W. Huang, Y. Mo, Y. Su, Q. Wang, Y. Li, J. Tang, J. Xu and K.-Y. Wong, Adv. Mater. Interfaces, 2014, 1, 1400215 CrossRef.
- C. Tengstedt, Appl. Phys. Lett., 2006, 88, 053502 CrossRef.
- Z. Hu, Z. Zhong, Y. Chen, C. Sun, F. Huang, J. Peng, J. Wang and Y. Cao, Adv. Funct. Mater., 2016, 26, 129–136 CrossRef CAS.
- P. Sehati, S. Braun, L. Lindell, X. Liu, L. M. Andersson and M. Fahlman, IEEE J. Sel. Top. Quantum Electron., 2010, 16, 1718–1724 CAS.
- A. K. K. Kyaw, X. W. Sun, C. Y. Jiang, G. Q. Lo, D. W. Zhao and D. L. Kwong, Appl. Phys. Lett., 2008, 93, 221107 CrossRef.
- Y. Zhou, C. Fuentes-Hernandez, J. Shim, J. Meyer, A. J. Giordano, H. Li, P. Winget, T. Papadopoulos, H. Cheun, J. Kim, M. Fenoll, A. Dindar, W. Haske, E. Najafabadi, T. M. Khan, H. Sojoudi, S. Barlow, S. Graham, J.-L. Brédas, S. R. Marder, A. Kahn and B. Kippelen, Science, 2012, 336, 327–332 CrossRef CAS.
- M. Nam, M. Cha, H. H. Lee, K. Hur, K.-T. Lee, J. Yoo, I. K. Han, S. J. Kwon and D.-H. Ko, Nat. Commun., 2017, 8, 14068 CrossRef CAS PubMed.
- S. Zhong, R. Wang, H. Y. Mao, Z. He, H. Wu, W. Chen and Y. Cao, J. Appl. Phys., 2013, 114, 113709 CrossRef.
- Q. Bao, O. Sandberg, D. Dagnelund, S. Sandén, S. Braun, H. Aarnio, X. Liu, W. M. Chen, R. Österbacka and M. Fahlman, Adv. Funct. Mater., 2014, 24, 6309–6316 CrossRef CAS.
- Q. Bao, X. Liu, E. Wang, J. Fang, F. Gao, S. Braun and M. Fahlman, Adv. Mater. Interfaces, 2015, 2, 500204 Search PubMed.
- X. Li, X. Liu, W. Zhang, H.-Q. Wang and J. Fang, Chem. Mater., 2017, 29, 4176–4180 CrossRef CAS.
- A. Kumatani, Y. Li, P. Darmawan, T. Minari and K. Tsukagoshi, Sci. Rep., 2013, 3, 1026 CrossRef PubMed.
- M. Kröger, S. Hamwi, J. Meyer, T. Riedl, W. Kowalsky and A. Kahn, Org. Electron., 2009, 10, 932–938 CrossRef.
- M. T. Greiner, M. G. Helander, W.-M. Tang, Z.-B. Wang, J. Qiu and Z.-H. Lu, Nat. Mater., 2012, 11, 76–81 CrossRef CAS PubMed.
- J. J. Jasieniak, J. Seifter, J. Jo, T. Mates and A. J. Heeger, Adv. Funct. Mater., 2012, 22, 2594–2605 CrossRef CAS.
- W. Tress and O. Inganäs, Sol. Energy Mater. Sol. Cells, 2013, 117, 599–603 CrossRef CAS.
- B. H. Lee, I. H. Jung, H. Y. Woo, H.-K. Shim, G. Kim and K. Lee, Adv. Funct. Mater., 2014, 24, 1100–1108 CrossRef CAS.
- S. van Reenen, S. Kouijzer, R. A. J. Janssen, M. M. Wienk and M. Kemerink, Adv. Mater. Interfaces, 2014, 1, 1400189 CrossRef.
- H. Zhang, W. Zhou, C. Yu, J. Guo and F. Li, Phys. Chem. Chem. Phys., 2019, 21, 20065–20072 RSC.
- C.-Z. Li, C.-C. Chueh, F. Ding, H.-L. Yip, P.-W. Liang, X. Li and A. K.-Y. Jen, Adv. Mater., 2013, 25, 4425–4430 CrossRef CAS PubMed.
- Z. He, C. Zhong, X. Huang, W.-Y. Wong, H. Wu, L. Chen, S. Su and Y. Cao, Adv. Mater., 2011, 23, 4636–4643 CrossRef CAS.
- J.-H. Lee, S. Y. Jeong, G. Kim, B. Park, J. Kim, S. Kee, B. Kim and K. Lee, Adv. Funct. Mater., 2018, 28, 1705079 CrossRef.
- A. Kumar, G. Lakhwani, E. Elmalem, W. T. S. Huck, A. Rao, N. C. Greenham and R. H. Friend, Energy Environ. Sci., 2014, 7, 2227–2231 RSC.
- R. Wang, D. Zhang, S. Xie, J. Wang, Z. Zheng, D. Wei, X. Sun, H. Zhou and Y. Zhang, Nano Energy, 2018, 51, 736–744 CrossRef CAS.
- S. Das, J. K. Keum, J. F. Browning, G. Gu, B. Yang, O. Dyck, C. Do, W. Chen, J. Chen, I. N. Ivanov, K. Hong, A. J. Rondinone, P. C. Joshi, D. B. Geohegan, G. Duscherd and K. Xiao, Nanoscale, 2015, 7, 15576–15583 RSC.
- C. Sun, Z. Wu, Z. Hu, J. Xiao, W. Zhao, H.-W. Li, Q.-Y. Li, S.-W. Tsang, Y.-X. Xu, K. Zhang, H.-L. Yip, J. Hou, F. Huang and Y. Cao, Adv. Mater., 2019, 31, 1805708 CrossRef PubMed.
- X. Bulliard, S.-G. Ihn, S. Yun, Y. Kim, D. Choi, J.-Y. Choi, M. Kim, M. Sim, J.-H. Park, W. Choi and K. Cho, Adv. Funct. Mater., 2010, 20, 4381–4387 CrossRef CAS.
- L. Huang, G. Wang, W. Zhou, B. Fu, X. Cheng, L. Zhang, Z. Yuan, S. Xiong, L. Zhang, Y. Xie, A. Zhang, Y. Zhang, W. Ma, W. Li, Y. Zhou, E. Reichmanis and Y. Chen, ACS Nano, 2018, 12, 4440–4452 CrossRef CAS PubMed.
- V. Vohra, K. Kawashima, T. Kakara, T. Koganezawa, I. Osaka, K. Takimiya and H. Murata, Nat. Photonics, 2015, 9, 403–408 CrossRef CAS.
