Iodine-induced synthesis of sulfonate esters from sodium sulfinates and phenols under mild conditions†
Abstract
An iodine-induced synthesis of sulfonate esters via cross-coupling reactions of sodium sulfinates with phenols is reported. This synthetic route is low-cost, facile, green and efficient, and could afford the target products with good to excellent yields under mild conditions.
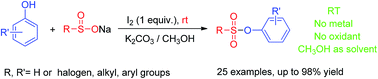

 Please wait while we load your content...
Please wait while we load your content...