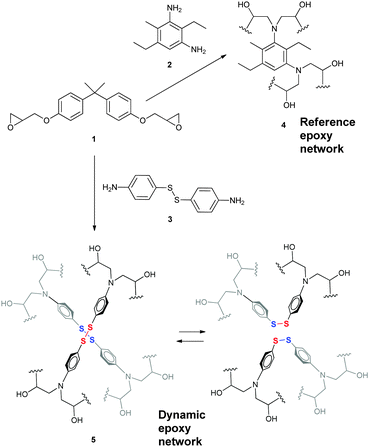
Epoxy resin with exchangeable disulfide crosslinks to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites†
Alaitz
Ruiz de Luzuriaga
,
Roberto
Martin
,
Nerea
Markaide
,
Alaitz
Rekondo
,
Germán
Cabañero
,
Javier
Rodríguez
and
Ibon
Odriozola
*
Materials Division, IK4-CIDETEC Research Centre, Paseo Miramón 196, 20009 Donostia-San Sebastián, Spain. E-mail: iodriozola@cidetec.es; Fax: +34 943309136; Tel: +34 943309022
First published on 7th March 2016
Abstract
Fiber-reinforced polymer composites (FRPCs) made of a “dynamic” epoxy resin possess good mechanical properties, while showing reprocessability, reparability and recyclability. The FRPCs are easily synthesized from readily available materials and could therefore be easily implemented in transportation, energy or construction industries, among others.
Conceptual insightsHere we present a new epoxy vitrimer which has many advantages compared to the existing ones (simplicity, low cost, scalability and excellent properties) and use it for the fabrication of reprocessable, repairable and recyclable fiber-reinforced polymer composites. To the best of our knowledge, this is the first time that a vitrimer material has been applied in the manufacture of fiber-reinforced polymer composites. Someday, fiber-reinforced polymer composites might live up to its hype and make all of our cars and airplanes more lightweight and efficient. Today, though, parts made of the material are very expensive to manufacture and are used mainly in race cars, high-end sports cars, and new jetliners. In addition, they cannot be reprocessed, repaired or recycled. The new technology demonstrated in this communication will open the way towards the high volume fabrication of fiber-reinforced polymer composites, which, although being thermoset, can be reprocessed, repaired and recycled. The impact of this technology will be tremendous for the automotive, aeronautic, construction and wind energy sectors, and will be a revolution in the composite field. |
1. Introduction
It is foreseen that the use of FRPCs in lightweight structures will strongly increase energy efficiency and conservation of resources. In this sense, constant demands for fuel-efficient vehicles and environmental regulations for low CO2 emissions drive the growth of the composite market. Among the different FRPCs, glass- or carbon-reinforced thermoset epoxy composites are usually preferred when structural performance is required. Epoxies generally outperform most other resin types in terms of mechanical properties and resistance to environmental degradation, which leads to their almost exclusive use in aircraft components. Other advantages include good thermal properties, high water resistance, low cure shrinkage, etc. However, due to their thermosetting nature, epoxy composites also have some very important drawbacks: once a thermoset composite is cured, it cannot be remolded, reshaped or dissolved, and therefore, their recycling or repair is extremely difficult. Moreover, their associated component manufacturing processes are mostly focused on low-medium volume productions, due to the relatively long curing-times required to chemically form the polymer network.Dynamic covalent chemistries1 have been recently applied for obtaining unconventional polymer networks with intriguing properties. Thus, new concepts such as dynamers,2,3 covalent adaptable networks (CANs)4–6 or vitrimers7 have emerged as hot topics in polymer and materials science. The latter concept, pioneered by Leibler and co-workers, is an especially interesting approach which could potentially overcome some of the above-mentioned limitations related to thermoset FRPCs. The idea underneath is to introduce exchangeable bonds in a polymer network, which can rearrange thermally (or under another stimulus), while maintaining network integrity. This enables unprecedented functionalities to such polymer networks, such as thermoforming, repairing, reprocessing or recycling. It is worth noticing that most of the state-of-the-art examples of dynamic thermosets are based on soft, non-structural materials,8,9 with a few exceptions.7,10–12
In a very recent and comprehensive minireview by Du Prez and co-workers,13 vitrimer materials developed so far have been overviewed according to the nature of the dynamic exchange reaction. The most popular dynamic chemistries used in the design of vitrimer or vitrimer-like materials have been carboxylate transesterification,7,14 transamination of vinylogous urethanes,12 transalkylation of triazolium salts,15 transcarbamoylation,16 siloxane silanol exchange,17 olefin metathesis,18 disulfide exchange19 and imine amine exchange.20 Despite the great scientific significance of all these systems, their use in real industrial applications, such as FRPCs, is far from being straightforward. This is due to the different issues which are inherent to the chemical nature of each system: catalyst insolubility, aging or leaching; long-term instability towards oxidation or hydrolysis; thermal degradation during reprocessing; low mechanical properties; scalability and cost; etc. In this scenario, the development of new industry-friendly vitrimer systems is still necessary, in order to obtain innovative thermoset structural materials which can be easily (re)processed, repaired and recycled.