- Y. Ge, L. Hu, L. Zhang, Q. Fu, G. Xu, Z. Xing, L. Huang, W. Zhou and Y. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 10706–10716 CrossRef PubMed.
- B. Qi, Z.-G. Zhang and J. Wang, Sci. Rep., 2015, 5, 7803 CrossRef CAS PubMed.
- X. Du, T. Heumueller, W. Gruber, A. Classen, T. Unruh, N. Li and C. J. Brabec, Joule, 2018, 3, 215–226 CrossRef.
- X. Xu, J. Xiao, G. Zhang, L. Wei, X. Jiao, H.-L. Yip and Y. Cao, Sci. Bull., 2020, 65, 208–216 CrossRef CAS.
- W. Zhao, S. Zhang and J. Hou, Sci. China: Chem., 2016, 59, 1574–1582 CrossRef CAS.
- J. Xiao, Z. Chen, G. Zhang, Q.-Y. Li, Q. Yin, X.-F. Jiang, F. Huang, Y.-X. Xu, H.-L. Yip and Y. Cao, J. Mater. Chem. C, 2018, 6, 4457–4463 RSC.
- K. Zhang, C. Zhong, S. Liu, C. Mu, Z. Li, H. Yan, F. Huang and Y. Cao, ACS Appl. Mater. Interfaces, 2014, 6, 10429–10435 CrossRef CAS.
- B. Ratier, J.-M. Nunzi, M. Aldissi, T. M. Kraft and E. Buncel, Polym. Int., 2012, 61, 342–354 CrossRef CAS.
- S.-H. Lee, S.-J. Ko, S. H. Eom, H. Kim, D. W. Kim, C. Lee and S. C. Yoon, ACS Appl. Mater. Interfaces, 2020, 12, 14244–14253 CrossRef CAS PubMed.
- M. B. Upama, N. K. Elumalai, M. A. Mahmud, C. Xu, D. Wang, M. Wright and A. Uddin, Sol. Energy Mater. Sol. Cells, 2018, 187, 273–282 CrossRef CAS.
- R. Peng, Z. Liu, Q. Guan, L. Hong, W. Song, Q. Wei, P. Gao, J. Huang, X. Fan, M. Wang and Z. Ge, J. Mater. Chem. A, 2018, 6, 6327–6334 RSC.
- Z. Li, X. Xu, G. Zhang, M. Deng, Y. Li and Q. Peng, Sol. RRL, 2018, 2, 1800182 CrossRef.
- Y. Wang, Z. Liang, X. Li, J. Qin, M. Ren, C. Yang, X. Bao, Y. Xia and J. Li, J. Mater. Chem. C, 2019, 7, 11152–11159 RSC.
- C. Sun, Z. Wu, Z. Hu, J. Xiao, W. Zhao, H.-W. Li, Q.-Y. Li, S.-W. Tsang, Y.-X. Xu, K. Zhang, H.-L. Yip, J. Hou, F. Huang and Y. Cao, Energy Environ. Sci., 2017, 10, 1784–1791 RSC.
- Z. Wu, C. Sun, S. Dong, X.-F. Jiang, S. Wu, H. Wu, H.-L. Yip, F. Huang and Y. Cao, J. Am. Chem. Soc., 2016, 138, 2004–2013 CrossRef CAS PubMed.
- P. Luo, K. An, L. Ying, G. Li, C. Zhu, B. Fan, F. Huang and Y. Cao, J. Mater. Chem. C, 2020, 8, 5273–5279 RSC.
- S. Guha, F. S. Goodson, L. J. Corson and S. Saha, J. Am. Chem. Soc., 2012, 134, 13679–13691 CrossRef CAS PubMed.
- R. Wang, B. Wang, J. Wang, X. Zhang, D. Zhang, D. Wei, X. Sun, H. Zhou and Y. Zhang, J. Mater. Chem. A, 2019, 7, 25808–25817 RSC.
- Y. Liu, V. V. Duzhko, Z. A. Page, T. Emrick and T. P. Russell, Acc. Chem. Res., 2016, 49, 2478–2488 CrossRef CAS.
- Z. Hu, Z. Chen, K. Zhang, N. Zheng, R. Xie, X. Liu, X. Yang, F. Huang and Y. Cao, Sol. RRL, 2017, 1, 1700055 CrossRef.
- Q. Kang, L. Ye, B. Xu, C. An, S. J. Stuard, S. Zhang, H. Yao, H. Ade and J. Hou, Joule, 2019, 3, 227–239 CrossRef CAS.
- Z. Wang, N. Zheng, W. Zhang, H. Yan, Z. Xie, Y. Ma, F. Huang and Y. Cao, Adv. Energy Mater., 2017, 7, 1700232 CrossRef.
- Q. Kang, Q. Wang, C. An, C. He, B. Xu and J. Hou, J. Energy Chem., 2020, 43, 40–46 CrossRef.
- F. Carulli, G. Scavia, E. Lassi, M. Pasini, F. Galeotti, S. Brovelli, U. Giovanella and S. Luzzati, J. Colloid Interface Sci., 2019, 538, 611–619 CrossRef CAS.
- Z. Zheng, R. Wang, H. Yao, S. Xie, Y. Zhang, J. Hou, H. Zhou and Z. Tang, Nano Energy, 2018, 50, 169–175 CrossRef CAS.
- Y. Bai, B. Yang, X. Chen, F. Wang, T. Hayat, A. Alsaedi and Z. Tan, Front. Chem., 2018, 6, 292 CrossRef PubMed.
- H. W. Cheng, P. Raghunath, K. L. Wang, P. Cheng, T. Huang, Q. Wu, J. Yuan, Y.-C. Lin, H.-C. Wang, Y. Zou, Z.-K. Wang, M. C. Lin, K.-H. Wei and Y. Yang, Nano Lett., 2020, 20, 715–721 CrossRef CAS PubMed.
- H. Liu, Z.-X. Liu, S. Wang, J. Huang, H. Ju, Q. Chen, J. Yu, H. Chen and C.-Z. Li, Adv. Energy Mater., 2019, 1900887 CrossRef.
- H. S. Park, Y. W. Han, H. S. Lee, S. J. Jeon and D. K. Moon, ACS Appl. Energy Mater., 2020, 3, 3745–3754 CrossRef CAS.
- T. Wang, M.-S. Niu, J.-J. Guo, K.-N. Zhang, Z.-C. Wen, J.-Q. Liu, C.-C. Qin and X.-T. Hao, Sol. RRL, 2020, 4, 2000047 CrossRef CAS.
- J. Wang, Z. Zheng, D. Zhang, J. Zhang, J. Zhou, J. Liu, S. Xie, Y. Zhao, Y. Zhang, Z. Wei, J. Hou, Z. Tang and H. Zhou, Adv. Mater., 2019, 31, 1806921 CrossRef PubMed.
- W. Yang, L. Ye, F. Yao, C. Jin, H. Ade and H. Chen, Nano Res., 2019, 12, 777–783 CrossRef CAS.
- Y.-A. Su, N. Maebayashi, H. Fujita, Y.-C. Lin, C.-I. Chen, W.-C. Chen, T. Michinobu, C.-C. Chueh and T. Higashihara, ACS Appl. Mater. Interfaces, 2020, 12, 12083–12092 CrossRef CAS PubMed.
- S. Park and H. J. Son, J. Mater. Chem. A, 2019, 7, 25830–25837 RSC.
- Y. Jiang, L. Sun, F. Jiang, C. Xie, L. Hu, X. Dong, F. Qin, T. Liu, L. Hu, X. Jiang and Y. Zhou, Mater. Horiz., 2019, 6, 1438–1443 RSC.
- A. Soultati, A. Verykios, S. Panagiotakis, K.-K. Armadorou, M. I. Haider, A. Kaltzoglou, C. Drivas, A. Fakharuddin, X. Bao, C. Yang, A. R. bin Mohd Yusoff, E. K. Evangelou, I. Petsalakis, S. Kennou, P. Falaras, K. Yannakopoulou, G. Pistolis, P. Argitis and M. Vasilopoulou, ACS Appl. Mater. Interfaces, 2020, 12, 21961–21973 CrossRef CAS PubMed.
- A. Soultati, A. Fakharuddin, E. Polydorou, C. Drivas, A. Kaltzoglou, M. I. Haider, F. Kournoutas, M. Fakis, L. C. Palilis, S. Kennou, D. Davazoglou, P. Falaras, P. Argitis, S. Gardelis, A. Kordatos, A. Chroneos, L. Schmidt-Mende and M. Vasilopoulou, ACS Appl. Energy Mater., 2019, 2, 1663–1675 CrossRef CAS.
- J. Guo, Y. Wu, R. Sun, W. Wang, J. Guo, Q. Wu, X. Tang, C. Sun, Z. Luo, K. Chang, Z. Zhang, J. Yuan, T. Li, W. Tang, E. Zhou, Z. Xiao, L. Ding, Y. Zou, X. Zhan, C. Yang, Z. Li, C. J. Brabec, Y. Li and J. Min, J. Mater. Chem. A, 2019, 7, 25088–25101 RSC.
- M. P. de Jong, L. J. van IJzendoorn and M. J. A. de Voigt, Appl. Phys. Lett., 2000, 77, 2255 CrossRef CAS.
- M. Jørgensen, K. Norrman and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2008, 92, 686–714 CrossRef.
- S. Wu, S. Han, Y. Zheng, H. Zheng, N. Liu, L. Wang, Y. Cao and J. Wang, Org. Electron., 2011, 12, 504–508 CrossRef CAS.
- X. Zhu, L. Hu, W. Wang, X. Jiang, L. Hu and Y. Zhou, ACS Appl. Energy Mater., 2019, 2, 7602–7608 CrossRef CAS.
- N. Y. Doumon, F. V. Houard, J. Dong, H. Yao, G. Portale, J. Hou and L. J. A. Koster, Org. Electron., 2019, 69, 255–262 CrossRef CAS.
- Y. Meng, Z. Hu, N. Ai, Z. Jiang, J. Wang, J. Peng and Y. Cao, ACS Appl. Mater. Interfaces, 2014, 6, 5122–5129 CrossRef CAS.
- L. Hu, Y. Liu, L. Mao, S. Xiong, L. Sun, N. Zhao, F. Qin, Y. Jiang and Y. Zhou, J. Mater. Chem. A, 2018, 6, 2273–2278 RSC.
- L. Hu, S. Xiong, W. Wang, L. Sun, F. Qin and Y. Zhou, J. Phys. Chem. C, 2020, 124, 2307–2312 CrossRef CAS.
- S. Xiong, L. Hu, L. Hu, L. Sun, F. Qin, X. Liu, M. Fahlman and Y. Zhou, Adv. Mater., 2019, 31, 1806616 CrossRef PubMed.
- D. Zhang, J. Wang, X. Zhang, J. Zhou, S.-U. Zafar, H. Zhou and Y. Zhang, J. Mater. Chem. C, 2020, 8, 158–164 RSC.
- Y. Cui, G. Jia, J. Zhu, Q. Kang, H. Yao, L. Lu, B. Xu and J. Hou, Chem. Mater., 2018, 30, 1078–1084 CrossRef CAS.
- N. S. Alharbi, C. Wang, F. E. Alsaadi, S. O. Rabah and Z. Tan, Adv. Sustainable Syst., 2020, 4, 2000054 CrossRef CAS.
- Y. Bai, B. Yang, F. Wang, H. Liu, T. Hayat, A. Alsaedi and Z. Tan, Org. Electron., 2018, 52, 323–328 CrossRef CAS.
- Y. Wang, Z. Shi, H. Liu, F. Wang, Y. Bai, X. Bian, B. Zhang, T. Hayat, A. Alsaedi and Z. Tan, Polymers, 2017, 9, 571 CrossRef PubMed.
- Z. Huang, J. Cheng, X. Ren, J. Zhuang, V. A. L. Roy, J. M. Burkhartsmeyer, K. S. Wong and W. C. H. Choy, Nano Energy, 2018, 47, 26–34 CrossRef CAS.
- B. Liu, Y. Wang, P. Chen, X. Zhang, H. Sun, Y. Tang, Q. Liao, J. Huang, H. Wang, H. Meng and X. Guo, ACS Appl. Mater. Interfaces, 2019, 11, 33505–33614 CrossRef CAS PubMed.
- Q. Kang, B. Yang, Y. Xu, B. Xu and J. Hou, Adv. Mater., 2018, 30, 1801718 CrossRef.
- Y. Zhu, Z. Yuan, W. Cui, Z. Wu, Q. Sun, S. Wang, Z. Kang and B. Sun, J. Mater. Chem. A, 2014, 2, 1436–1442 RSC.
- X. Jia, L. Shen, M. Yao, Y. Liu, W. Yu, W. Guo and S. Ruan, ACS Appl. Mater. Interfaces, 2015, 7, 5367–5372 CrossRef CAS.
- G. Ji, Y. Wang, Q. Luo, K. Han, M. Xie, L. Zhang, N. Wu, J. Lin, S. Xiao, Y.-Q. Li, L.-Q. Luo and C.-Q. Ma, ACS Appl. Mater. Interfaces, 2018, 10, 943–954 CrossRef CAS PubMed.