Reversible epoxy networks based on Diels–Alder chemistries have been previously reported.21–23 However, even though these systems are reprocessable and recyclable, they are not considered vitrimers due to the dissociative nature of the Diels–Alder moieties. In these systems there is a temporary loss of cross-links, which results in a sudden viscosity drop, as is normally observed for thermoplastic materials. Having all this in mind, here we report a new epoxy vitrimer system, whose uniqueness compared to other related vitrimers relies on several aspects, such as (i) ease of synthesis from readily available starting materials in a scalable manner, (ii) fast stress relaxation at high temperature (vitrimer behavior) without the need of a catalyst, and (iii) easy applicability for the manufacture of (re)processable, repairable and recyclable FRPCs. This dynamic epoxy system is based on the reversible exchange of aromatic disulfides, an approach that we have recently reported for the creation of self-healing19 and reprocessable8 poly(urea-urethane) elastomers. Such an exchange reaction has been recently proposed to proceed via a radical-mediated [2+1] mechanism.24
2. Results and discussion
2.1. Synthesis and characterization of reference and dynamic epoxy resins
A wide range of epoxy monomers with different functionalities are commercially available and widely used in the composites industry. The reaction of these monomers with different hardeners (typically having amine, anhydride or carboxylic acid functionalities) gives access to a wide choice of epoxy networks, which can be tuned according to the targeted material properties by varying the monomers, hardeners or the stoichiometry. In order to constitute a representative example of a material with good mechanical properties and high glass transition temperature (Tg), diglycidyl ether of bisphenol A (DGEBA, 1) was chosen for being one of the most widely used epoxy monomers in the market. Diethyltoluenediamine (DETDA, 2) was chosen as the reference hardener, whereas 4-aminophenyl disulfide (AFD, 3) was chosen as the dynamic hardener (see Scheme 1).The epoxy networks were prepared by mixing DGEBA with the corresponding hardener in the bulk at 60–80 °C followed by a curing process at higher temperatures. The reaction was followed by Fourier transform infrared spectroscopy (FTIR), where the disappearance of bands corresponding to the epoxy group at 915 cm−1 (C–O stretching of the oxirane ring) and 3056 cm−1 (C–H stretching of the oxirane ring) was used as the criteria to establish that the curing was complete (see Fig. S1 in the ESI†).
No residual curing exothermic peak was observed by differential scanning calorimetry (DSC), confirming the complete curing of both epoxy networks (Fig. S2 and S3, ESI†). From the DSC thermograms, comparable Tg values were determined for the reference network 4 and the dynamic network 5, respectively (Table 1).
From the dynamic mechanical analysis (DMA), Tg and storage modulus (E′) values are shown to be comparable for both the reference network 4 (Fig. S4, ESI†) and the dynamic network 5 (Fig. S5, ESI†). A rubbery plateau of 20 MPa at 150 °C, typically attributed to chemically crosslinked polymer systems, confirmed the presence of a network (Table 1).