- A. Cominetti, G. Serrano, A. Savoini, C. Carbonera, F. Melchiorre, S. Perucchini, A. Congiu, G. Corso, R. Barbieri, E. Trippodo, A. Caneschi and R. Po, Phys. Status Solidi A, 2020, 217, 1901023 CrossRef CAS.
- Q. Kang, Y. Zu, Q. Liao, Z. Zheng, H. Yao, S. Zhang, C. He, B. Xu and J. Hou, J. Mater. Chem. A, 2020, 8, 5580–5586 RSC.
- Y. Lin, B. Adilbekova, Y. Firdaus, E. Yengel, H. Faber, M. Sajjad, X. Zheng, E. Yarali, A. Seitkhan, O. M. Bakr, A. El-Labban, U. Schwingenschlögl, V. Tung, I. McCulloch, F. Laquai and T. D. Anthopoulos, Adv. Mater., 2019, 31, 1902965 CrossRef CAS PubMed.
- J. Wang, H. Yu, C. Hou and J. Zhang, Sol. RRL, 2020, 4, 1900428 CrossRef CAS.
- L. Reshmaa and K. Santhakumar, Org. Electron., 2017, 47, 35–43 CrossRef.
- Z. Zheng, Q. Hu, S. Zhang, D. Zhang, J. Wang, S. Xie, R. Wang, Y. Qin, W. Li, L. Hong, N. Liang, F. Liu, Y. Zhang, Z. Wei, Z. Tang, T. P. Russell, J. Hou and H. Zhou, Adv. Mater., 2018, 30, 1801801 CrossRef.
- Q. Yang, S. Yu, P. Fu, W. Yu, Y. Liu, X. Liu, Z. Feng, X. Guo and C. Li, Adv. Funct. Mater., 2020, 30, 1910205 CrossRef CAS.
- C. Hou and H. Yu, J. Mater. Chem. C, 2020, 8, 4169–4180 RSC.
- C. Cui, Y. Li and Y. Li, Adv. Energy Mater., 2017, 7, 1601251 CrossRef.
- K. Yan, Z.-X. Liu, X. Li, J. Chen, H. Chen and C.-Z. Li, Org. Chem. Front., 2018, 5, 2845–2851 RSC.
- J. Li, F. Qin, W. Zeng, L. Sun, W. Wang and Y. Zhou, Sustainable Energy Fuels, 2020, 4, 1984–1990 RSC.
- E. Kozma and M. Catellani, Dyes Pigm., 2013, 98, 160–179 CrossRef CAS.
- Y. Yin, Z. Zheng, M. Liu, S. Gao, F. Guo, G. T. Mola, J. Wang, L. Zhao and Y. Zhang, Dyes Pigm., 2020, 175, 108119 CrossRef CAS.
- Y. Li, M. Han, W. Yang, J. Guo, K. Chang, J. Wang, J. Min, Q. Li and Z. Li, Mater. Chem. Front., 2019, 3, 1840–1848 RSC.
- X. Zhang, W. Li, J. Yao and C. Zhan, ACS Appl. Mater. Interfaces, 2016, 8, 15415–15421 CrossRef CAS.
- J. Yao, B. Qui, Z.-G. Zhang, L. Xue, R. Wang, C. Zhang, S. Chen, Q. Zhou, C. Sun, C. Yang, M. Xiao, L. Meng and Y. Li, Nat. Commun., 2020, 11, 2726 CrossRef CAS.
- R. Qin, D. Guo, L. Hu, Z. Liu, J. Yang, H. Liu, L. Jiang and Y. Jiang, Energy Technol., 2020, 4, 2000072 CrossRef.
- M. Li, J. Lv, L. Wang, J. Liu, X. Yu, R. Xing, L. Wang, Y. Geng and Y. Han, Colloids Surf., A, 2015, 469, 326–332 CrossRef CAS.
- X. Yin, X. Liu, Y. Peng, W. Zeng, C. Zhong, G. Xie, L. Wang, J. Fang and C. Yang, Adv. Funct. Mater., 2019, 29, 1806125 CrossRef.
- S. Wang, Z. Li, X. Xu, M. Zhang, G. Zhang, Y. Li and Q. Peng, J. Mater. Chem. A, 2018, 6, 22503–22507 RSC.
- Q. Liao, Q. Kang, Y. Yang, C. An, B. Xu and J. Hou, Adv. Mater., 2020, 32, 1906557 CrossRef CAS PubMed.
- W. Li, D. Yan, W. Liu, J. Chen, W. Xu, C. Zhan and J. Yao, Sol. RRL, 2017, 1, 1700014 CrossRef.
- M. Gupta, D. Yan, J. Yao and C. Zhan, ACS Appl. Mater. Interfaces, 2018, 10, 35896–35903 CrossRef CAS.
- M. Gupta, D. Yan, D. Yong, F. Shen, J. Xu and C. Zhan, Acta Phys.-Chim. Sin., 2019, 35, 496–502 Search PubMed.
- M. Gupta, D. Yan, J. Yao and C. Zhan, Mater. Chem. Front., 2018, 2, 1876–1883 RSC.
- Q. Guan, R. Peng, Z. Liu, W. Song, R. Yang, L. Hong, T. Lei, X. Fan, Q. Wei and Z. Ge, J. Mater. Chem. A, 2018, 6, 464–468 RSC.
- J.-S. Yeo, M. Kang, Y.-S. Jung, R. Kang, S.-H. Lee, Y.-J. Heo, S.-H. Jin, D.-Y. Kim and S.-I. Na, Nano Energy, 2016, 21, 26–38 CrossRef CAS.
- Y. Wang, B. Jia, F. Qin, Y. Wu, W. Meng, S. Dai, Y. Zhou and X. Zhan, Polymer, 2016, 107, 108–112 CrossRef CAS.
- Z. Zhang, Z. Zhang, Y. Yu, B. Zhao, S. Li, J. Zhang and S. Tan, J. Energy Chem., 2020, 47, 196–202 CrossRef.
- B. Yang, S. Zhang, S. Li, H. Yao, W. Li and J. Hou, Adv. Mater., 2019, 31, 1804657 CrossRef PubMed.
- G. Sai-Anand, A.-I. Gopalan, K.-P. Lee, S. Venkatesan, Q. Qiao, B.-H. Kang, S.-W. Lee, J.-S. Lee and S.-W. Kang, Sol. Energy Mater. Sol. Cells, 2016, 153, 148–163 CrossRef CAS.
- J. Vinokur, I. Deckman, T. Sarkar, L. Nouzman, B. Shamieh and G. L. Frey, Adv. Mater., 2018, 30, 1706803 CrossRef PubMed.
- B. Shamieh, T. Sarkar and G. L. Frey, J. Mater. Chem. C, 2020, 8, 8992–8998 RSC.
- Y. Lin, L. Yu, Y. Xia, Y. Firdaus, S. Dong, C. Müller, O. Inganäs, F. Huang, T. D. Anthopoulos, F. Zhang and L. Hou, Sol. RRL, 2019, 3, 1900179 CrossRef.
- M. Lv, Y. Li, X. Wei, Y. Xu, Z. Ge and X. Chen, ACS Appl. Energy Mater., 2019, 2, 2238–2245 CrossRef CAS.
- Q. Yin, K. Zhang, L. Zhang, J. Jia, X. Zhang, S. Pang, Q.-H. Xu, C. Duan, F. Huang and Y. Cao, J. Mater. Chem. A, 2019, 7, 12426–12433 RSC.
- J. Jia, B. Fan, M. Xiao, T. Jia, Y. Jin, Y. Li, F. Huang and Y. Cao, Macromolecules, 2018, 51, 2195–2202 CrossRef CAS.
- Y. Shi, Z. Yu, X. Lu and J. Zhang, Macromol. Chem. Phys., 2020, 221, 1900554 CrossRef CAS.
- Y. Shi, X. Lu, Y. Zhang and J. Xi, Org. Electron., 2020, 77, 105542 CrossRef CAS.
- Y. Shi, E. Yang, J. Zhang and Z. Ji, Colloid Polym. Sci., 2019, 297, 1313–1319 CrossRef CAS.
- Y. Liu, M. Sheri, M. D. Cole, T. Emrick and T. P. Russell, Angew. Chem., Int. Ed., 2018, 57, 9675–9678 CrossRef CAS.
- Y. Liu, M. Sheri, M. D. Cole, D. M. Yu, T. Emrick and T. P. Russell, Angew. Chem., Int. Ed., 2019, 58, 5677–5681 CrossRef CAS.
- M. Liu, P. Fan, Q. Hu, T. R. Russell and Y. Liu, Angew. Chem., Int. Ed., 2020, 59, 18131–18135 CrossRef CAS PubMed.
- M. Rafiq, Z. Chen, H. Tang, Z. Hu, X. Zhang, Y. Xing, Y. Li and F. Huang, ACS Appl. Polym. Mater., 2020, 2, 12–18 CrossRef CAS.
- J. Wu, X. Che, H.-C. Hu, H. Xu, B. Li, Y. Liu, J. Li, Y. Ni, X. Zhang and X. Ouyang, J. Mater. Chem. A, 2020, 8, 5442–5448 RSC.
- P.-C. Lin, Y.-T. Wong, Y.-A. Su, W.-C. Chen and C.-C. Chueh, ACS Sustainable Chem. Eng., 2018, 6, 14621–14630 CrossRef CAS.
- J. Wu, Y. Liu, A. Islam, Q. Zheng, J. Li, W. Ji, L. Chen and X. Ouyang, Adv. Sci., 2020, 7, 1902269 CrossRef CAS.
- X. Wang, S. Yi, Z. He, X. Ouyang, H.-B. Wu, W. Zhu, B. Zhang and Y. Cao, Sustainable Energy Fuels, 2020, 4, 1234–1241 RSC.
- K.-T. Huang, C.-C. Shih, B.-H. Jiang, R.-J. Jeng, C.-P. Chen and W.-C. Chen, J. Mater. Chem. C, 2019, 7, 12572–12579 RSC.
- H.-C. Hu, H. Xu, J. Wu, L. Li, F. Yue, L. Huang, L. Chen, X. Zhang and X. Ouyang, Adv. Funct. Mater., 2020, 30, 2001494 CrossRef CAS.
- S. Bai, Y. Jin, X. Liang, Z. Ye, Z. Wu, B. Sun, Z. Ma, Z. Tang, J. Wang, U. Würfel, F. Gao and F. Zhang, Adv. Energy Mater., 2015, 5, 1401606 CrossRef.
- B. A. MacLeod, B. J. Tremolet de Villers, P. Schulz, P. F. Ndione, H. Kim, A. J. Giordano, K. Zhu, S. R. Marder, S. Graham, J. J. Berry, A. Kahn and D. C. Olson, Energy Environ. Sci., 2015, 8, 592–601 RSC.
- S. Trost, A. Behrendt, T. Becker, A. Polywka, P. Görrn and T. Riedl, Adv. Energy Mater., 2015, 5, 1500277 CrossRef.
- B. Bob, T.-S. Song, C.-C. Chen, Z. Xu and Y. Yang, Chem. Mater., 2013, 25, 4725–4730 CrossRef CAS.
- V.-H. Tran, R. B. Ambade, S. B. Ambade, S.-H. Lee and I.-H. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 1645–1653 CrossRef CAS PubMed.
- T. Bu, J. Li, F. Zheng, W. Chen, X. Wen, Z. Ku, Y. Peng, J. Zhong, Y.-B. Cheng and F. Huang, Nat. Commun., 2018, 9, 4609 CrossRef PubMed.
- M. Ylikunnari, M. Välimäki, K.-L. Väisänen, T. M. Kraft, R. Sliz, G. Corso, R. Po, R. Barbieri, C. Carbonera, G. Gorni and M. Vilkman, Flexible Printed Electron., 2020, 5, 014008 CrossRef CAS.
- Y. Sun, S. Lu, R. Xu, K. Liu, Z. Zhou, S. Yue, M. Azam, K. Ren, Z. Wei, Z. Wang, S. Qu, Y. Lei and Z. Wang, J. Power Sources, 2019, 412, 465–471 CrossRef CAS.
- Y. Bai, C. Zhao, X. Chen, S. Zhang, S. Zhang, T. Hayat, A. Alsaedi, Z. Tan, J. Hou and Y. Li, J. Mater. Chem. A, 2019, 7, 15887–15894 RSC.
- Z. Shi, H. Liu, Y. Wang, J. Li, Y. Bai, F. Wang, X. Bian, T. Hayat, A. Alsaedi and Z. Tan, ACS Appl. Mater. Interfaces, 2017, 9, 43871–43879 CrossRef CAS PubMed.
- M. Vasilopoulou, A. R. B. M. Yusoff, N. Kuganathan, X. Bao, A. Verykios, E. Polydorou, K.-K. Armadorou, A. Soultati, G. Papadimitropoulos, M. I. Haider, A. Fakharuddin, L. C. Palilis, S. Kennou, A. Chroneos, P. Argitis and D. Davazoglou, Nano Energy, 2020, 70, 104508 CrossRef CAS.