Thermogravimetric analysis (TGA) showed that both resins possess thermal stabilities up to 300 °C (Fig. S7 and S8, ESI†). The dynamic network 5 showed a slightly inferior degradation temperature (Td) (Table 1), which could be attributed to the presence of the disulfide species, which are energetically less stable than carbon–carbon bonds.
Tensile tests were performed in order to characterize the mechanical properties of both reference and dynamic epoxy resins (Fig. S9, ESI†). Both materials showed similar stress and strain at break (Table 1), which means that the mechanical properties of both systems are similar at temperatures below Tg.
Thus, the thermal and mechanical properties of both reference and dynamic networks resulted in being comparable. This indicates that the use of AFD instead of a classical diamine hardener (DETDA) does not, in principle, alter the thermal or mechanical performances of the resulting epoxy thermosets. This is quite important for potential industrial applications, as the substitution of the current resins for dynamic resins would not affect the performance of the final components.
2.2. Dynamic properties
As shown above, AFD could be used to obtain epoxy networks that are thermally and mechanically comparable to classical networks obtained using commercial diamine hardeners. However, for containing such reversible disulfide bridges, the dynamic network 5 was expected to show vitrimer behavior and thus some interesting features derived from it. As shown in the following subsections, stress relaxation was studied first, followed by reprocessing, repair and recycling tests. | (1) |
 | ||
| Fig. 1 (a) Normalized stress relaxation curves of dynamic epoxy network 5 at different temperatures. (b) Fitting of the relaxation times to the Arrhenius' equation (R-square = 0.946). | ||
A key characteristic for vitrimer materials is the topology freezing transition temperature (Tv). Tv corresponds to the temperature at which the transition from solid to liquid occurs as a result of bond exchange reactions in the network. Such a transition is considered at the point at which the viscosity becomes higher than 1012 Pa s.27–29 In our case, Tv could be determined by the extrapolation of the Arrhenius' fitted line (Fig. 1b) when τ* = 3 × 105 s.‡ For our dynamic epoxy system 5, the hypothetical Tv value obtained was −13 °C, which is well below its Tg (127 °C from DSC). This is indicative of a fast exchange reaction embedded in a rigid polymer matrix. In these cases, Tv is calculated via the extrapolation of stress relaxation.13 It is worth noticing that this transition is hypothetical, since the network is not frozen from the reaction kinetics, but by the lack of segmental motions associated with Tg. Thus, this would explain why at temperatures above Tg the relaxation of this system was very fast.
For comparison, the stress relaxation of the reference epoxy network 4 was measured at 160 °C, where no significant relaxation was observed (Fig. S11, ESI†).
In order to study the mechanical recycling of the dynamic epoxy network 5, the material was ground to fine powder, which was then compacted into a mold by applying heat and pressure. Fig. 2c shows photographs of the complete recycling sequence. Samples obtained after recycling showed perfect visual appearance. In addition, the mechanical properties of the mechanically recycled materials resulted to be comparable to the pristine materials (Fig. S10, ESI†), as tensile tests showed no changes in stress and strain at break after recycling. This could be explained by the fact that the less energetically stable disulfide bonds would preferentially break during the grinding process, and due to their reversible nature, the reshuffling of such bonds would lead to a full recovery of mechanical properties during the compacting process.
As can be concluded from Fig. 2a, the reference epoxy resin 4 could not be recycled from powder.
2.3. Application in fiber-reinforced polymer composites
As mentioned in the Introduction, FRPCs based on thermoset epoxy resins are a class of high-performance structural materials with great potential in a broad variety of industrial sectors. However, the difficulties in (re)processing, repair or recycling, which are inherent to their thermoset nature, are limiting their fast growth in applications such as automotive. In the following sections, FRPCs made of the dynamic epoxy system 5 and glass- or carbon-fiber reinforcements are studied.The dynamic character of the epoxy resin 5 described here would permit the manufacture of a new class of “enduring prepregs”, as totally cured prepreg sheets could still be valid for the fabrication of multilayered composite parts. Thus, prepreg sheets made of the dynamic epoxy resin 5 would no longer need to be stored in refrigerators, avoiding all the above-mentioned drawbacks.