- R. Peng, T. Yan, J. Chen, S. Yang, Z. Ge and M. Wang, Adv. Electron. Mater., 2020, 6, 1901245 CrossRef CAS.
- R. Shivanna, S. Rajaram and K. S. Narayan, Appl. Phys. Lett., 2015, 106, 123301 CrossRef.
- X. Wen, A. Nowak-Króll, O. Nagler, F. Kraus, N. Zhu, N. Zheng, M. Müller, D. Schmidt, Z. Xie and F. Würthner, Angew. Chem., Int. Ed., 2019, 58, 13051–13055 CrossRef CAS PubMed.
- J. Yu, Y. Xi, C.-C. Chueh, D. Zhao, F. Lin, L. D. Pozzo, W. Tang and A. K.-Y. Jen, Adv. Mater. Interfaces, 2016, 3, 1600476 CrossRef.
- M. Abd-Ellah, J. Cann, S. V. Dayneko, A. Laventure, E. Cieplechowicz and G. C. Welch, ACS Appl. Electron. Mater., 2019, 1, 1590–1596 CrossRef CAS.
- X. Guan, K. Zhang, F. Huang, G. C. Bazan and Y. Cao, Adv. Funct. Mater., 2012, 22, 2846–2854 CrossRef CAS.
- X. Liu, W. Jiao, M. Lei, Y. Zhou, B. Song and Y. Li, J. Mater. Chem. A, 2015, 3, 9278–9284 RSC.
- M. Wu, J. Zhou, Y. Luo, N. Zheng, C. Wang, L. Liu, Z. Xie and Y. Ma, Org. Chem. Front., 2018, 5, 3324–3330 RSC.
- J. Li, J. Liu, J. Zhang, J. Ma, Y. Hu, G. Xie, J. Zhang and G. Tu, Sol. Energy, 2019, 191, 219–226 CrossRef CAS.
- X. Zhu, B. Guo, J. Fang, T. Zhai, Y. Wang, G. Li, J. Zhang, Z. Wei, S. Duhm, X. Guo, M. Zhang and Y. Li, Org. Electron., 2019, 70, 25–31 CrossRef CAS.
- D. Zhou, S. Xiong, L. Chen, X. Cheng, H. Xu, Y. Zhou, F. Liu and Y. Chen, Chem. Commun., 2018, 54, 563–566 RSC.
- C. Adachi, T. Tsutsui and S. Saito, Appl. Phys. Lett., 1989, 55, 1489–1491 CrossRef CAS.
- A. P. Kulkarni, C. J. Tonzola, A. Babel and S. A. Jenekhe, Chem. Mater., 2004, 16, 4556–4573 CrossRef CAS.
- B. Fanady, W. Song, R. Peng, T. Wu and Z. Ge, Org. Electron., 2020, 76, 105483 CrossRef CAS.
- T.-W. Chen, C.-C. Chang, Y.-T. Hsiao, C. K. Chan, L. Hong, L. Zhong, W.-T. Chuang, J. Hou, Y. Li and C.-S. Hsu, ACS Appl. Mater. Interfaces, 2019, 11, 31069–31077 CrossRef CAS.
- Y. Y. Lai, Y.-J. Cheng and C.-S. Hsu, Energy Environ. Sci., 2014, 7, 1866–1883 RSC.
- C. Tao, J. Van Der Velden, L. Cabau, N. F. Montcada, S. Neutzner, A. Ram Srimath Kandada, S. Marras, L. Brambilla, M. Tommasini, W. Xu, R. Sorrentino, A. Perinot, M. Caironi, C. Bertarelli, E. Palomares and A. Petrozza, Adv. Mater., 2017, 29, 1604493 CrossRef PubMed.
- H. Ma, H.-L. Yip, F. Huang and A. K.-Y. Jen, Adv. Funct. Mater., 2010, 20, 1371–1388 CrossRef CAS.
- S. Khodabakhsh, B. M. Sanderson, J. Nelson and T. S. Jones, Adv. Funct. Mater., 2006, 16, 95–100 CrossRef CAS.
- B. de Boer, A. Hadipour, M. M. Mandoc, T. van Woudenbergh and P. W. M. Blom, Adv. Mater., 2005, 17, 621–625 CrossRef CAS.
- G. Heimel, L. Romaner, E. Zojer and J. L. Bredas, Acc. Chem. Res., 2008, 41, 721–729 CrossRef CAS.
- S. Zhang, L. Zhan, S. Li, C.-Z. Li and H. Chen, Org. Electron., 2018, 63, 143–148 CrossRef CAS.
- H. Liu, Z.-X. Liu, S. Wang, J. Huang, H. Ju, Q. Chen, J. Yu, H. Chen and C.-Z. Li, Adv. Energy Mater., 2019, 9, 1900887 CrossRef.
- G. Xie, Z. Zhang, J. Li, Y. Hu, X. Ge, G. Tu, X. Zhang, X. Zhang and J. Zhang, Phys. Status Solidi RRL, 2019, 13, 1900372 CrossRef CAS.
- B. R. Lee, E. D. Jung, Y. S. Nam, M. Jung, J. S. Park, S. Lee, H. Choi, S.-J. Ko, N. R. Shin, Y.-K. Kim, S. O. Kim, J. Y. Kim, H.-J. Shin, S. Cho and M. H. Song, Adv. Mater., 2014, 26, 494–500 CrossRef CAS PubMed.
- S. Huang, N. Ali, Z. Huai, J. Ren, Y. Sun, X. Zhao, G. Fu, W. Kong and S. Yang, Org. Electron., 2020, 78, 105555 CrossRef CAS.
- Y. Tan, L. Chen, F. Wu, B. Huang, Z. Liao, Z. Yu, L. Hu, Y. Zhou and Y. Chen, Macromolecules, 2018, 51, 8197–8204 CrossRef CAS.
- D. Zhou, Y. Qin, R. Zhong, H. Xu, Y. Tong, B. Hu and Y. Xie, J. Mater. Sci.: Mater. Electron., 2018, 29, 18458–18464 CrossRef CAS.
- J. Li, J. Liu, X. Liu, R. Wang, J. Zhang and G. Tu, ChemSusChem, 2019, 12, 1401–1409 CrossRef CAS.
- N. Ahmad, X. Zhang, S. Yang, D. Zhang, J. Wang, S. uz Zafar, Y. Li, Y. Zhang, S. Hussain, Z. Cheng, A. Kumaresan and H. Zhou, J. Mater. Chem. C, 2019, 7, 10795–10801 RSC.
- P. F. Cheng, H. C. Liu and Y. T. Zhang, Adv. Mater. Res., 2011, 393–395, 135–138 Search PubMed.
- L. J. A. Koster, Appl. Phys. Lett., 2005, 87, 203502 CrossRef.
- P. Tiwana, P. Docampo, M. B. Johnston, H. J. Snaith and L. M. Herz, ACS Nano, 2011, 5, 5158–5166 CrossRef CAS PubMed.
- F. A. Selim, M. H. Weber, D. Solodovnikov and K. G. Lynn, Phys. Rev. Lett., 2007, 99, 085502 CrossRef CAS PubMed.
- A. Janotti and C. G. Van de Walle, Rep. Prog. Phys., 2009, 72, 126501 CrossRef.
- S. Chen, C. E. Small, C. M. Amb, J. Subbiah, T. Lai, S.-W. Tsang, J. R. Manders, J. R. Reynolds and F. So, Adv. Energy Mater., 2012, 2(11), 1333–1337 CrossRef CAS.
- F. Zhao, L. Deng, K. Wang, C. Han, Z. Liu, H. Yu, J. Li and B. Hu, ACS Appl. Mater. Interfaces, 2020, 12, 5120–5127 CrossRef CAS.
- Y. Luo, S. Fang, N. Zheng, L. Liu, F. Würthner and Z. Xie, ACS Appl. Energy Mater., 2020, 3, 1694–1701 CrossRef CAS.
- M. Zhou and K. Hou, Thin Solid Films, 2019, 692, 137577 CrossRef CAS.
- Z. Zheng, S. Zhang, J. Wang, J. Zhang, D. Zhang, Y. Zhang, Z. Wei, Z. Tang, J. Hou and H. Zhou, J. Mater. Chem. A, 2019, 7, 3570–3576 RSC.
- F. Bu, W. Shen, X. Zhang, Y. Wang, L. A. Belfiore and J. Tang, Nanomaterials, 2020, 10, 80 CrossRef CAS.
- J. Li, Y. Bai, B. Yang, J. Zhang, X. Chen, T. Hayat, A. Alsaedi, Y. Yang, J. Hou and Z. Tan, J. Mater. Chem. A, 2019, 7, 2667–2676 RSC.
- A. Seitkhan, M. Neophytou, M. Kirkus, E. Abou-Hamad, M. N. Hedhili, E. Yengel, Y. Firdaus, H. Faber, Y. Lin, L. Tsetseris, I. McCulloch and T. D. Anthopoulos, Adv. Funct. Mater., 2019, 29, 1905810 CrossRef CAS.
- J. E. Carlé, M. Helgesen, O. Hagemann, M. Hösel, I. M. Heckler, E. Bundgaard, S. A. Gevorgyan, R. R. Søndergaard, M. Jørgensen, R. Garcìa-Valverde, S. Chaouki-Almagro, J. A. Villarejo and F. C. Krebs, Joule, 2017, 1, 274–289 CrossRef.
- https://www.nrel.gov/pv/module-efficiency.html, accessed 10.10.2020.
- Y.-C. Huang, H.-C. Cha, C.-Y. Chen and C.-S. Tsao, Prog. Photovoltaics, 2017, 25, 928–935 CAS.
- M. Jørgensen, J. E. Carlé, R. R. Søndergaard, M. Lauritzen, N. A. Dagnæs-Hansen, S. L. Byskov, T. R. Andersen, T. T. Larsen-Olsen, A. P. L. Böttiger, B. Andreasen, L. Fu, L. Zuo, Y. Liu, E. Bundgaard, X. Zhan, H. Chen and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2013, 119, 84–93 CrossRef.
- R. Po, A. Bernardi, A. Calabrese, C. Carbonera, G. Corso and A. Pellegrino, Energy Environ. Sci., 2014, 7, 925–943 RSC.
- R. Po, G. Bianchi, C. Carbonera and A. Pellegrino, Macromolecules, 2015, 48, 453–461 CrossRef CAS.
- X. Li, F. Pan, C. Sun, M. Zhang, Z. Wang, J. Du, J. Wang, M. Xiao, L. Xue, Z.-G. Zhang, C. Zhang, F. Liu and Y. Li, Nat. Commun., 2019, 10, 519 CrossRef CAS PubMed.
- A. S. Gertsen, M. Fernández Castro, R. R. Søndergaard and J. W. Andreasen, Flexible Printed Electron., 2020, 5, 014004 CrossRef CAS.
- L. Lucera, P. Kubis, F. W. Fecher, C. Bronnbauer, M. Turbiez, K. Forberich, T. Ameri, H.-J. Egelhaaf and C. J. Brabec, Energy Technol., 2015, 3, 373–384 CrossRef.
- M. J. Tan, S. Zhong, J. Li, Z. Chen and W. Chen, ACS Appl. Mater. Interfaces, 2013, 5, 4696–4701 CrossRef CAS PubMed.
- C.-Y. Liao, Y. Chen, C.-C. Lee, G. Wang, N.-W. Teng, C.-H. Lee, W.-L. Li, Y.-K. Chen, C.-H. Li, H.-L. Ho, P. H.-S. Tan, B. Wang, Y.-C. Huang, R. M. Young, M. R. Wasielewski, T. J. Marks, Y.-M. Chang and A. Facchetti, Joule, 2020, 4, 1–18 CrossRef PubMed.
- Y. Wu, H. Yang, Y. Zou, Y. Dong, J. Yuan, C. Cui and Y. Li, Energy Environ. Sci., 2019, 12, 675–683 RSC.
- I. Jeon, R. Sakai, S. Seo, G. E. Morse, H. Ueno, T. Nakagawa, Y. Qian, S. Maruyama and Y. Matsuo, J. Mater. Chem. A, 2018, 6, 5746–5751 RSC.
- F. Hermerschmidt, A. Savva, E. Georgiou, S. M. Tuladhar, J. R. Durrant, I. McCulloch, D. D. C. Bradley, C. J. Brabec, J. Nelson and S. A. Choulis, ACS Appl. Mater. Interfaces, 2017, 9, 14136–14144 CrossRef CAS PubMed.
- S. Song, K. T. Lee, C. W. Koh, H. Shin, M. Gao, H. Y. Woo, D. Vak and J. Y. Kim, Energy Environ. Sci., 2018, 11, 3248–3255 RSC.
- W. Liu, H. Shi, T. R. Andersen, N. K. Zawacka, P. Cheng, E. Bundgaard, M. Shi, X. Zhan, F. C. Krebs and H. Chen, RSC Adv., 2015, 5, 36001–36006 RSC.