In order to demonstrate the above, eight single enduring prepreg sheets made from totally cured dynamic epoxy resin 5 were laid up and hot pressed. The result was the formation of a perfectly compacted multilayer composite (Fig. 3a). When equivalent prepreg sheets made of the reference epoxy resin 4 were subjected to the same process, no multilayered composite was obtained (Fig. 3b).
In order to demonstrate the effectiveness of such a manufacturing process, an equivalent multilayered composite laminate was made by manual lay-up (see Section 3.7. in the ESI†). As shown in Table 2, the mechanical properties of the laminate made from enduring prepregs showed superior compression, interlaminar shear, flexural and impact strengths. This could be attributed to a better compacting of such laminates, due to the higher pressure used in the processing of enduring prepregs.
| Composite preparation | Compression strength [MPa] | Interlaminar shear strength [MPa] | Flexural strength [MPa] | Impact strength [kJ m−2] |
|---|---|---|---|---|
| Manual composite lay-up | 242 ± 18 | 29 ± 1 | 557 ± 7 | 159 ± 18 |
| Enduring prepregs | 292 ± 16 | 37 ± 3 | 595 ± 39 | 194 ± 18 |
In order to demonstrate this, a 50 × 60 × 2 mm sheet of a multilayered dynamic carbon-fiber reinforced composite was manufactured using resin transfer molding (RTM). The sheet was then placed inside a preheated zig-zag mold and reprocessed in a hot press. After cooling to room temperature, a zig-zag shaped 3D composite was obtained (Fig. 4). An equivalent composite made from the reference epoxy 4 could not be reprocessed, but the composite was broken instead when subjected to the same process.
Delaminations are probably the most common failures in multilayered composite parts, which cannot be repaired easily. An approach to solve this problem could be the use of a dynamic epoxy resin, which could self-adhere by applying heat and pressure.
In order to investigate the repairing ability of FRPCs made of dynamic epoxy 5, the following experiment was carried out. First, 20 × 10 × 1.8 mm specimens (ISO 14130) were cut from an FRPC sheet manufactured from enduring prepregs (see Section 3.6. in the ESI†). Then, delamination was induced while performing interlaminar shear strength (ILSS) tests. ILSS tests are usually made to quantify the strength necessary to induce a delamination in the specimen. Thus, pristine specimens made of dynamic GFR epoxy composites gave an average strength value of 37 ± 3 MPa, showing visual evidence of delamination (Fig. 5i). The specimens were then subjected to 200 °C and 100 bar for 5 minutes in a hot press. After this, the reparation of the delamination was visible to the eye, as can be observed in Fig. 5ii. Finally the samples were subjected again to ILSS tests, showing an average strength value of 38 ± 2 MPa, which indicated a quantitative recovery of the damage.
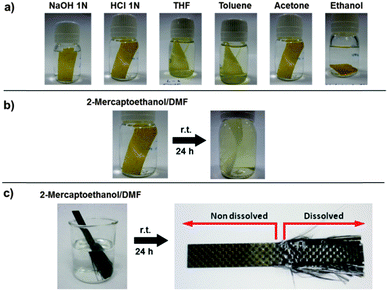
Chemical recycling. Similarly to conventional epoxy composites, dynamic epoxy composites showed good resistance to different chemical media such as THF, toluene, acetone, ethanol, NaOH or HCl (Fig. 6a). However, due to the reversible nature of the disulfide crosslinks, the resin could be easily dissolved in the presence of a thiol, due to the thiol–disulfide exchange reaction which disrupts such crosslinks.33 In order to prove this, a small fragment of the enduring epoxy prepreg was immersed in a solution of 2-mercaptoethanol in DMF. The system was magnetically stirred at room temperature for 24 hours, after which the epoxy matrix was completely dissolved (Fig. 6b). The glass-fiber reinforcement was recovered undamaged by drying in an oven at 100 °C under vacuum for 24 hours. The same experiment was performed using a multilayered carbon-fiber composite made of epoxy resin 5. As shown in Fig. 6c, the carbon fibers were recovered undamaged after matrix dissolution.
Mechanical recycling. As another interesting approach, FRPCs made of dynamic epoxy resin 5 could also be recycled mechanically. This involved a two-step process, consisting of (i) grinding FRPC scraps into fine powder and (ii) compression molding of the powder to obtain “2nd generation” parts (Fig. 7). It is worth noticing that in this case, the resulting short-fiber composite parts are not expected to have the mechanical performance of the initial continuous-fiber composite materials, and would therefore be used for other non-structural applications.
3. Conclusions
In the present communication we have shown that the combination of DGEBA and AFD leads to an epoxy vitrimer with very fast relaxation times and low activation energy. Unlike other reported vitrimer systems, here the starting materials are readily available and the synthetic process is easily scalable, which makes this system easily applicable for the manufacturing of FRPCs. The so prepared FRPCs show equivalent mechanical properties as the reference epoxy counterparts, while showing new features such as (re)processability, reparability and recyclability, as demonstrated in the above examples. The FRPCs presented here are manufactured using standard manufacturing processes and equipment, by just substituting the conventional hardener by AFD, which is readily available in tonnes at a competitive price. Thus, such a system constitutes a step towards the implementation of vitrimers in industrial applications, and offers the possibility of obtaining a new generation of fiber-reinforced composite structures with enhanced functional properties.Acknowledgements
Financial support from the Basque Government through the ELKARTEK ACTIMAT 2015 project is acknowledged. Izaskun Azcarate-Ascasua is acknowledged for technical help.Notes and references
- Y. Jin, C. Yu, R. J. Denman and W. Zhang, Chem. Soc. Rev., 2013, 42, 6634–6654 RSC.
- W. G. Skene and J.-M. Lehn, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 8270–8275 CrossRef CAS PubMed.
- N. Roy, B. Bruchmann and J.-M. Lehn, Chem. Soc. Rev., 2015, 44, 3786–3807 RSC.
- C. J. Kloxin, T. F. Scott, B. J. Adzima and C. N. Bowman, Macromolecules, 2010, 43, 2643–2653 CrossRef CAS PubMed.
- C. J. Kloxin and C. N. Bowman, Chem. Soc. Rev., 2013, 42, 7161–7173 RSC.
- C. N. Bowman and C. J. Kloxin, Angew. Chem., Int. Ed., 2012, 51, 4272–4274 CrossRef CAS PubMed.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965–968 CrossRef CAS PubMed.
- R. Martin, A. Rekondo, A. Ruiz de Luzuriaga, G. Cabanero, H. J. Grande and I. Odriozola, J. Mater. Chem. A, 2014, 2, 5710–5715 CAS.
- Z. Q. Lei, H. P. Xiang, Y. J. Yuan, M. Z. Rong and M. Q. Zhang, Chem. Mater., 2014, 26, 2038–2046 CrossRef CAS.
- J. M. García, G. O. Jones, K. Virwani, B. D. McCloskey, D. J. Boday, G. M. ter Huurne, H. W. Horn, D. J. Coady, A. M. Bintaleb, A. M. S. Alabdulrahman, F. Alsewailem, H. A. A. Almegren and J. L. Hedrick, Science, 2014, 344, 732–735 CrossRef PubMed.
- S. Billiet, K. De Bruycker, F. Driessen, H. Goossens, V. Van Speybroeck, J. M. Winne and F. E. Du Prez, Nat. Chem., 2014, 6, 815–821 CrossRef CAS PubMed.
- W. Denissen, G. Rivero, R. Nicolaÿ, L. Leibler, J. M. Winne and F. E. Du Prez, Adv. Funct. Mater., 2015, 25, 2451–2457 CrossRef CAS.
- W. Denissen, J. M. Winne and F. E. Du Prez, Chem. Sci., 2016, 7, 30–38 RSC.
- K. Yu, P. Taynton, W. Zhang, M. L. Dunn and H. J. Qi, RSC Adv., 2014, 4, 10108–10117 RSC.
- M. M. Obadia, B. P. Mudraboyina, A. Serghei, D. Montarnal and E. Drockenmuller, J. Am. Chem. Soc., 2015, 137, 6078–6083 CrossRef CAS PubMed.
- D. J. Fortman, J. P. Brutman, C. J. Cramer, M. A. Hillmyer and W. R. Dichtel, J. Am. Chem. Soc., 2015, 137, 14019–14022 CrossRef CAS PubMed.
- P. Zheng and T. J. McCarthy, J. Am. Chem. Soc., 2012, 134, 2024–2027 CrossRef CAS PubMed.
- Y.-X. Lu, F. Tournilhac, L. Leibler and Z. Guan, J. Am. Chem. Soc., 2012, 134, 8424–8427 CrossRef CAS PubMed.
- A. Rekondo, R. Martin, A. Ruiz de Luzuriaga, G. Cabanero, H. J. Grande and I. Odriozola, Mater. Horiz., 2014, 1, 237–240 RSC.
- P. Taynton, K. Yu, R. K. Shoemaker, Y. Jin, H. J. Qi and W. Zhang, Adv. Mater., 2014, 26, 3938–3942 CrossRef CAS PubMed.
- X. Kuang, G. Liu, X. Dong, X. Liu, J. Xu and D. Wang, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 2094–2103 CrossRef CAS.
- Y. Min, S. Huang, Y. Wang, Z. Zhang, B. Du, X. Zhang and Z. Fan, Macromolecules, 2015, 48, 316–322 CrossRef CAS.
- X. Chen, M. A. Dam, K. Ono, A. Mal, H. Shen, S. R. Nutt, K. Sheran and F. Wudl, Science, 2002, 295, 1698–1702 CrossRef CAS PubMed.
- J. M. Matxain, J. M. Asua and F. Ruiperez, Phys. Chem. Chem. Phys., 2016, 18, 1758–1770 RSC.
- L. H. Sperling, Introduction to Physical Polymer Science, John Wiley & Sons, New Jersey, USA, 2005 Search PubMed.
- J. P. Brutman, P. A. Delgado and M. A. Hillmyer, ACS Macro Lett., 2014, 3, 607–610 CrossRef CAS.
- J. C. Dyre, Rev. Mod. Phys., 2006, 78, 953–972 CrossRef CAS.
- C. A. Angell, Science, 1995, 267, 1924–1935 CAS.
- M. D. Ediger, C. A. Angell and S. R. Nagel, J. Phys. Chem., 1996, 100, 13200–13212 CrossRef CAS.
- G. C. Tesoro and V. Sastri, J. Appl. Polym. Sci., 1990, 39, 1425–1437 CrossRef CAS.
- V. R. Sastri and G. C. Tesoro, J. Appl. Polym. Sci., 1990, 39, 1439–1457 CrossRef CAS.
- A. Takahashi, T. Ohishi, R. Goseki and H. Otsuka, Polymer, 2016, 82, 319–326 CrossRef CAS.
- L. M. Johnson, E. Ledet, N. D. Huffman, S. L. Swarner, S. D. Shepherd, P. G. Durham and G. D. Rothrock, Polymer, 2015, 64, 84–92 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures; FT-IR, DSC, DMA, TGA and stress–strain graphics. See DOI: 10.1039/c6mh00029k |
| ‡ We used the Maxwell relation: η = G·τ*, G being the shear modulus. G was calculated from the tensile modulus as measured by DMA from the relation: G = E′/(2(1 + v)), with v ≈ 0.5, the Poisson's ratio usually used for rubbers, and E′ ≈ 10 MPa. |
| This journal is © The Royal Society of Chemistry 2016 |