- K. Liu, T. T. Larsen-Olsen, Y. Lin, M. Beliatis, E. Bundgaard, M. Jørgensen, F. C. Krebs and X. Zhan, J. Mater. Chem. A, 2016, 4, 1044–1051 RSC.
- Y. W. Han, S. J. Jeon, H. S. Lee, H. Park, K. S. Kim, H.-W. Lee and D. K. Moon, Adv. Energy Mater., 2019, 9, 1902065 CrossRef CAS.
- X. Guo, H. Li, Y. Han, Y. Yang, Q. Luo, C.-Q. Ma and J. Yang, Org. Electron., 2020, 82, 105725 CrossRef CAS.
- M. Yi, S. Hong, J.-R. Kim, H. Kang, J. Lee, K. Yu, S. Kee, W. Lee and K. Lee, Sol. Energy Mater. Sol. Cells, 2016, 153, 117–123 CrossRef CAS.
- F. C. Krebs, T. Tromholt and M. Jørgensen, Nanoscale, 2010, 2, 873–886 RSC.
- M. Välimäki, P. Apilo, R. Po, E. Jansson, A. Bernardi, M. Ylikunnari, M. Vilkman, G. Corso, J. Puustinen, J. Tuominen and J. Hast, Nanoscale, 2015, 7, 9570–9580 RSC.
- P. Apilo, M. Välimäki, R. Po, K.-L. Väisänen, H. Richter, M. Ylikunnari, M. Vilkman, A. Bernardi, G. Corso, H. Hoppe, R. Roesch, R. Meitzner, U. S. Schubert and J. Hast, Sol. RRL, 2018, 2, 1700160 CrossRef.
- K. Chang, Y. Li, G. Du, M. Zhong, P. Yang, Y. Zhu, F. He, B. Mi, X. Zhao and W. Deng, ACS Appl. Mater. Interfaces, 2020, 12, 27405–27415 CrossRef CAS PubMed.
- T. R. Andersen, H. F. Dam, M. Hösel, M. Helgesen, J. E. Carlé, T. T. Larsen-Olsen, S. A. Gevorgyan, J. W. Andreasen, J. Adams, N. Li, F. Machui, G. D. Spyropoulos, T. Ameri, N. Lemaître, M. Legros, A. Scheel, D. Gaiser, K. Kreul, S. Berny, O. R. Lozman, S. Nordman, M. Välimäki, M. Vilkman, R. R. Søndergaard, M. Jørgensen, C. J. Brabec and F. C. Krebs, Energy Environ. Sci., 2014, 7, 2925–2933 RSC.
- C. Kapnopoulos, E. D. Mekeridis, L. Tzounis, C. Polyzoidis, A. Zachariadis, S. Tsimikli, C. Gravalidis, A. Laskarakis, N. Vouroutzis and S. Logothetidis, Sol. Energy Mater. Sol. Cells, 2016, 144, 724–731 CrossRef CAS.
- S. Strohm, F. Machui, S. Langner, P. Kubis, N. Gasparini, M. Salvador, I. McCulloch, H. Egelhaaf and C. J. Brabec, Energy Environ. Sci., 2018, 11, 2225–2234 RSC.
- J. Lee, Y. Seo, S. Kwon, D. Kim, S. Jang, H. Jung, Y. Lee, H. Weerasinghe, T. Kim, J. Y. Kim, D. Vak and S. Na, Adv. Energy Mater., 2019, 9, 1901805 CrossRef.
- M. Vilkman, K.-L. Väisänen, P. Apilo, R. Po, M. Välimäki, M. Ylikunnari, A. Bernardi, T. Pernu, G. Corso, J. Seitsonen, S. Heinilehto, J. Ruokolainen and J. Hast, ACS Appl. Energy Mater., 2018, 1, 5977–5985 CrossRef.
- S. Alem, N. Graddage, J. Lu, T. Kololuoma, R. Movileanu and Y. Tao, Org. Electron., 2018, 52, 146–152 CrossRef CAS.
- T. H. Lee, S. Oh, S. Rasool, S. Hong, C. E. Song, D. Kim, S. K. Lee, W. S. Shin and E. Lim, J. Mater. Chem. A, 2020, 8, 10318–10330 RSC.
- S.-I. Na, Y.-H. Seo, Y.-C. Nah, S.-S. Kim, H. Heo, J.-E. Kim, N. Rolston, R. H. Dauskardt, M. Gao, Y. Lee and D. Vak, Adv. Funct. Mater., 2018, 29, 1805825 CrossRef.
- M. O. Reese, S. A. Gevorgyan, M. Jørgensen, E. Bundgaard, S. R. Kurtz, D. S. Ginley, D. C. Olson, M. T. Lloyd, P. Morvillo, E. A. Katz, A. Elschner, O. Haillant, T. R. Currier, V. Shrotriya, M. Hermenau, M. Riede, K. R. Kirov, G. Trimmel, T. Rath, O. Inganäs, F. Zhang, M. Andersson, K. Tvingstedt, M. Lira-Cantu, D. Laird, C. McGuiness, S. Gowrisanker, M. Pannone, M. Xiao, J. Hauch, R. Steim, D. M. DeLongchamp, R. Rösch, H. Hoppe, N. Espinosa, A. Urbina, G. Yaman-Uzunoglu, J.-B. Bonekamp, A. J. J. M. van Breemen, C. Girotto, E. Voroshazi and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2011, 95, 1253–1267 CrossRef CAS.
- M. V. Khenkin, E. A. Katz, A. Abate, G. Bardizza, J. J. Berry, C. Brabec, F. Brunetti, V. Bulović, Q. Burlingame, A. Di Carlo, R. Cheacharoen, Y.-B. Cheng, A. Colsmann, S. Cros, K. Domanski, M. Dusza, C. J. Fell, S. R. Forrest, Y. Galagan, D. Di Girolamo, M. Grätzel, A. Hagfeldt, E. von Hauff, H. Hoppe, J. Kettle, H. Köbler, M. S. Leite, S. Liu, Y.-L. Loo, J. M. Luther, C.-Q. Ma, M. Madsen, M. Manceau, M. Matheron, M. McGehee, R. Meitzner, M. K. Nazeeruddin, A. F. Nogueira, Ç. Odabaşı, A. Osherov, N.-G. Park, M. O. Reese, F. De Rossi, M. Saliba, U. S. Schubert, H. J. Snaith, S. D. Stranks, W. Tress, P. A. Troshin, V. Turkovic, S. Veenstra, I. Visoly-Fisher, A. Walsh, T. Watson, H. Xie, R. Yıldırım, S. M. Zakeeruddin, K. Zhu and M. Lira-Cantu, Nat. Energy, 2020, 5, 35–49 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |